Abstract
Background: Reliable non-invasive methods for detection of Helicobacter pylori infection are required to investigate the incidence, transmission, and clearance of infection in childhood.
Aim: To evaluate a new monoclonal enzyme immunoassay (EIA) (FemtoLab H pylori Cnx) for detection of H pylori antigen in stool in a large cohort of children compared with invasive diagnostic methods and the 13C urea breath test.
Patients and methods: A total of 302 symptomatic previously untreated children (aged 0.5–18.7 years; 148 girls) were recruited at three centres. H pylori status was defined by results of culture, histology, the rapid urease test, and the 13C urea breath test. Stool samples were investigated locally by the EIA using two different production lots. According to the manufacturer’s recommendations, an optical density (OD) of 0.150 was used as a cut off value.
Results: OD values clearly differentiated between the 92 H pylori infected and the 210 non-infected children (median (5th–95th percentiles) 2.729 (0.232–>4.000) v 0.021 (0.009–0.075)). Only two false positive and two false negative results occurred, giving a sensitivity, specificity, positive predictive value, and negative predictive value of 98%, 99%, 98%, and 99%, respectively. No significant relation was found between age and OD values in infected or non-infected children.
Conclusions: The monoclonal stool antigen EIA was excellent in diagnosing H pylori infection in symptomatic children. Accuracy was independent of the laboratory, production lot used, or the child’s age. Because only 18/116 children <6 years of age were infected with H pylori, further validation of the test is needed in young infected children.
Keywords: children, Helicobacter pylori, stool test, monoclonal enzyme immunoassay
Helicobacter pylori infection is almost always acquired in early childhood and usually persists throughout life unless a specific treatment is given. To investigate transmission, incidence, spontaneous clearance, and preventive measures in H pylori infection, non-invasive tests are required which must be reliable in all age groups, including toddlers, in which the incidence of new infections is highest.1,2 Serological tests show a low sensitivity in young children; also, serology cannot be considered a non-invasive test in children.3–5 The 13C urea breath test (UBT) has an excellent sensitivity regardless of age but specificity decreases in infants and young children.5–7 In addition, in this age group UBT requires trained staff for air sampling with a face mask, which may be unpleasant for the child. An enzyme immunoassay (EIA) to detect H pylori antigen in stool would circumvent these difficulties as stool samples can be obtained from children without their active collaboration. In addition, EIA tests do not require expensive instruments such as a mass spectrometer for UBT. Much experience has been gained with the HpSA (Meridian Diagnostics, Cincinnati, USA) which uses polyclonal antibodies to detect antigens in stool.8,9 Overall performance in diagnosing H pylori infection or evaluating the success of eradication therapy has been good10–12 but some limitations and discrepancies have been reported with respect to inter charge variability, cut off values, and lower accuracy after eradication therapy.13–15 The FemtoLab H pylori Cnx is a novel test based on monoclonal antibodies to detect H pylori antigen in faeces. Preliminary results using developmental test kits have been reported in children.13 The aim of this prospective multicentre study was to evaluate the monoclonal EIA for detection of H pylori infection in a large number of children compared with well defined H pylori status established by invasive diagnostic techniques and UBT.
PATIENTS AND METHODS
Patients
Over two years, 302 unselected children (148 girls, 154 boys) with abdominal symptoms were enrolled in three paediatric hospitals (Munich n=173; Amsterdam n=97; Paris n=32). All children underwent upper gastrointestinal endoscopy because of abdominal symptoms suggestive of organic disease. In all 302 children, stool test results were compared with H pylori status defined by the results of biopsy based methods (rapid urease test, culture, and histology) and/or UBT.
Children were excluded if they had taken antibiotic or acid suppressive drugs (proton pump inhibitors, H2 receptor antagonists, antacids, bismuth preparations) within four weeks prior to testing, if they had received previous antiH pylori therapy, or if the H pylori status was not clearly defined. The study was approved by the local ethics committees, and informed consent was obtained from the parents and children, if appropriate.
Definition of H pylori status
During upper endoscopy, two biopsies each from the gastric antrum and corpus were taken for histological examination, formalin fixed, stained with haematoxylin-eosin and modified Giemsa, and viewed for the presence of H pylori by a local pathologist who was blinded to the results of the other tests (n=302). One antral specimen each was obtained for the rapid urease test (n=222) and for bacterial culture (n= 309). Biopsies were transported to the local microbiological laboratory in transport media and processed within four hours. UBT (n=274) was performed according to a standardised protocol, as described previously.6 The test was defined as positive when changes from baseline values after 30 minutes were ≥5%.
H pylori status was defined as positive if culture and/or at least two of the other methods (histology, rapid urease test, UBT) gave positive results. A negative H pylori status was considered if all tests gave concordant negative results.
