Abstract
Background: The exocrine pancreas secretes large volumes of isotonic fluid, most of which originates from the ductal system. The role of aquaporin (AQP) water channels in this process is unknown.
Methods: Expression and localisation of known AQP isoforms was examined in normal human pancreas, pancreatic adenocarcinoma, and pancreatic cell lines of ductal origin (Capan-1, Capan-2, and HPAF) using reverse transcriptase-polymerase chain reaction and immunohistochemistry.
Results: Messenger RNAs for AQP1, -3, -4, -5, and -8 were detected in normal pancreas and in pancreatic adenocarcinoma. The cell lines expressed AQP3, -4, and -5 but lacked AQP1 and AQP8. Immunohistochemistry of normal pancreas revealed that AQP1 is strongly expressed in centroacinar cells and in both the apical and basolateral domains of intercalated and intralobular duct epithelia. AQP1 expression declined with distance along the small interlobular ducts and was not detectable in larger interlobular ducts. AQP3 and AQP4 were not detectable by immunohistochemistry. AQP5 was observed at the apical membrane of intercalated duct cells and also in duct associated mucoid glands. AQP8 was confined to the apical pole of acinar cells. Both AQP1 and AQP5 were colocalised with cystic fibrosis transmembrane conductance regulator (CFTR) at the apical membrane of intercalated duct cells.
Conclusions: AQP1 and AQP5 are strongly expressed in the intercalated ducts of the human pancreas. Their distribution correlates closely with that of CFTR, a marker of ductal electrolyte secretion. This suggests that fluid secretion is concentrated in the terminal branches of the ductal tree and that both AQP1 and AQP5 may play a significant role.
Keywords: aquaporin water channels, pancreatic duct, fluid secretion, cystic fibrosis transmembrane conductance regulator
The human pancreas secretes approximately 1 litre of bicarbonate rich juice into the duodenum every day. Much of the fluid is thought to be produced by epithelial cells lining the ductal system rather than by acinar cells.1 The near isotonicity of pancreatic juice2 suggests that transepithelial water movement is coupled osmotically to the active transport of electrolytes. However, such a mechanism requires a high transepithelial water permeability.
Aquaporin (AQP) water channels, first discovered in 1991,3,4 are known to enhance the water permeabilities of a wide range of epithelia. The aquaporins are small integral membrane proteins whose six α-helical membrane spanning domains surround a highly selective aqueous pore.5–7 Currently, at least 10 mammalian members of the family have been identified and many show very specific tissue localisation.8–10
Until recently, only AQP8 was thought to be associated with the secretory epithelia of the exocrine pancreas.11 However, immunohistochemistry of the rat pancreas revealed that this isoform is localised in the acinar cells, which are not thought to be the principal site of fluid secretion.12 Although AQP1 was known to be present in the vasculature, recent studies have provided evidence that AQP1 is also expressed in interlobular ducts.13 Low levels of mRNA for AQP5, an isoform associated with fluid secretion in other exocrine glands,14,15 have also been detected in rat pancreas but protein expression could not be demonstrated by immunohistochemistry.12,13 The only information on aquaporin expression in the human pancreas is an early immunofluorescence study which claimed to detect AQP1 expression in acinar cells.16
In order to resolve these conflicting observations, we have used immunohistochemistry in combination with reverse transcriptase-polymerase chain reaction (RT-PCR) to explore expression and localisation of a number of different aquaporin isoforms in the human pancreas, and also in the rat and mouse pancreas for comparison. As the cystic fibrosis transmembrane conductance regulator (CFTR) protein is a key transporter involved in secretin evoked pancreatic electrolyte secretion,1 we have used this as a functional marker to identify the ductal segments most likely to be the principal sites of fluid secretion.
METHODS
Tissue samples
Samples of human pancreas, pancreatic adenocarcinoma, salivary gland, and brain were taken from adult patients undergoing surgery who had previously given informed consent according to approved local procedures. Tissue samples destined for RT-PCR analysis were frozen in liquid nitrogen and stored at −70°C. Samples for immunohistochemistry were immersion fixed in formalin and processed for paraffin embedding.
Cell culture
Three human pancreatic adenocarcinoma cell lines of ductal origin were obtained from the American Type Culture Collection. Capan-1 and Capan-2 cells17,18 were maintained in RPMI 1640 medium (Sigma, St Louis, Missouri, USA). HPAF cells19 were maintained in Dulbecco’s modified Eagle’s medium (Sigma). Culture media were supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma). Cells were routinely cultivated in a humidified atmosphere of 5% CO2 and 95% air at 37°C.
RNA extraction and RT-PCR
Total RNA was extracted from human pancreas, pancreatic adenocarcinoma, and pancreatic cell lines using conventional methods.20 Reverse transcription was carried out on 5 μg RNA at 42°C using 200 U reverse transcriptase (SuperScript II; Gibco, Grand Island, New York, USA) according to the manufacturer’s instructions.
The PCR reactions for AQP1, -3, -4, and -8 were performed in a final volume of 20 μl using 0.5 U Takara Taq polymerase (Takara, Shiga, Japan), 0.5 μM each of the primers (table 1 ▶), and 200 μM dNTP in 10 mM Tris HCl buffer (pH 8.3) containing 50 mM KCl and 1.5 mM MgCl2. For AQP5, 0.5 U Promega Taq polymerase (Promega, Madison, Wisconsin, USA) was used together with 0.3 U Pfu DNA polymerase (Promega) in 20 mM Tris HCl buffer (pH 8.8) containing 10 mM KCl, 10 mM (NH4)2SO4, 2 mM MgSO4, 0.1% Triton X-100, and 0.1 mg/ml nuclease free bovine serum albumin. After denaturation at 94°C for three minutes, 30 cycles of amplification were performed for AQP1, -4, and -8 (94°C, 30 seconds; 63°C, 45 seconds; 72°C, 60 seconds), for AQP3 (94°C, 30 seconds; 65°C, 45 seconds; 72°C, 60 seconds), and for AQP5 (94°C, 30 seconds; 63°C, 30 seconds; 72°C, 120 seconds). Amplification was terminated by a final extension step at 72°C for 10 minutes in all cases. RT-PCR products were visualised on 1.5% agarose gels stained with ethidium bromide and were sequenced with the PRISM BigDye Terminator v3.0 sequencing kit (Applied Biosystems, Foster City, California, USA).
Table 1.
