Abstract
p21WAF1/Cip1 is one of the best characterized downstream targets of p53, and the growth suppressing function of this cyclin-dependent kinase inhibitor is well established. However, whether p21 exerts a tumor-suppressing function of its own remains to be established. We report here that, similarly to loss of p53, disruption of the p21WAF1/Cip1 gene results in a markedly increased susceptibility to chemically induced skin carcinoma formation, whereas the number of papillomas is reduced. Previous evidence indicates that malignant versus benign keratinocyte tumor formation is likely to involve distinct target-cell populations with a different commitment to differentiation. In parallel with the increased susceptibility to carcinoma formation, loss of p21WAF1/Cip1 was found to promote keratinocyte subpopulations with increased growth/differentiation potential, including clonal growth capability, reversible commitment to differentiation, and capability to generate all types of terminally differentiated keratinocytes present in vivo, not only in the interfollicular epidermis but also in hair follicles. Thus, these findings have revealed a function of p21 as a suppressor of malignant but not benign skin-tumor formation and a determinant of the growth/differentiation potential of keratinocyte subpopulations.
Like other self-renewing tissues, the epidermis contains a compartment of cells that are capable of continuous self-regeneration and can replenish the populations of keratinocytes that are lost with differentiation. At least two kinds of proliferating keratinocyte populations are thought to exist: uncommitted “stem cells,” with an indefinite proliferation potential and capability to generate all other types of growing and differentiating keratinocytes, and “transit amplifying cells,” capable of a limited number of cell divisions and already committed toward differentiation (1). The existence of keratinocyte populations with different growth/differentiation potential has been well demonstrated in culture, and has been related, in vivo, to the presence of specific slow-cycling populations that reside at privileged sites of the epidermis and hair follicles and that may function as reservoir to generate all other keratinocytes (2–9).
These putative stem cells can be recruited into cycle under conditions of accelerated epidermal proliferation, such as during wound healing or in response to inflammatory or tumor-promoting stimuli (2). Slow-cycling stem-cell populations are also the likely target cells for chemically induced skin tumors (refs. 10 and 11, and refs. therein). In a conventional initiation–promotion protocol, most of the benign tumors or papillomas grow to a large size but do not progress to carcinomas, whereas the carcinomas arise from a distinct subset of papillomas with a high risk of malignant conversion (12, 13). Experimental evidence indicates that malignant versus benign keratinocyte tumor formation is likely to involve distinct target-cell populations with a different commitment to differentiation (14). This situation is similar to human pathology, where in many cases malignant epithelial tumors develop independently from their benign counterparts, whereas the latter rarely convert into malignancy (15).
Although the existence of epidermal stem cells has been well demonstrated, there are no unique biochemical markers for these cells, nor is it known what controls their growth/differentiation potential. The cyclin-dependent kinase inhibitor p21WAF1/Cip1 has been shown to play an important role in control of keratinocyte growth and differentiation. Like in other cell types, p21 expression is increased at the onset of keratinocyte differentiation, and this increase may contribute to the differentiation-associated growth arrest (16), even if it is by itself not essential (17). On the other hand, in the skin as in a number of other tissues, expression of p21 is induced in postmitotic cells immediately adjacent to the proliferative compartment but is decreased in cells further along the differentiation pathway (18, 19). A similar down-modulation of p21 expression occurs in cultured keratinocytes at late stages of differentiation, and direct overexpression of p21 in these cells inhibits differentiation in a manner that is not simply amenable to cell-cycle arrest (20). Thus, p21 may be part of both a positive mechanism triggering differentiation-associated growth arrest and a negative regulatory loop, which needs to be inactivated for differentiation to proceed. In the present communication, we show that p21 has additional regulatory functions in the skin as a suppressor of malignant, but not benign, tumor formation and as a determinant of the growth/differentiation potential of keratinocyte stem cell populations.
MATERIALS AND METHODS
Cells, Growth, and Attachment Assays.
Primary keratinocytes were derived either from wild-type and knockout newborn littermates generated by heterozygous p21+/− parents or from wild-type Sencar mice and mice carrying a homozygous disruption of the p21 gene and back-crossed up to the sixth or seventh generation into a Sencar background. Conditions for primary keratinocyte cultivation and induction of differentiation by Ca2+ are as described (16).