Stool antigen test
Parents of children scheduled for endoscopy were asked to bring a stool sample of their child at the time of the procedure or to send it in by mail within three days after endoscopy before any therapy was initiated. Samples were stored at −20°C until analysed.
The stool antigen test (FemtoLab H pylori Cnx (Martinsried, Germany), identical to HpStAR (DakoCytomation GmbH, Hamburg, Germany) and Ridascreen FemtoLab (R-Biopharm AG, Darmstadt, Germany) was performed according to the manufacturer’s recommendations at the three local microbiological laboratories using two different production lots. Those performing and reading the test were unaware of the H pylori status of the children tested. The stool antigen test is an EIA which uses monoclonal mouse anti-H pylori antibodies adsorbed to microwells as capture antibody. Firstly, 50 μl of supernatant of the diluted stool sample (0.1 g stool in 0.5 ml sample diluent) and thereafter 50 μl conjugated monoclonal antibody solution were added to the wells and incubated for one hour at room temperature on a shaker. Unbound material was removed by washing four times with a washing buffer. After washing, 100 μl of a substrate solution were added and incubated for 10 minutes. After addition of 100 μl of a stopping solution, the results were read by spectrophotometry (450/630 nm double wavelength).
According to the manufacturer’s guidelines, an optical density (OD) <0.150 was defined as a negative and an OD ≥0.150 as a positive test result.
Statistics
Sensitivity and specificity with confidence intervals, and positive and negative predictive values of the stool test were calculated against the defined H pylori status as gold standard. Statistical analysis was performed using SPSS (Statistical Package for Social Sciences version 9.1; SPSS Inc., Chicago, Illinois, USA). Correlation between age and OD values was analysed by the Spearman-Rho test. The likelihood ratios for a positive and negative test result were determined.16
RESULTS
According to the predefined criteria, 92 (30.5%) of 302 patients were H pylori positive; in 88/92 children, culture was positive for H pylori. The remaining 210 (69.5%) children had negative results in all diagnostic tests performed and were therefore considered H pylori negative.
Age of the children ranged from 0.5 to 18.7 years. A total of 116 patients were <6 years of age (18 were positive and 98 negative for H pylori), 106 were ≥6–<12 years (42 positive, 64 negative), and the remaining 80 children were ≥12 years of age (32 positive, 48 negative) (table 1▶). The proportions of infected to non-infected children in the three centres were as follows: in Munich, 55 to 118; in Amsterdam, 27 to 70; and in Paris, 10 to 22. The geographical background of the children’s families was Northern or Western Europe in 188 cases, 23 families came from Southern Europe, three from Eastern Europe, 45 from Turkey, nine from Asia, 27 from Africa, and seven from America.
Table 1 .
Performances of the monoclonal stool antigen test according to age group, and for all children
| Age group (y) | n (Hp+) | Sensitivity | Specificity | Accuracy | PPV | NPV |
|---|---|---|---|---|---|---|
| <6 | 116 (18) | 94.4 [72.7–99.9] | 98.0 [92.8–99.8] | 97.4 [92.6–99.5] | 89.5 [66.9–98.7] | 99.0 [94.4–99.9] |
| ≥6–<12 | 106 (42) | 97.6 [87.4–99.9] | 100 [94.4–100] | 99.1 [94.9–99.9] | 100 [91.4–100] | 98.5 [91.7–99.9] |
| ≥12–<18 | 80 (32) | 100 [89.1–100] | 100 [92.6–100] | 100 [95.5–100] | 100 [89.1–100] | 100 [92.6–100] |
| All children | 302 (92) | 97.8 [92.4–99.7] | 99.0 [96.6–99.9] | 98.7 [96.6–99.6] | 97.8 [92.4–99.7] | 99.0 [96.6–99.9] |
PPV, positive predictive value; NPV, negative predictive value.
Hp+, Helicobacter pylori positive.
Values are median [5th–95th percentiles].
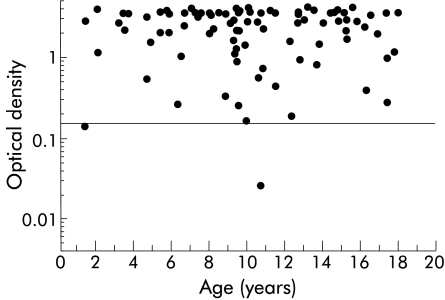
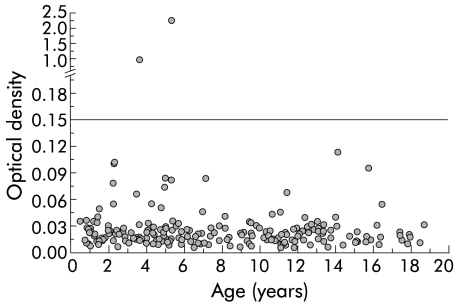
OD values in the EIA clearly differentiated between H pylori infected (median 2.729 (5th–95th percentiles 0.232–>4.000)) and non-infected children (0.021 (0.009–0.076)). No significant correlation was seen between age of the patient and OD values in the H pylori positive (r=−0.077, p=0.265) or negative (r=−0.024, p=0.821) groups (figs 1, 2▶▶).