Primer sequences used for polymerase chain reactions for aquaporins AQP1, -3, -4, -5, and -8
| Sense | Antisense | Product size (bp) | |
| XS13 | 5′-CTGGCTAAGTTGGTTGCTTT-3′ | 5′-GCAGCTGATCAAGACTGGA-3′ | 500 |
| AQP1 | 5′-GTCTTCATCAGCATCGGTTC-3′ | 5′-GTCGGCATCCAGGTCATACT-3′ | 701 |
| AQP3 | 5′-CCTTTGGCTTTGCTGTCACTC-3′ | 5′-ACGGGGTTGTTGTAAGGGTCA-3′ | 373 |
| AQP4 | 5′-TCCCTTTGCTTTGGACTCAG-3′ | 5′-TTCCCCTTCTTCTCCTCTCC-3′ | 719 |
| AQP5 | 5′-CTTCCTCAAGGCCGTGTTC-3′ | 5′-GCTGGAAGGTCAGAATCAGC-3′ | 398 |
| AQP8 | 5′-GGTACGAACGGTTTGTGCAG-3′ | 5′-CCATCTCCAATGAAGCACCT-3′ | 667 |
XS13, acidic ribosomal phosphoprotein used as an internal control.
Antibodies
Previously characterised affinity purified polyclonal primary antibodies were used as follows: rabbit antirat/human AQP1 (Alpha Diagnostic International); rabbit antirat AQP3, LL178AP21; rabbit antirat AQP4, LL182AP22; rabbit antirat AQP5 (Alpha Diagnostic International, San Antonio, Texas, USA); rabbit antihuman AQP5 (kindly provided by Peter Agre and Landon King, Johns Hopkins University Medical School)23; and rabbit antihuman AQP8, 2669AP. For CFTR, a monoclonal mouse antihuman antibody was used (25031; R&D Systems, Minneapolis, Minnesota, USA). Non-immune IgG and non-immune rabbit serum were obtained from Dako (Glostrup, Denmark).
Immunohistochemistry
Paraffin embedded blocks of human pancreas were kindly provided by the Departments of Pathology, Aarhus University Hospitals. Non-tumour tissue was obtained from patients with pancreatic carcinoma. As described in detail elsewhere,24 2 μm sections were exposed to the primary antibody overnight, then to a horseradish peroxidase conjugated goat antirabbit secondary antibody (P448; Dako), and were finally counterstained with Mayer’s haematoxylin. For fluorescence microscopy, labelling was visualised with Alexa Fluor 488 goat antirabbit IgG (Molecular Probes, Leiden, the Netherlands) for AQP1 and -5, and with Alexa Fluor 546 goat antimouse IgG (Molecular Probes) for human CFTR. Sections were examined with a Leica SP2 confocal laser microscope.
Tissue was prepared for electron microscopy by freeze substitution, as described previously.12 Immunolabelling was performed on ultrathin sections (60 nm) which were incubated with rabbit antirat AQP1 overnight. Labelling was visualised with goat antirabbit IgG conjugated to 10 nm colloidal gold particles (BioCell Research Laboratories, Cardiff, UK). Control sections were incubated with non-immune IgG at 0.2 μg/ml dilution. Sections were stained with uranyl acetate for 10 minutes and examined on a Phillips CM100 electron microscope.
RESULTS
RT-PCR analysis
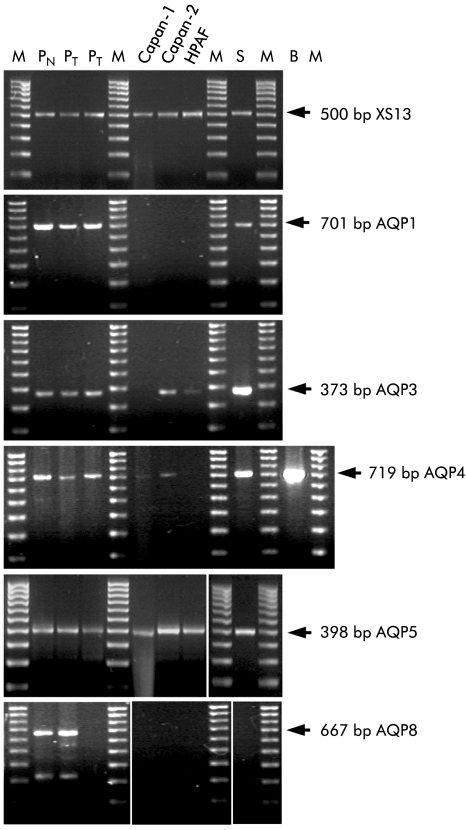
To examine aquaporin expression at the mRNA level, PCR primers were designed to amplify cDNA sequences specific to individual human AQP isoforms. The ribosomal phosphoprotein XS13 was used as an internal reference.25 PCR products of the expected size were obtained from normal human pancreas using primers specific for AQP1, -3, -4, -5, and - 8 (fig 1 ▶). The nucleotide sequences of the PCR products were all at least 98% identical with the published human sequences.
Figure 1.
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of aquaporin (AQP) expression in human pancreas, pancreatic tumours, and ductal cell lines. Agarose gels stained with ethidium bromide showing PCR products obtained with RNA extracted from human tissues and cell lines using specific primers for XS13 (an acidic ribosomal phosphoprotein used as an internal control), AQP1, -3, -4, -5, and -8. The concentration of the pancreatic cDNA was increased threefold for the AQP5 reaction. The arrows indicate the expected sizes of the products based on published sequences. Lane 1, normal pancreas (PN); lanes 2, 3, pancreatic tumours (PT); lane 4, Capan-1 cells; lane 5, Capan-2 cells; lane 6, HPAF cells; lane 7, parotid salivary gland (S); M, marker. The additional lane for AQP4 shows the positive control from human hippocampus (B).
Expression levels varied between samples (fig 1 ▶). AQP1 mRNA was readily detected in normal pancreas and pancreatic adenocarcinoma but was not present in any of the three cell lines. Messenger RNAs for AQP3 and AQP4 were clearly present in the normal pancreas and in the adenocarcinoma but were expressed at lower levels in the pancreatic cell lines. AQP5 mRNA was expressed at low levels in all of the samples while AQP8 mRNA was detected in normal human pancreas, and in one of the two adenocarcinoma samples, but not in the cell lines.