Clonal growth assays were performed by trypsinizing freshly confluent primary keratinocyte cultures (2–3 days after plating), counting, and plating decreasing amounts of cells (106, 105, and 104) in triplicate onto 60-mm dishes. Cells were cultured up to 12–14 days, with medium changes every other day, fixed with 100% ethanol at −20°C, and stained with 0.1% crystal violet.
Attachment assays were performed essentially as described by Rousselle and Aumalley (21). Briefly, 96-well Petri plates (Costar) were coated (100 μl per well) with serial dilutions (0–10 μg/ml) of collagen type IV, laminin, or fibronectin (Becton - Dickinson;) by overnight adsorption at 4°C. Plates were washed with PBS and incubated with 0.1% BSA for 30 min at room temperature to block nonspecific binding. Freshly confluent primary keratinocyte cultures (2–3 days after plating) were trypsinized, washed in medium containing 4% serum, and resuspended in serum-free medium. Cells were then plated onto the coated wells (1 × 105 cells per well) in triplicate and incubated for 90 min at 34°C. Wells were rinsed once with serum-free medium and once with 70% ethanol. Attached cells were fixed with 100% ethanol for 10 min at −20°C, stained with 0.1% crystal violet, and quantified by light absorbance by using an ELISA reader at 595 nm.
BrdUrd-labeling assays were performed by incubating cells with BrdUrd (1:300 dilution; cell proliferation reagent; Amersham Pharmacia) for the last 6 h before fixation in 2% paraformaldehyde in PBS. After cell permeabilization with 0.1% Nonidet P-40 and denaturation in 50 mM NaOH, BrdUrd-labeled DNA was detected by incubation with anti-BrdUrd mouse mAbs (1:4; Becton–Dickinson) and anti-mouse rhodamine-conjugated secondary antibodies (1:100; Vector Laboratories). Nuclei were stained with a 1 μg/ml solution of Hoechst dye 33258, and the fraction of BrdUrd-positive cells was determined by double-fluorescence microscopy.
Tumor-Induction Experiments.
p21 knockout mice (on an NIH Swiss background) were crossed with Sencar mice. The F1 p21+/− heterozygous were interbred, and the resulting littermates were divided into p21−/−, p21+/−, and p21+/+ groups. Sixteen to twenty mice of each group, in the resting phase of the hair cycle (7–8 weeks old), were shaved and treated with 7,12-dimethylbenz[a]anthracene (DMBA) (20 μg in 200 ml acetone) 48 hrs after shaving. Mice were subsequently treated twice a week with 12-O-tetradecanoylphorbol 13-acetate (PMA) (10−4 M in acetone) for 2 months. DMBA was dissolved in acetone just before use. PMA solution was stored at −70°C. Mice were sacrificed at the end of the experiment (32 weeks) or at earlier times in cases of excessive tumor load or other adverse signs. Papilloma-versus-carcinoma formation was assessed throughout the duration of the experiments, by macroscopic examination and palpation of the tumor base, to determine the onset of an invasive pattern of growth as well as infiltration of the surrounding tissues. Macroscopic analysis was confirmed by histological examination of all presumptive carcinomas and most aggressive-looking papillomas when the animals were sacrificed.
Skin/Hair Reconstitution Assay.
Skin/hair reconstitution assays were performed essentially as described by Lichti et al. (22) as modified by Kamimura et al. (23) and Prouty et al. (24). Primary keratinocytes from p21 +/+ and p21 −/− mice were infected with an alkaline phosphatase-transducing retrovirus 2 days after plating. Three days later, cells were trypsinized and mixed (1 × 107 per graft) with fresh dermal cells (8 × 106 per graft), which were prepared from the skin of newborn CD1 mice as described by Prouty et al. (24). Dense suspensions were injected into silicon transplantation chambers implanted onto the dorsal surface of nude mice. The transplantation chambers were removed 1 week after grafting, and mice were sacrificed 6 weeks later. Grafts were fixed in 4% paraformaldehyde at 4°C for 8 h, rinsed in PBS, and placed in 30% sucrose in PBS containing 2 mM MgCl2 overnight at 4°C. Samples were then embedded in OCT compound. Frozen sections were fixed in 4% paraformaldehyde for 10 min, rinsed twice in PBS at 4°C, and heated at 68°C for 30 min to destroy endogenous alkaline phosphatase (AP) activity. AP colorimetric reactions were performed as described by Cepko et al. (25). Essentially, sections were rinsed in X-P detection buffer (100 mM Tris⋅HCl, pH 9.5/100 mM NaCl/50 mM MgCl2) for 10 min and incubated in X-P reaction mix (0.1 mg of 5-bromo-4-chloro-3-indolyl phosphate per ml, 1 mg of nitroblue tetrazolium per ml in detection buffer) for 12 h in the dark. Sections were rinsed in PBS containing 20 mM EDTA three times for 10 min. Slides were then prepared for microscopic examination.