Figure 1 .
Optical density (OD) values in relation to age in 92 children with a positive Helicobacter pylori status. The cut off value was set at an OD of 0.150.
Figure 2 .
Optical density (OD) values on a log scale in relation to age in 210 children with a negative Helicobacter pylori status. The cut off value was set at an OD of 0.150.
Four children were misclassified by the stool antigen test. Two patients from Munich had false positive results; one was infected by Campylobacter jejunii at the time of endoscopy. The two patients with false negative results came from Amsterdam; both had positive bacterial cultures. One of the four false results had an OD value that was close to the cut off value (fig 1▶). Sensitivity, specificity, accuracy, and positive and negative predictive values (with 95% confidence intervals) are presented in table 1▶ according to the three different age groups and globally for the total cohort. The likelihood ratio for a positive test result was calculated as 103.
DISCUSSION
To the best of our best knowledge, this is the first prospective multicentre based study of a novel monoclonal EIA stool test used to establish a diagnosis of H pylori infection in children. For every child, H pylori status was assessed using three different tests. In fact, culture, which is considered to be 100% specific, was successful in 88/92 children with a positive H pylori status. The monoclonal EIA on stool samples correctly classified 298 of 302 children, giving an accuracy of 98%.
To date, only one study has been published using this monoclonal EIA in children prior to treatment. Makristathis et al used a developmental kit provided by the manufacturer at a time when the test was not yet marketed.13 The authors performed the test in 79 children, 39 of whom were considered to be H pylori positive according to positive results from UBT and serology. The test yielded a sensitivity of 98% and a specificity of 97%, which is similar to our results of 98% and 99%, respectively. In our study, these excellent results were obtained in spite of the fact that the test was performed in three different laboratories using two different production lots. In contrast, the HpSA, which is of polyclonal origin, seems to have problems with lot to lot variability.13 This variability is reflected by the wider range of sensitivity and specificity values, reported in some studies to be as low as 63%, even in patients before therapy.8 In a recent multicentre European trial involving non-invasive tests in 316 children with a biopsy based H pylori status, HpSA achieved a sensitivity of only 72.7%.17
Differentiation between positive and negative results (figs 1, 2▶▶) is valuable. In contrast with the HpSA test, no grey zone is necessary. To improve the accuracy of the HpSA, some investigators have suggested adapting the cut off value.12,14,15
In accordance with our previous experience with the polyclonal test, we did not find any relation between OD values and patient age. This is particular advantageous in paediatric settings as both serology and UBT are less accurate in younger children.3,5,6,18 With respect to specificity, we are confident that the monoclonal EIA for detection of H pylori antigen in stool is excellent in children younger than six years of age, as 98 of the non-infected children in our study belonged to this age group, and 47 of those were less than three years of age. In contrast, only 18 H pylori infected children were less than six years of age and only four were less than three years of age. This is reflected by the larger 95% confidence intervals for sensitivity compared with specificity in this age group. Therefore, for final conclusions regarding sensitivity, more H pylori infected infants and toddlers need to be studied with the monoclonal antigen test.
We cannot judge how the test will perform in developing countries with a high prevalence of diarrhoea in young children due to gastrointestinal infections. It is noteworthy that one of the two children with a false positive test result suffered from Campylobacter jejunii infection at the time of testing, indicating that there might be some cross reactivity between the two bacterial species. Also, we need to establish how acid suppressive drugs or recent intake of different antibiotics influence the test results. We excluded all patients who had taken any of these medications during the four weeks prior to testing to obtain a well defined H pylori status, as both types of substances may suppress growth of H pylori and cause false negative results.8
In conclusion, the monoclonal EIA stool test is easy to perform and provides excellent differentiation between positive and negative test results. In symptomatic children the test is well suited for evaluation of H pylori status. The high accuracy seems to be independent of the laboratory, production lots, and age of the child. An age specific cut off value is not required, even in young children. Therefore, if further studies in children confirm our results, this test may become an excellent tool to study the incidence, spontaneous clearance of H pylori infection, and effect of preventive measures such as vaccination.
Acknowledgments
The study was supported by the Child Health Foundation, Munich. Connex GmbH, Martinsried, Germany, provided free test kits for determination of stool antigen.