Immunohistochemical localisation of AQP1
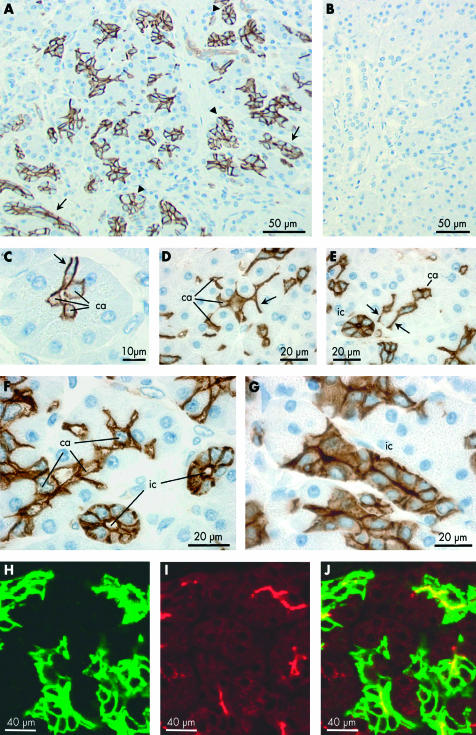
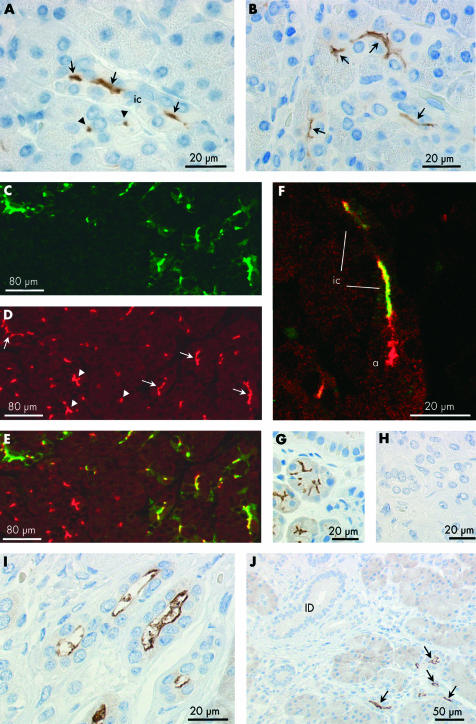
Immunoperoxidase labelling was performed on paraffin sections of normal human pancreas using an affinity purified primary antibody for AQP1 (fig 2A ▶). Labelling was completely abolished when the primary antibody was pre-adsorbed with the immunising peptide (fig 2B ▶). In fig 2A ▶, clusters of small AQP1 labelled cells and, occasionally, isolated cells can be seen within and among the acini throughout the lobular exocrine tissue. None of the secretory cells within the acini, identifiable by the zymogen granules present at their apical pole, was labelled. However, centroacinar cells, located at the centre of many of the acini, and sometimes extending to the edges of the acini, showed strong labelling of the plasma membranes (fig 2C–F ▶).
Figure 2.
Immunolocalisation of aquaporin 1 (AQP1) expression in centroacinar cells and intercalated ducts of human pancreas. (A–G) Immunoperoxidase labelling of human pancreas with an affinity purified antibody to AQP1 (2 μm paraffin sections). (A) Low magnification survey field showing scattered clusters of labelled cells, some clearly identifiable as intercalated or intralobular ducts in transverse (arrowheads) or longitudinal (arrows) section. (B) Similar section exposed to primary antibody pre-adsorbed with the immunising peptide. (C–E) Labelling of centroacinar (ca) cells. Some extend to the perimeter of the acinus (arrows), between neighbouring acinar cells, and show strong labelling of their lateral surfaces. Others show an extensive branching morphology with interdigitations between several acinar cells (D). (F, G) Apical and basolateral labelling of intercalated duct (ic) cells in transverse (F) and longitudinal (G) section. Intercalated ducts are identifiable by their small diameter (<20 μm), the small number of surrounding cells (typically 3–7 nuclei in transverse section), and very small luminal space. In (F), a chain of AQP1 labelled centroacinar cells extends along the lumen of a tubular acinus. (H–J) Immunofluorescence labelling of a paraffin section of normal human pancreas showing apical and basolateral labelling of intercalated duct cells with AQP1 antibody (H, green), apical labelling with cystic fibrosis transmembrane conductance regulator (CFTR) antibody (I, red), and superimposed (J) showing colocalisation (yellow) of AQP1 and CFTR at the apical membrane. Because of the very small luminal diameter of the intercalated ducts, CFTR labelling is observed at isolated points of fluorescence in ducts cut transversely and only occasionally in longitudinal profiles of the duct lumen.
Also strongly labelled were the intercalated ducts (fig 2A ▶). These are seen as elongated and occasionally branching groups of cells in longitudinal section (fig 2G ▶) and as cartwheel-like structures in transverse section (fig 2E, F ▶). Both the apical and basolateral plasma membrane domains exhibited AQP1 labelling, and there was also some diffuse labelling of the cytoplasm. The abrupt transition from unlabelled acinar cells to strongly labelled intercalated duct cells, shown in fig 2G ▶, is particularly striking.
AQP1 was also detected in capillaries and other small blood vessels but was absent from the larger vessels and also from the endocrine cells in the islets of Langerhans (fig 3B ▶).
Figure 3.
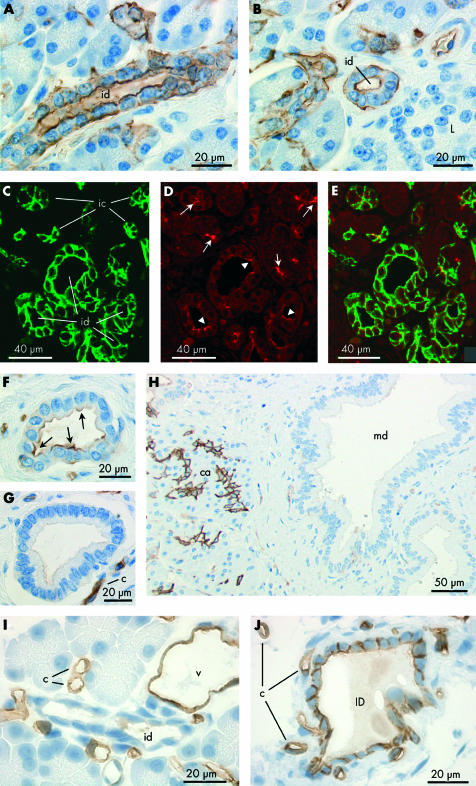
Immunolocalisation of aquaporin 1 (AQP1) expression in intra- and interlobular ducts of human pancreas (A–H) and rat pancreas (I–J). (A, B) Immunoperoxidase labelling of apical and basolateral membranes of intralobular ducts (id) in longitudinal (A) and transverse (B) section. Intralobular ducts are larger than intercalated ducts and have a clear luminal space surrounded by 8–14 small cuboidal cells when viewed in cross section. Note the absence of labelling from the islets of Langerhans (L) in (B). (C–E) Immuno- fluorescence labelling of intralobular ducts (id) with antibodies to AQP1 (C) and cystic fibrosis transmembrane conductance regulator (CFTR) (D), and superimposed (E). While AQP1 labelling is uniform over the apical and basolateral surfaces (C), CFTR labelling at the apical membrane is patchy (arrowheads, D). Intercalated duct cells (ic) also show colocalisation of AQP1 and CFTR (arrows, D) at the apical membrane. (F–H) Immuno- peroxidase labelling of AQP1 in interlobular and main ducts. Labelling is mainly at the apical membrane in small interlobular ducts (arrows, F) and virtually absent from the larger interlobular ducts (G) and main duct (md, H). Note the strong labelling of capillaries (c, G) and centroacinar cells (ca, H) which provide a positive internal control. (I, J) Immuno- peroxidase labelling of AQP1 in rat pancreas. Within the lobules (I), labelling was present in capillaries (c), venules (v), and intralobular ducts (ID). Labelling in the ductal system was confined to the interlobular ducts (id, J) and shows slightly stronger labelling at the apical and lateral surfaces of the epithelial cells than at the basal surface.