RESULTS
Loss of p21 Function Results in Increased Rate, Number, and Multiplicity of Carcinoma Formation.
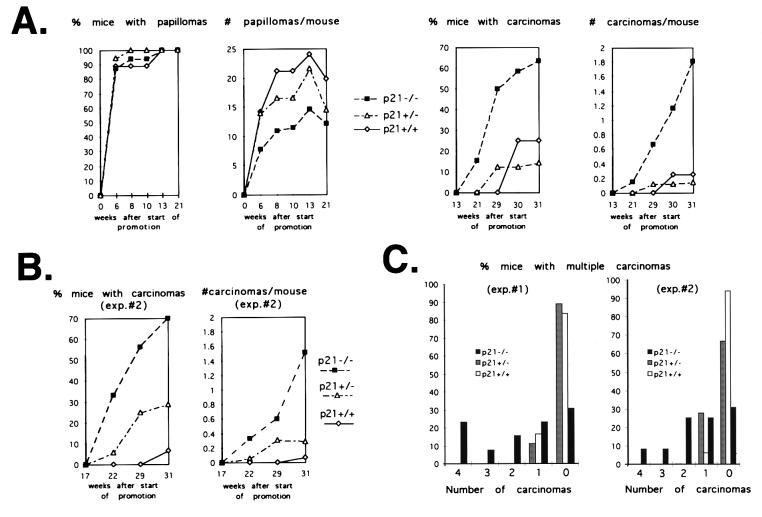
Initial studies of keratinocytes with a disruption of the p21 gene revealed that these cells have an intrinsic reduction of differentiation marker expression and produce rapidly growing tumors when transformed with a ras oncogene and injected subcutaneously into nude mice (17). Thus, although the skin of p21-deficient mice is apparently normal, loss of p21 function may still have important consequences for skin carcinogenesis and favor benign and/or malignant tumor development. To test these possibilities, p21 knockout mice and their wild-type and heterozygous littermates were subjected to a conventional chemical carcinogenesis protocol with a single dose of DMBA for initiation and biweekly treatments with PMA for promotion. All three groups of mice developed papillomas with a similar latency period (6–8 weeks), and the number of mice with papillomas was approximately the same. Interestingly, the number of papillomas per mouse was significantly lower in the p21 knockouts than in the wild-type mice, with intermediate values being found with the p21+/− heterozygous (Fig. 1A).
Figure 1.
Papilloma and carcinoma formation in p21−/− versus p21+/− and p21+/+ littermates. (A) The three groups of mice were subjected to a classical chemical carcinogenesis protocol as described in Materials and Methods. The arising papillomas and carcinomas were counted regularly, and the results of macroscopic analysis were confirmed histologically when the animals were sacrificed. Each group consisted of 15–20 animals. Statistical significance was determined as indicated in the text. (B) Carcinoma formation of p21−/− versus p21+/− and p21+/+ littermates in a second independent experiment performed similarly to the first. (C) Percentage of mice with multiple independent carcinomas, as counted in the two experiments at 30 weeks after the start of tumor promotion.
Unlike papillomas, carcinomas arose with a shorter latency period in the p21 knockouts than in the wild-type or heterozygous mice (21 vs. 29 weeks), and the number of mice with carcinomas was 5- to 6-fold higher in the p21−/− animals (Fig. 1A). Significantly, a substantial fraction of p21 knockouts developed several independent carcinomas per mouse, whereas such multiplicity of malignant tumor formation was very rare with the p21+/+ or p21+/− mice (Fig. 1C). Papilloma and carcinoma values within each individual group and differences among the three groups were evaluated by using ANOVA and Tukey-HSD tests (26). The higher carcinoma formation and the lower number of papillomas in the p21−/− versus p21+/+ and p21+/− groups were both highly significant (P = 0.005 and 0.0024, respectively). Similar conclusions were obtained by using a more stringent nonparametric test (Kruskal–Wallis test; ref. 26). The same differences in latency, number, and multiplicity of carcinoma formation were found in a second independent experiment between p21−/− and p21+/+ mice, whereas an intermediate number of tumors was produced by the p21+/− heterozygous (Fig. 1 B and C). A selected number of papillomas from the three groups of mice, and all carcinomas, were analyzed histologically. This analysis indicated that the degree of squamous-cell differentiation in the various tumors was not significantly different among the three groups of mice. Terminal deoxynucleotidyltransferase-mediated UTP end labeling (TUNEL) assays on tumors derived from the three groups of mice were also performed, and numbers of TUNEL-positive cells were found to vary among individual tumors, irrespective of genotype.
