Abstract
The prevalence of antibodies against hepatitis C virus (anti-HCV) among intravenous drug users (IVDU) has consistently been very high. Cross challenge studies in chimpanzees provide evidence that reinfection with different HCV strains may occur. In humans, reinfection with different HCV strains has been reported in multitransfused haemophiliacs and recently in IVDU but no case has been reported while on interferon (IFN) therapy. We report on a 22 year old woman who was treated with IFN alpha for HCV genotype 3a chronic infection. At six months, HCV RNA was undetectable by reverse transcription-polymerase chain reaction. In October 1997, while still on IFN, she developed an acute hepatitis after an intravenous drug injection and HCV genotype 1a infection was identified using genotyping and sequencing methods. IFN therapy was continued until August 1998, and in January 1999 HCV-RNA was not detectable. Our case indicates that the previous HCV infection might have prevented development of chronicity. An alternative explanation is that IFN, while not preventing acute hepatitis C, may prevent chronicity. The risk of multiple infection in IVDU underlines the need for preventive strategies.
Keywords: hepatitis C virus, reinfection, interferon, drug, genotype
Hepatitis C virus (HCV) is a major cause of chronic liver disease worldwide, and current or past intravenous drug use (IVDU) is the most common risk factor for infection in the Western world.1,2
HCV has been classified into six major genotypes, each with a variable number of more closely related subtypes.3 Studies in chimpanzees provide evidence that HCV reinfection may occur, suggesting a lack of protective immunity against HCV.4 In humans, reinfection with different HCV strains has been reported in multitransfused haemophiliacs.5
Second HCV infection after IVDU has been documented6–8 but no case has been reported while on interferon (IFN) therapy. We report on a 22 year old woman who developed a second HCV infection while she was undergoing IFN therapy.
CASE REPORT
In November 1996, a 22 year old woman was referred for chronic hepatitis C. She had a history of IVDU from 1994 to 1996. Alcohol consumption was negligible and she was not receiving any medication; neither had she been transfused. Physical examination showed no abnormality. Laboratory tests showed an alanine aminotransferase (ALT) level of 160 IU/l (normal <40 IU/l).
The presence of antibodies against HCV was detected with a third generation enzyme linked immunosorbent assay (Ortho HCV 3.0 ELISA test system; Ortho-Clinical Diagnostics, Germany) and confirmed by a third generation recombinant immunoblot assay (Chiron RIBA HCV 3.0; Ortho Diagnostic Systems). Serum HCV RNA was detectable with reverse transcription-polymerase chain reaction (RT-PCR) (Amplicor HCV test; Roche Diagnostic Systems, Neuilly sur seine, France). HCV genotyping performed in the 5′ non-coding region of the HCV genome using reverse hybridisation with the line probe assay (InGeN, Rungis, France)9 revealed a genotype 3a infection.
Complete hepatitis B virus (HBV) markers were negative. She refused vaccination against HBV. There was no marker of human immunodeficiency virus infection and no autoimmune antibodies. A liver biopsy was performed and histological examination revealed mild chronic hepatitis.
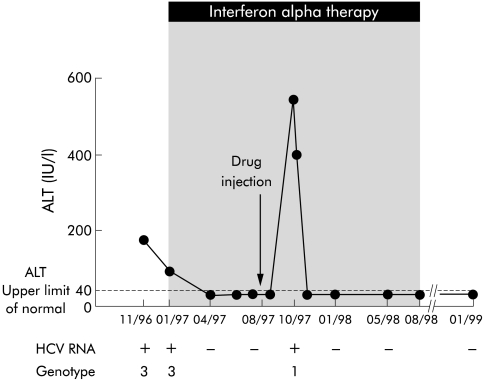
IFN therapy was started in January 1997 at a dose of 3 MU, three times a week (fig 1▶). At six months, ALT levels were normal and HCV RNA was undetectable. In October 1997, while still on IFN, she described general fatigue with increased ALT (peaks of 530 IU/l) and detectable serum HCV RNA. The notion of a single intravenous drug injection eight weeks previously was elicited. HCV genotype 1a was identified during this second HCV infection using a genotyping assay. Seroconversion for IgM antibodies to hepatitis B core antigen (anti-HBc) in the serum sample of October 1997 confirmed that HBV infection had occurred and resolved spontaneously. In all of these samples, HBV DNA was not detectable. IgG anti-HBc were detectable in the serum samples of January 1998 and January 1999. Markers for hepatitis delta virus were negative. IFN was continued until August 1998. In January 1999, she had normal ALT levels and absence of HCV-RNA.
Figure 1 .
Biochemical and virological profile of the patient. Serum hepatitis C virus (HCV) RNA was detected by reverse transcription-polymerase chain reaction. The horizontal line indicates the upper limit of normal for alanine aminotransferase (ALT). Interferon therapy was started in January 1997 and continued until August 1998.