Colocalisation of AQP1 and CFTR in intercalated ducts
To compare localisation of AQP1 with the expected site of fluid secretion in the ductal system, CFTR was chosen as a functional marker of the secretory cells for double label immunofluorescence experiments. Figure 2H ▶ shows localisation of AQP1 (green) and this clearly follows the same general pattern as in fig 2A ▶. The more restricted distribution of CFTR is shown in the same section (red, fig 2I ▶) where it is confined to the apical membrane of the intercalated ducts and also acinar cells. When the two images are merged (fig 2J ▶), it is evident that AQP1 and CFTR are colocalised apically in the intercalated ducts. The one or two points of CFTR labelling that do not coincide with AQP1 labelling in fig 2J ▶ indicate CFTR expression at the apical membrane of the acinar cells. Extensive regions of AQP1 labelling that do not coincide with CFTR labelling, on the other hand, are clearly due to the presence of AQP1 in the basolateral membrane domains of the intercalated duct cells where CFTR is absent.
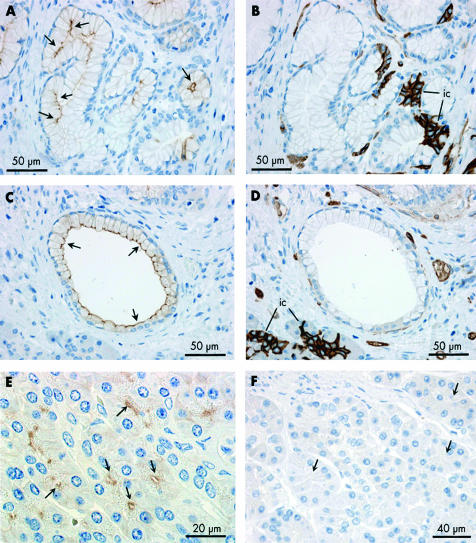
AQP1 distribution in intralobular and interlobular ducts of human pancreas
Although less common than intercalated ducts, intralobular ducts were occasionally observed between acini within lobules. These were labelled by the AQP1 antibody at both the apical and basolateral membranes although less strongly at the latter (fig 3A ▶, 3B ▶). The immunofluorescence images in fig 3C ▶–E show a group of five intralobular ducts, probably close to where they converge and emerge from the hilus of the lobule. All show AQP1 labelling of the apical and basolateral membranes (fig 3C ▶). CFTR labelling of the apical plasma membrane domains (fig 3D ▶) is much weaker here than in intercalated ducts that are visible elsewhere in the section.
Interlobular ducts, which are larger and located within the connective tissue septa between the lobules, showed much weaker AQP1 labelling (fig 3F ▶–H). In the small and medium sized interlobular ducts, AQP1 was observed at the apical membrane but rarely at the basolateral membrane (fig 3F ▶, 3G ▶). In the larger interlobular ducts (fig 3H ▶), there was no evidence of any AQP1 expression in the ductal epithelium.
AQP1 distribution in rat and mouse pancreas
In a previous study of aquaporin expression in rat pancreas,12 we failed to detect any AQP1 expression in ductal cells despite strong labelling of endothelial cells in capillaries and other blood vessels. However, by raising the concentration of the AQP1 antibody, we have now been able to detect weak labelling of interlobular ducts (fig 3J ▶). Within the lobules of the gland however AQP1 expression could only be detected in the blood vessel endothelia and appeared to be totally absent from acini and intralobular ducts (fig 3I ▶). A similar pattern was observed in the mouse pancreas (data not shown).
Immunoelectron microscopy of AQP1 localisation in human pancreas
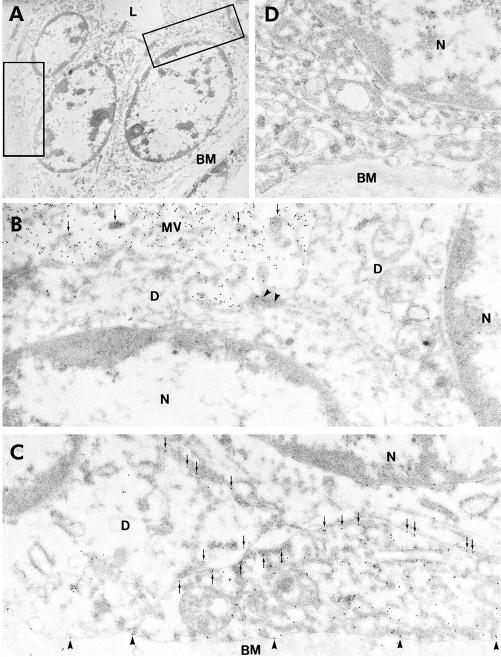
Figure 4A ▶ shows part of an intercalated duct which has been subjected to immunoelectron microscopy with an anti-AQP1 primary antibody. The apical regions of two adjacent cells are shown at higher magnification in fig 4B ▶ where dense labelling with gold particles is clearly associated with the microvilli at the apical membrane of intercalated duct cells. Figure 4C ▶ shows clear labelling of AQP1 in the basal and lateral plasma membrane domains.
Figure 4.