Abbreviations
EIA, enzyme immunoassay
UBT, 13C urea breath test
OD, optical density
REFERENCES
- 1.Rothenbacher D, Inceoglu J, Bode G, et al. Acquisition of Helicobacter pylori infection in a high-risk population occurs within the first 2 years of life. J Pediatr 2000;136:744–8. [PubMed] [Google Scholar]
- 2.Thomas JE, Dale A, Harding M, et al. Helicobacter pylori colonization in early life. Pediatr Res 1999;45:218–23. [DOI] [PubMed] [Google Scholar]
- 3.Oliveira AMR, Rocha GA, Queiroz DM, et al. Evaluation of enzyme-linked immunosorbent assay for the diagnosis of Helicobacter pylori infection in children from different age groups with and without duodenal ulcer. J Pediatr Gastroenterol Nutr 1999;28:157–61. [DOI] [PubMed] [Google Scholar]
- 4.Kindermann A, Konstantopoulos N, Lehn N, et al. Evaluation of two commercial enzyme immunoassays, testing immunoglobulin G (IgG) and IgA responses, for diagnosis of Helicobacter pylori infection in children. J Clin Microbiol 2001;39:3591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koletzko S, Feydt-Schmidt A. Infants differ from teenagers: use of non-invasive tests for detection of Helicobacter pylori infection in children. Eur J Gastroenterol Hepatol 2001;13:1047–52. [DOI] [PubMed] [Google Scholar]
- 6.Kindermann A, Demmelmair H, Koletzko B, et al. Influence of age on 13C-urea breath test results in children. J Pediatr Gastroenterol Nutr 2000;30:85–91. [DOI] [PubMed] [Google Scholar]
- 7.Imrie C, Rowland M, Bourke B, et al. Limitations to carbon 13-labeled urea breath testing for Helicobacter pylori in infants. J Pediatr 2001;139:734–7. [DOI] [PubMed] [Google Scholar]
- 8.Gisbert JP, Pajares JM. Diagnosis of Helicobacter pylori infection by stool antigen determination: a systematic review. Am J Gastroenterol 2001;96:2829–38. [DOI] [PubMed] [Google Scholar]
- 9.Kabir S. Detection of Helicobacter pylori in faeces by culture, PCR and enzyme immunoassay. J Med Microbiol 2001;50:1021–9. [DOI] [PubMed] [Google Scholar]
- 10.Konstantopoulos N, Russmann H, Tasch C, et al. Evaluation of the Helicobacter pylori stool antigen test (HpSA) for detection of Helicobacter pylori infection in children. Am J Gastroenterol 2001;96:677–83. [DOI] [PubMed] [Google Scholar]
- 11.van Doorn OJ, Bosman DK, van’t Hoff BW, et al. Helicobacter pylori stool antigen test: a reliable non-invasive test for the diagnosis of Helicobacter pylori infection in children. Eur J Gastroenterol Hepatol 2001;13:1061–5. [DOI] [PubMed] [Google Scholar]
- 12.Oderda G, Rapa A, Ronchi B, et al. Detection of Helicobacter pylori in stool specimens by non-invasive antigen enzyme immunoassay in children: multicentre Italian study. BMJ 2000;320:347–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makristathis A, Barousch W, Pasching, et al. Two enzyme immunoassays and PCR for detection of Helicobacter pylori in stool specimens from pediatric patients before and after eradication therapy. J Clin Microbiol 2000;38:3710–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leodolter A, Agha-Amiri K, Peitz U, et al. Validity of a Helicobacter pylori stool antigen assay for the assessment of H. pylori status following eradication therapy. Eur J Gastroenterol Hepatol 2001;13:673–6. [DOI] [PubMed] [Google Scholar]
- 15.Forne M, Dominguez J, Fernandez-Banares F, et al. Accuracy of an enzyme immunoassay for the detection of Helicobacter pylori in stool specimens in the diagnosis of infection and posttreatment check-up. Am J Gastroenterol 2000;95:2200–5. [DOI] [PubMed] [Google Scholar]
- 16.Jaeschke R, Guyatt G, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA 1994;271:389–91. [DOI] [PubMed] [Google Scholar]
- 17.Megraud F, on behalf of the Paediatric Task Force of H. pylori infection. Non-invasive diagnostic test for Helicobacter pylori in children. Evaluation in a multicentric European study. Gut 2002;51(suppl ii):A81. [Google Scholar]
- 18.Imrie C, Rowland M, Bourke B, et al. Limitations to carbon 13-labeled urea breath testing for Helicobacter pylori in infants. J Pediatr 2001;139:734–7. [DOI] [PubMed] [Google Scholar]