VIROLOGICAL METHODS AND RESULTS
Serum samples were stored during clinical visits and were used for virological testing (fig 1▶). Serum HCV RNA level was 7 Meq genomes/ml in January 1997 (first infection) and 6 Meq genomes/ml in October 1997 (second infection) (Versant HCV RNA 3.0 Assay (bDNA); Bayer, Puteaux, France). Sequencing in the 5′ non-coding and the NS5B region of the HCV genome confirmed the results of genotyping. The viral sequence isolated in January 1997 was genotype 3a and that isolated in October 1997 was genotype 1a. To exclude the persistence of low levels of HCV RNA between the two infections, all RT-PCR negative serum samples were tested using two methods: the new RT-PCR assay (Cobas Amplicor HCV monitor assay) with a detection limit of 100 copies/ml, and the transcription mediated amplification assay (TMA, Versant HCV RNA qualitative assay, Gen-Probe; Bayer) with a sensitivity of 50 copies/ml. HCV RNA was not detectable in any of the samples tested.
To rule out the fact that the patient originally had a mixed infection with genotype 1a and 3, we developed an allele specific fluorescent amplification on a LightCycler (Roche Diagnostics) which enables fluorescent resonance energy transfer to be monitored during PCR. Briefly, 10 μl of a 10−6 Amplicor positive product were amplified using two sets of primers, located in the 5′UTR region, and specific for HCV genotypes 1 and 3, respectively. Fluorescence was generated by hybridisation of a 5′ TAMRA, 3′ FAM labelled TaqMan probe, common to all genotypes. Primers and probes were chosen with the assistance of the computer program Oligo 4.0 (National Bioscience, Plymouth, Minnesota, USA).
Quantitative values were obtained from the crossing point (CP) number at which the increase in the signal associated with exponential growth of PCR products begins to be detected using LightCycler analysis software, according to the manufacturer’s manual. This numerical value, which represent a number of PCR cycles, is proportional to the number of generated amplicons and reflects the specificity of the reaction.
To determine if we could detect a mixture of HCV genotype 1 and 3 infection (with genotype 1 minority), we pooled under the following conditions two serum samples genotyped as 1 and 3 and quantified at the same level (7 Meq genomes/ml), respectively: pool A, 1 part of genotype 1 added to 102 parts of genotype 3; pool B, 1 part of genotype 1 added to 103 parts of genotype 3; pool C, 1 part of genotype 1 added to 104 parts of genotype 3.
The patient samples before treatment (January 1997) and during the ALT peak (October 1997) were tested in the same run. CPs obtained with both sets of primers on pools A, B, and C indicated that we were able to specifically detect 1 copy of genotype 1 in 104 copies of genotype 3 (table 1▶). Before treatment (November 96 and January 97), amplification permitted detection of HCV genotype 3, and HCV genotype 1 was undetectable. In the serum sample corresponding to the new infection (October 1997), amplification permitted detection of HCV genotype 1, and HCV genotype 3 was undetectable.
Table 1 .
Results of amplification using real time polymerase chain reaction (PCR) with specific probes for hepatitis C virus (HCV) genotype 1 or HCV genotype 3
| Amplification product |
||
|---|---|---|
| Genotype 1 fragment | Genotype 3 fragment | |
| November 1996 | Negative | Positive (26) |
| January 1997 | Negative | Positive (28) |
| October 1997 | Positive (23) | Negative |
| Pool A | Positive (28) | Positive (22) |
| Pool B | Positive (33) | Positive (22) |
| Pool C | Positive (36) | Positive (24) |
| HCV genotype 1 | Positive (23) | Negative |
| HCV genotype 3 | Negative | Positive (25) |
| Non-template control | Negative | Negative |
Pool A, 1 part of genotype 1 added to 102 parts of genotype 3; pool B, 1 part of genotype 1 added to 103 parts of genotype 3; pool C, 1 part of genotype 1 added to 104 parts of genotype 3.
Numbers in parentheses are quantitative values (representing a number of PCR cycles) obtained from the crossing point (CP) number at which the increase in the signal associated with exponential growth of PCR products begins to be detected using LightCycler analysis software, according to the manufacturer’s manual.
DISCUSSION
The diagnosis of second HCV infection was based on the following: (a) virological response of HCV genotype 3 infection under therapy, as demonstrated by HCV-RNA clearance for more than six months; (b) a second peak of ALT with detectable HCV-RNA; (c) definite exposure to infection eight weeks prior to the second infection; (d) appearance of anti-HBc indicated that there was contact with contaminated material; (e) sequencing data confirmed the genotyping results and demonstrated that the acute hepatitis was the result of a second infection with another strain; and (f) finally, we developed a genotype specific amplification and ruled out a pre-existing coinfection.
Studies in chimpanzees provide evidence that reinfection with either homologous or heterologous strains of HCV may occur.4 In humans, reinfection with different HCV strains have been reported in multitransfused haemophiliacs.5 Cases of HCV second infection after intravenous drug use have been described previouslyl6–8 but no case has been reported while on IFN therapy. Recently, Mehta et al in a study of IVDU reported that occurrence of a second infection was lower in a previously infected group than in an initially uninfected group.8 Thus it is well known that a previous HCV infection does not confer a sterilising immunity. However, both human and chimpanzee data show that a previous infection or vaccination may prevent development of chronicity.