Immunoelectron microscopy of aquaporin 1 (AQP1) expression in intercalated duct of human pancreas. (A) Survey micrograph of an intercalated duct. Frames indicate areas magnified in (B) and (C). (B) Apical regions of two intercalated duct cells showing AQP1 labelling of microvilli (arrows) at the apical membrane. Arrowheads indicate the tight junction between adjacent duct cells. (C) Basal regions of neighbouring intercalated duct cells showing immunolabelling of AQP1 at both lateral (arrows) and basal membrane domains (arrowheads). (D) Control showing absence of labelling with non-immune IgG. L, lumen; BM, basement membrane; D, intercalated duct cell; N, nucleus; MV, microvilli. Magnification: A, ×3500; B–D, ×45 000.
Immunohistochemical localisation of AQP5
Immunoperoxidase labelling revealed that AQP5 is confined to the apical plasma membrane of intercalated duct cells (fig 5 ▶). Centroacinar cells did not exhibit any AQP5 immunolabelling. Although the very narrow lumen of intercalated ducts is only occasionally seen in longitudinal section, fig 5A ▶ shows the converging intercalated ducts from two acini with strong apical membrane labelling of AQP5. In the same section, a group of acinar cells and intercalated duct cells, sharing a common lumen, is seen in transverse section, as is another narrow intercalated duct. Both of these structures show dense apical AQP5 labelling. More examples of labelling in the intercalated ducts are shown in fig 5B ▶. AQP5 labelling was also observed at the apical membrane of intralobular ducts (fig 5I ▶) but was totally absent from interlobular ducts (fig 5J ▶).
Figure 5.
Immunolocalisation of aquaporin 5 (AQP5) in intercalated and intralobular ducts of human pancreas. (A, B) Immunoperoxidase labelling of AQP5 at the apical membrane of intercalated ducts (ic) in longitudinal (arrows) and transverse (arrowheads) section. Both antirat and antihuman AQP5 antibodies gave the same labelling pattern. The field in (A) corresponds to fig 2G ▶ which shows AQP1 labelling in a contiguous section. (C) Immuno- fluorescence labelling of AQP5 (green) at the apical membrane of intercalated ducts. (D) Cystic fibrosis transmembrane conductance regulator (CFTR) (red) at similar locations (arrows) and also in the lumen of the acini (arrowheads). (E) Merged images showing colocalisation of AQP5 and CFTR at the apical membrane of intercalated ducts (yellow). (F) Colocalisation of AQP5 (green) and CFTR (red) in the lumen of the intercalated duct (ic) leading from a single acinus. CFTR, but not AQP5, is seen at the apical membrane of the acinar cells (a). (G) Immunoperoxidase labelling of the apical membrane and secretory canaliculi of human submandibular acinar cells confirming the specificity of the AQP5 antibody. (H) Section of human pancreas exposed to the AQP5 antibody pre-adsorbed with the immunising peptide. (I) Immuno- peroxidase labelling of AQP5 at the apical membrane of intralobular duct cells in longitudinal profile. (J) Absence of AQP5 labelling in interlobular ducts (ID) despite strong labelling of intercalated and intralobular ducts (arrows).
Colocalisation of AQP5 and CFTR in intercalated ducts
To compare the distributions of AQP5 and CFTR, we again used the double label immunofluorescence technique. AQP5 labelling was confined to the apical plasma membrane in intercalated duct cells (fig 5C ▶) where it was colocalised with CFTR (fig 5D ▶, 5E ▶). In addition, some CFTR labelling at the centre of the acini was found not to be associated with AQP5 fluorescence. This is also evident in fig 5F ▶ which shows an intercalated duct leading into a single acinus. It is clear that CFTR labelling extends further into the centre of the acinus than AQP5 labelling.
AQP5 in mucoid ducts and glands
Mucoid glands were also observed occasionally in sections of human pancreas (fig 6A ▶). Their secretory cells have flattened basally located nuclei and an enlarged apical region. As shown in fig 6A ▶, the luminal membrane was labelled strongly with the AQP5 antibody. No AQP1 labelling was observed in these cells in a contiguous section (fig 6B ▶). A few larger ducts lined with similar cells also showed distinct apical AQP5 labelling (fig 6C ▶) and no AQP1 (fig 6D ▶).
Figure 6.
Immunolocalisation of aquaporin 5 (AQP5) and AQP1 in duct associated mucoid glands (A–D), AQP8 localisation in acinar cells of human pancreas (E), and immunolabelling control in human pancreas using non-immune IgG (F). (A, C) Immunoperoxidase labelling of AQP5 at the apical membrane of the glycoprotein secreting cells (arrows) of a mucoid gland (A) and a large mucoid duct (C). (B, D) Absence of AQP1 labelling of mucoid cells in a contiguous section. But note the characteristic strong labelling of AQP1 in intercalated ducts (ic) associated with the mucoid gland (B). The morphology of the mucoid duct epithelium (C, D) was quite different from that of the columnar epithelium which is typical of interlobular ducts (fig 3G ▶, 3H ▶). (E) Immunoperoxidase labelling of AQP8 (arrows) at the apical pole of acinar cells in human pancreas. (F) Immunolabelling control using non-immune IgG on section of human pancreas. No labelling was observed (arrows indicate acinar cells).
Other aquaporins
Despite the positive RT-PCR results for AQP3 and AQP4, we were unable to confirm expression of these isoforms by immunohistochemistry. An affinity purified antibody for AQP3, which gave positive results in human salivary glands as described previously,24 failed to produce plasma membrane labelling in human pancreas (not shown). Moreover, no labelling was detected with antibodies to rat or human AQP4 (not shown).
An antihuman AQP8 antibody showed distinct labelling of the apical plasma membrane domains of the acinar cells in close proximity to the zymogen granules (fig 6E ▶). The pattern of expression was similar to what we have reported previously in rat pancreas12 and there was no obvious labelling of intracellular sites or of other cell types. Immunolabelling controls using non-immune IgG or non-immune serum revealed no labelling (fig 6F ▶).
We therefore conclude that while AQP8 is expressed exclusively in acinar cells, AQP1 and AQP5 are abundantly expressed in intercalated duct cells, which are probably the main site of fluid secretion in human pancreas.
DISCUSSION
Our observations on the distribution of AQP1, -5, -8, and CFTR in the human exocrine pancreas are summarised in table 2 ▶. The fact that AQP1 is expressed in both the apical and basolateral membrane domains of intercalated duct cells, and also in centroacinar cells, suggests that these are major sites of transepithelial water flow. However, we found no evidence for the presence of AQP1 in acinar cells, as previously reported by Hasegawa and colleagues.16 AQP5 immunolabelling was observed in the apical plasma membrane of intercalated duct cells and colocalised with CFTR and AQP1, strongly indicating that AQP5 also participates in pancreatic fluid production.
Table 2.
Distribution of aquaporin (AQP) water channels and cystic fibrosis transmembrane conductance regulator (CFTR) in human pancreas
| Acinar cells | Intercalated ducts | Intralobular ducts | Small interlobular ducts | Large interlobular ducts | |||||||
| AP | BL | Centroacinar cells | AP | BL | AP | BL | AP | BL | AP | BL | |
| CFTR | ++ | − | ? | ++ | − | + | − | − | − | − | − |
| AQP1 | − | − | ++ | ++ | ++ | ++ | + | + | − | − | − |
| AQP5 | − | − | − | ++ | − | ++ | − | − | − | − | − |
| AQP8 | ++ | − | − | − | − | − | − | − | − | − | − |
AP, apical (luminal) membrane; BL, basolateral membrane.
When stimulated with secretin, the exocrine pancreas secretes large volumes of isotonic HCO3− rich fluid containing relatively little enzyme activity.1 As early as 1952, Hollander and Birnbaum proposed that this fluid originates from “flat centro-acinar and terminal-duct cells” rather than from acinar cells.26 Subsequent studies have supported the ductal origin of the HCO3− rich fluid—notably the observation that secretin evoked secretion persists in rats with severe acinar cell atrophy27 and that fluid and HCO3− secretion can be evoked in short segments of interlobular duct isolated from rat and guinea pig pancreas.28,29
The aquaporins normally associated with fluid secretion in exocrine glands are AQP5 and AQP8 at the apical membrane10,12,23,24,30 and AQP3 and AQP4 at the basolateral membrane.10,24 Most of these glands also express AQP1 but this is generally confined to the endothelia of blood vessels.31 It is therefore perhaps surprising to find AQP1 expressed apically and basolaterally in the ductal epithelial cells of the pancreas. On the other hand, its distribution in the pancreas is quite similar to that in the ductal system of the liver. Cholangiocytes, the epithelial cells that line the bile ductules, share many common features with pancreatic duct cells32 and they too express AQP1 at both the apical and basolateral membranes31,33 and at diffuse intracellular sites.34,35 Interestingly, Marinelli et al have suggested that AQP1 is subject to translocation from intracellular membranes to the apical plasma membrane in response to secretin.34,35 The cytoplasmic labelling that we have observed in intercalated duct cells might indicate a similar regulated translocation of AQP1 in the pancreas.
The fact that both centroacinar cells and intercalated duct cells show labelling for AQP1 supports the widely held view that centroacinar cells have a phenotype that is similar to that of intercalated duct cells.36–39 Indeed, centroacinar cells can probably be regarded as terminal intercalated duct cells which invaginate into the acinar lumen. In addition, we have shown that they may penetrate deeply, sometimes as far as the basement membrane, between adjacent acinar cells. This raises the interesting question of whether centroacinar cells have functionally distinct apical and basolateral membrane domains.40
Further down the ductal system, AQP1 expression appears to decline (table 2 ▶). This suggests that while intercalated and intralobular ducts are likely to be major sites of transepithelial water flow, interlobular and main ducts act principally as conduits for conveying the secreted fluid to the duodenum. Comparing localisation of AQP1 with that of CFTR, which plays an important role in HCO3− secretion, supports this hypothesis. In agreement with previous studies,41–43 we observed CFTR immunolabelling in the apical membrane of intercalated ducts but it was generally absent from interlobular ducts (table 2 ▶). Colocalisation of AQP1 and CFTR in the smaller ducts suggests that both electrolyte secretion and osmotic water flow mainly occur here. Within the acini we also detected some CFTR labelling which did not colocalise with AQP1. This indicates that CFTR is expressed at the apical membrane of acinar cells, as reported in rodents,44,45 although previously discounted in human pancreas.42,43
A remarkable feature of the human pancreas is coexpression of AQP1 and AQP5 at the apical membrane of intercalated duct cells. Although AQP5 immunolabelling appears relatively sparse compared with AQP1, this is partly because the lumen of intercalated ducts is extremely small, and partly because AQP5, unlike AQP1, is not expressed at the basolateral membrane, which has a much larger area. None the less, this result suggests that water transport across the very small apical surface of the intercalated ducts is facilitated by the presence of two aquaporin isoforms. Such redundancy might account for the lack of any obvious defect in pancreatic function in AQP1 null humans.46
We also observed strong AQP5 labelling of mucoid glands in the human pancreas where AQP5 was clearly expressed at the apical and lateral surfaces of the cells (fig 6A ▶). Glands of this type have been compared with Brunner’s glands39 and therefore it is interesting that AQP5 expression has recently been reported at the same cellular locations in Brunner’s glands of the duodenum in the rat.47
In our previous immunohistochemical study of aquaporin expression in the rat pancreas, AQP1 was clearly present in blood vessels but we failed to detect it in the ductal system.12 AQP1 has however recently been shown to be expressed, albeit at a low level, in interlobular ducts of the rat pancreas.13,48 We have now confirmed this, in both the rat and mouse, by using increased concentrations of AQP1 antibody (fig 3J ▶). There is however a striking difference in the distribution of AQP1 between the human and rodent pancreas. In the rat, AQP1 is absent from centroacinar cells, intercalated ducts, and intralobular ducts but present in the small and medium sized interlobular ducts.48 In humans, AQP1 is strongly expressed in centroacinar cells, intercalated ducts, and intralobular ducts, and declines with distance along the interlobular ducts. This suggests that there is a difference in the distribution of transepithelial water flow along the pancreatic ductal systems of humans and rodents. It may of course simply reflect the different sizes of the glands and the presence of a more extensive intralobular ductal system in the larger lobules of the human pancreas.
As anticipated from previous observations in the rat,12 immunolabelling of AQP8 was observed apically in acinar cells, in close proximity to zymogen granules (fig 6E ▶). AQP8 may therefore contribute to the production of a small amount of Cl − rich fluid that is thought to be associated with enzyme secretion. Although positive RT-PCR results were also obtained with primers for AQP3 and AQP4, neither of these isoforms was detected in normal human pancreas by immunohistochemistry. If they are present, they are presumably expressed at low levels and are therefore unlikely to participate significantly in pancreatic fluid secretion.