Approximately 85% of patients with acute hepatitis C develop persistent viraemia.10 Given the high rate of progression to chronic infection and the limited efficacy of therapy for chronic infection, treatment of acute infection is recommended.11 Recently, very high efficacy of IFN in acute hepatitis C was reported.12
The antiviral effects of IFN can be classified as direct and indirect. Direct antiviral effects are initiated by interaction of IFN and its receptor, which leads to production of antiviral polypeptides. Indirect antiviral effects result from the host immune response against infected cells. In a recent publication, the highest numbers of IFN-gamma producing HCV specific CD8+ T cells were found in patients with acute hepatitis C and a self limited course of disease.13
Resolution of acute infection is associated with induction of broad helper and cytotoxic T lymphocyte responses to the virus.14 We may postulate that the second acute infection resolved because she recovered from a previous HCV chronic infection and still had immune memory. It is important to keep in mind that on a sequence level genotypes 1a and 3a are quite similar. Thus it is fully plausible that a genotype 3a infection may prime T cells that are active against the genotype 1a infection. In fact, the development of acute hepatitis suggests that the patient did have an immune response against the new infection. This hypothesis is consistent with the results of the study from Mehta et al which showed that the frequency of viral persistence was lower in those who were previously infected than in those who were not.8
As it is established that HCV spontaneously evolves to chronicity in about 85% of cases, the alternative interpretation could be that IFN administration might have prevented chronicity. IFN may have boosted the immune response. However, there is no evidence to support the fact that the genotype 1a infection would not have been cleared in the absence of therapy (the pre-existing genotype 3a specific immunity may have been responsible).
In conclusion, our case indicates that a previous infection with one HCV genotype, while not preventing a new acute hepatitis with another genotype, may have prevented development of chronicity. In addition, an alternative explanation in our patient is that IFN administration might have prevented chronicity. The risk of multiple infection in intravenous drug users underlines the need for preventive strategies through education and use of sterile equipment.
Abbreviations
HCV, hepatitis C virus
HBV, hepatitis B virus
RT-PCR, reverse transcription-polymerase chain reaction
ALT, alanine aminotransferase
IFN, interferon
IVDU, intravenous drug users
anti-HBc, antibody to hepatitis B core antigen
CP, crossing point
REFERENCES
- 1.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001;345:41–52. [DOI] [PubMed] [Google Scholar]
- 2.Serfaty MA, Lawrie A, Smith B, et al. Risk factors and medical follow up of drug users tested for hepatitis C—can the risk of transmission be reduced? Drug Alcohol Rev 1997;16:339–47. [DOI] [PubMed] [Google Scholar]
- 3.Simmonds P, Holmes EC, Cha TA, et al. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J Gen Virol 1993;74:2391–9. [DOI] [PubMed] [Google Scholar]
- 4.Farci P, Alter HJ, Govindarajan S, et al. Lack of protective immunity against reinfection with hepatitis C virus. Science 1992;258:135–40. [DOI] [PubMed] [Google Scholar]
- 5.Lai ME, Mazzoleni AP, Argiolu F, et al. Hepatitis C virus in multiple episodes of acute hepatitis in polytransfused thalassaemic children. Lancet 1994;343:388–90. [DOI] [PubMed] [Google Scholar]
- 6.Payen JL, Izopet J, Barange K, et al. Hepatitis C virus reinfection after an intravenous drug injection. Gastroenterol Clin Biol 1998;22:469–70. [PubMed] [Google Scholar]
- 7.Proust B, Dubois F, Bacq Y, et al. Two successive hepatitis C virus infections in an intravenous drug user. J Clin Microbiol 2000;38:3125–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta SH, Cox A, Hoover DR, et al. Protection against persistence of hepatitis C. Lancet 2002;359:1478–83. [DOI] [PubMed] [Google Scholar]
- 9.Stuyver L, Rossau R, Wyseur A, et al. Typing of hepatitis C virus isolates and characterization of new subtypes using a line probe assay. J Gen Virol 1993;74:1093–102. [DOI] [PubMed] [Google Scholar]
- 10.Villano SA, Vlahov D, Nelson KE, et al. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology 1999;29:908–14. [DOI] [PubMed] [Google Scholar]
- 11.EASL International Consensus Conference on Hepatitis C. Paris, 26–27 February 1999. Consensus statement. J Hepatol 1999;31(suppl 1):3–8. [PubMed] [Google Scholar]
- 12.Jaeckel E, Cornberg M, Wedemeyer H, et al. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med 2001;345:1452–7. [DOI] [PubMed] [Google Scholar]
- 13.Gr]uner NH, Gerlach TJ, Jung MC, et al. Association of hepatitis C virus-specific CD8+ T cells with viral clearance in acute hepatitis C. J Infect Dis 2000;181:1528–36. [DOI] [PubMed] [Google Scholar]
- 14.Cerny A, Chisari FV. Pathogenesis of chronic hepatitis C: immunological features of hepatic injury and viral persistence. Hepatology 1999;30:595–601. [DOI] [PubMed] [Google Scholar]