The two samples of pancreatic adenocarcinoma examined for AQP expression by RT-PCR resembled the normal pancreas closely (fig 1 ▶). However, only one of the two showed expression of AQP8 mRNA. As AQP8 is associated with the acinar cell phenotype, and as most tumours are of ductal origin, the absence of AQP8 from one of the samples is not surprising. It is possible that AQP8 mRNA detected in the other sample was derived from some normal acinar tissue associated with the tumour.
An interesting feature of the RT-PCR results from the pancreatic cell lines is the absence of mRNA for AQP1. Many of the well differentiated “ductal” adenocarcinomas of the pancreas are thought to derive from the epithelia of the larger interlobular ducts.49 As these ducts do not express AQP1, it is perhaps not surprising that the cell lines lack this isoform. Strangely however all three cell lines examined appear to express mRNA for AQP5. In our hands, immunolabelling of AQP5 in normal human pancreas was largely confined to the intercalated ducts, which also expressed abundant AQP1. Furthermore, Capan-1 cells have been shown to express CFTR50 which is also characteristic of intercalated and intralobular ducts rather than the large interlobular ducts. Taken together, these results suggest that the phenotypes of the cell lines do not exactly correspond to any particular segment of the pancreatic ductal system.
In summary, the principal finding of this study was that both AQP1 and AQP5 are strongly expressed in intercalated ducts of the human exocrine pancreas. Their expression correlates closely with the distribution of CFTR, a marker of ductal electrolyte secretion, and all three decline with distance downstream from the intercalated ducts. This suggests that ductal fluid secretion in the human pancreas is concentrated in the smallest terminal branches of the ductal tree and that both AQP1 and AQP5 may play a significant role in coupling the flow of water to active electrolyte secretion.
Acknowledgments
We thank Inger Merete Paulsen and Merete Pedersen for excellent technical assistance, Andi Mig for help with the RT-PCR studies, and Dr Peter Agre, Johns Hopkins University, for providing the antihuman AQP1 and AQP5 antibodies. The Water and Salt Research Center at the University of Aarhus was established and is supported by the Danish National Research Foundation (Danmarks Grundforskningsfond). This study was supported by grants from the Hungarian Ministry of Social Welfare (ETT 2001/33), the Karen Elise Jensen Foundation, the Human Frontier Science Program, the European Commission (QLRT 2000 00778), the Novo Nordic Foundation, the Danish Medical Research Council, the University of Aarhus Research Foundation, the University of Aarhus, the Dongguk University, and the Wellcome Trust, UK.
Abbreviations
AQP, aquaporin
CCK, cholecystokinin
CFTR, cystic fibrosis transmembrane conductance regulator
PBS, phosphate buffered saline
RT-PCR, reverse transcriptase-polymerase chain reaction
REFERENCES
- 1.Case RM, Argent BE. Pancreatic duct secretion: control and mechanisms of transport. In: Go VLW, DiMagno EP, Gardner JD, et al, eds. The Pancreas: Biology, Pathobiology, and Disease, 2nd edn. New York: Raven Press, 1993:301–50.
- 2.Case RM, Harper AA, Scratcherd T. Water and electrolyte secretion by the perfused pancreas of the cat. J Physiol 1968;196:133–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preston GM, Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci U S A 1991;88:11110–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Preston GM, Carroll TP, Guggino WB, et al. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 1992;256:385–7. [DOI] [PubMed] [Google Scholar]
- 5.Agre P, King LS, Yasui M, et al. Aquaporin water channels—from atomic structure to clinical medicine. J Physiol 2002;542:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borgnia M, Nielsen S, Engel A, et al. Cellular and molecular biology of the aquaporin water channels. Ann Rev Biochem 1999;68:425–58. [DOI] [PubMed] [Google Scholar]
- 7.Verkman AS, Mitra AK. Structure and function of aquaporin water channels. Am J Physiol 2000;278:F13–28. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen S, Frokiaer J, Marples D, et al. Aquaporins in the kidney: From molecules to medicine. Physiol Rev 2002;82:205–44. [DOI] [PubMed] [Google Scholar]
- 9.Ma TH, Verkman AS. Aquaporin water channels in gastrointestinal physiology. J Physiol 1999;517:317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen S, King LS, Christensen BM, et al. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Amer J Physiol 1997;273:C1549–61. [DOI] [PubMed] [Google Scholar]
- 11.Koyama Y, Yamamoto T, Kondo D, et al. Molecular cloning of a new aquaporin from rat pancreas and liver. J Biol Chem 1997;272:30329–33. [DOI] [PubMed] [Google Scholar]
- 12.Hurley PT, Ferguson CJ, Kwon TH, et al. Expression and immunolocalization of aquaporin water channels in rat exocrine pancreas. Am J Physiol 2001;280:G701–9. [DOI] [PubMed] [Google Scholar]
- 13.Ko SBH, Naruse S, Kitagawa M, et al. Aquaporins in rat pancreatic interlobular ducts. Amer J Physiol 2002;282:G324–31. [DOI] [PubMed] [Google Scholar]
- 14.Raina S, Preston GM, Guggino WB, et al. Molecular-cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J Biol Chem 1995;270:1908–12. [DOI] [PubMed] [Google Scholar]
- 15.Ma TH, Song YL, Gillespie A, et al. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem 1999;274:20071–4. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa H, Lian SC, Finkbeiner WE, et al. Extrarenal tissue distribution of CHIP28 water channels by in situ hybridization and antibody staining. Am J Physiol 1994;266:C893–903. [DOI] [PubMed] [Google Scholar]
- 17.Dahiya R, Kwak KS, Byrd JC, et al. Mucin synthesis and secretion in various human epithelial cancer cell lines that express the MUC-1 mucin gene. Cancer Res 1993;53:1437–43. [PubMed] [Google Scholar]
- 18.Fanjul M, Hollande E. Morphogenesis of duct-like structures in 3-dimensional cultures of human cancerous pancreatic duct cells (Capan-1). In Vitro Cell Dev Biol 1993;29A:574–84. [DOI] [PubMed] [Google Scholar]
- 19.Kim YW, Kern HF, Mullins TD, et al. Characterization of clones of a human pancreatic adenocarcinoma cell line representing different stages of differentiation. Pancreas 1989;4:353–62. [DOI] [PubMed] [Google Scholar]
- 20.Burghardt B, Wenger C, Barabás K, et al. GRP-receptor-mediated signal transduction, gene expression and DNA synthesis in the human pancreatic adenocarcinoma cell line HPAF. Peptides 2001;22:1119–28. [DOI] [PubMed] [Google Scholar]
- 21.Ecelbarger CA, Terris J, Frindt G, et al. Aquaporin-3 water channel localization and regulation in rat kidney. Amer J Physiol 1995;269:F663–72. [DOI] [PubMed] [Google Scholar]
- 22.Terris J, Ecelbarger CA, Nielsen S, et al. Long-term regulation of four renal aquaporins in rats. Amer J Physiol 1996;271:F414–22. [DOI] [PubMed] [Google Scholar]
- 23.Nejsum LN, Kwon TH, Jensen UB, et al. Functional requirement of aquaporin-5 in plasma membranes of sweat glands. Proc Natl Acad Sci U S A 2002;99:511–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gresz V, Kwon TH, Hurley PT, et al. Identification and localization of aquaporin water channels in human salivary glands. Am J Physiol 2001;281:G247–54. [DOI] [PubMed] [Google Scholar]
- 25.Wallrapp C, MullerPillasch F, SolinasToldo S, et al. Characterization of a high copy number amplification at 6q24 in pancreatic cancer identifies c-myb as a candidate oncogene. Cancer Res 1997;57:3135–9. [PubMed] [Google Scholar]
- 26.Hollander F, Birnbaum D. The role of carbonic anhydrase in pancreatic secretion. Trans NY Acad Sci 1952;15:56–8. [DOI] [PubMed] [Google Scholar]
- 27.Smith PA, Sunter JP, Case RM. Progressive atrophy of pancreatic acinar tissue in rats fed a copper-deficient diet supplemented with D-penicillamine or triethylenetetramine: morphological and physiological studies. Digestion 1982;23:16–30. [DOI] [PubMed] [Google Scholar]
- 28.Argent BE, Arkle S, Cullen MJ, et al. Morphological, biochemical and secretory studies on rat pancreatic ducts maintained in tissue culture. Q J Exp Physiol 1986;71:633–48. [DOI] [PubMed] [Google Scholar]
- 29.Ishiguro H, Naruse S, Steward MC, et al. Fluid secretion in interlobular ducts isolated from guinea-pig pancreas. J Physiol 1998;511:407–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He XJ, Tse CM, Donowitz M, et al. Polarized distribution of key membrane transport proteins in the rat submandibular gland. Pflugers Arch 1997;433:260–8. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen S, Smith BL, Christensen EI, et al. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci U S A 1993;90:7275–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyer JL. Bile-duct epithelium—frontiers in transport physiology. Am J Physiol 1996;33:G1–5. [DOI] [PubMed] [Google Scholar]
- 33.Roberts SK, Yano M, Ueno Y, et al. Cholangiocytes express the aquaporin chip and transport water via a channel-mediated mechanism. Proc Natl Acad Sci U S A 1994;91:13009–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marinelli RA, Tietz PS, Pham LD, et al. Secretin induces the apical insertion of aquaporin-1 water channels in rat cholangiocytes. Am J Physiol 1999;276:G280–6. [DOI] [PubMed] [Google Scholar]
- 35.Marinelli RA, Pham L, Agre P, et al. Secretin promotes osmotic water transport in rat cholangiocytes by increasing aquaporin-1 water channels in plasma membrane—Evidence for a secretin-induced vesicular translocation of aquaporin-1. J Biol Chem 1997;272:12984–8. [DOI] [PubMed] [Google Scholar]
- 36.Ekholm R, Edlund Y. Ultrastructure of the human exocrine pancreas. J Ultrastruct Res 1959;2:453–81. [Google Scholar]
- 37.Ekholm R, Zelander T, Edlund Y. The ultrastructural organization of the rat exocrine pancreas. II. Centroacinar cells, intercalary and intralobular ducts. J Ultrastruct Res 1962;7:73–83. [DOI] [PubMed] [Google Scholar]
- 38.Kodama T. A light and electron microscopic study on the pancreatic ductal system [DOI] [PubMed]
- 39.Egerbacher M, Bock P. Morphology of the pancreatic duct system in mammals. Microsc Res Tech 1997;37:407–17. [DOI] [PubMed] [Google Scholar]
- 40.Orci L, Perrelet A, Montesano R. Differential filipin labeling of the luminal membranes lining the pancreatic acinus. J Histochem Cytochem 1983;31:952–5. [DOI] [PubMed] [Google Scholar]
- 41.Strong TV, Boehm K, Collins FS. Localization of cystic fibrosis transmembrane conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J Clin Invest 1994;93:347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marino CR, Matovcik LM, Gorelick FS, et al. Localization of the cystic fibrosis transmembrane conductance regulator in pancreas. J Clin Invest 1991;88:712–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crawford I, Maloney PC, Zeitlin PL, et al. Immunocytochemical localization of the cystic fibrosis gene product CFTR. Proc Natl Acad Sci U S A 1991;88:9262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng WZ, Lee MG, Yan M, et al. Immuno and functional characterization of CFTR in submandibular and pancreatic acinar and duct cells. Am J Physiol 1997;273:C442–55. [DOI] [PubMed] [Google Scholar]
- 45.Kopelman H, Ferretti E, Gauthier C, et al. Rabbit pancreatic acini express CFTR as a cAMP-activated chloride efflux pathway. Am J Physiol 1995;38:C626–31. [DOI] [PubMed] [Google Scholar]
- 46.Preston GM, Smith BL, Zeidel ML, et al. Mutations in aquaporin-1 in phenotypically normal humans without functional CHIP water channels. Science 1994;265:1585–7. [DOI] [PubMed] [Google Scholar]
- 47.Parvin MN, Tsumura K, Akamatsu T, et al. Expression and localization of AQP5 in the stomach and duodenum of the rat. Biochim Biophys Acta 2002;1542:116–24. [DOI] [PubMed] [Google Scholar]
- 48.Furuya S, Naruse S, Ko SBH, et al. Distribution of aquaporin 1 in the rat pancreatic duct system examined with light- and election-microscopic immunohistochemistry. Cell Tiss Res 2002;308:75–86. [DOI] [PubMed] [Google Scholar]
- 49.Adler G, Kern HF. The human exocrine pancreas in health and disease. In: Riva A, Motta PM, eds. Ultrastructure of the Extraparietal Glands of the Digestive Tract. Boston: Kluwer, 1990:115–46.
- 50.Chambers JA, Harris A. Expression of the cystic fibrosis gene and the major pancreatic mucin gene, MUC1, in human ductal epithelial cells. J Cell Sci 1993;105:417–22. [DOI] [PubMed] [Google Scholar]