Thus, loss of p21 results in a markedly enhanced susceptibility to chemically induced skin carcinogenesis. Under our protocol conditions, p21 knockout mice exhibited a much higher number of carcinomas than the controls, whereas the number of papillomas was reduced. Increased carcinoma formation was not accompanied by a more undifferentiated phenotype, as was reported for p53-deficient mice (27).
Loss of p21 Function Results in Keratinocyte Populations with Increased Proliferation Potential.
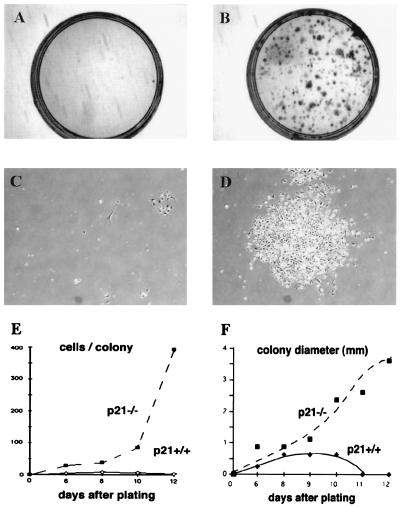
Previous evidence indicates that malignant versus benign keratinocyte tumor formation is likely to involve distinct target-cell populations with a different commitment to differentiation (14). The size of keratinocyte populations with high growth/differentiation potential may increase as a consequence of p21 deficiency, thereby explaining the enhanced susceptibility to skin carcinoma formation. In the human keratinocyte system, one important characteristic of stem-cell populations is that of unlimited growth potential with production of rapidly expanding colonies (4, 5, 7, 8). Unlike human keratinocytes, mouse primary keratinocytes cultured under standard conditions do not contain cells capable of clonal expansion. We tested whether the loss of p21 may result in populations with clonogenic potential. As reported (17), the vast majority of p21−/− keratinocytes plated at low concentrations (105 cells per 60-mm dish) failed to survive and grow. However, unlike in the p21+/+ controls, in the p21−/− cultures, a significant number of cells produced dense and actively proliferating colonies after 1–2 weeks of incubation (Fig. 2). An average of 100 dense colonies was found after plating 105 cells onto a dish, indicating that the fraction of clonogenic keratinocytes in the p21−/− cultures amounted to ≈0.1% of the total cells. No such colonies were found in parallel cultures with wild-type keratinocytes.
Figure 2.
Clonogenic growth potential of p21-deficient versus wild-type keratinocytes. Primary keratinocytes derived from p21−/− and p21+/+ mice were plated under sparse conditions (105 cells per 60-mm dish) and cultivated in growth medium at low calcium concentrations (0.05 mM) for up to 2 weeks. (A and B) Culture dishes fixed and stained with 0.1% crystal violet at the end of the experiment. (C and D) Photographs of representative colonies at 10 days after plating. (E and F) Average colony size and colony cell number as a function of time, as determined by examination of several random colonies at daily intervals. Cells were tested in triplicate dishes, and similar results were observed in another independent experiment.
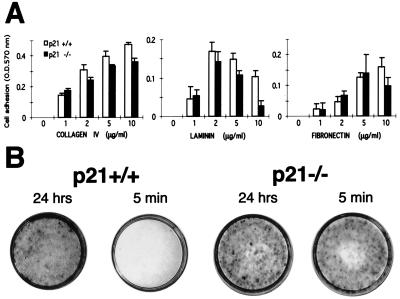
Recent studies with murine keratinocytes have shown that specific stem-cell populations, identified as label-retaining cells at 1 month after BrdUrd in vivo labeling, are characterized by a high growth potential. These label-retaining populations can be selected by rapid attachment to the substrate, regardless of specific integrin function (9). Although all keratinocytes attach after 24 h of incubation, when cells are left to attach for 10 min, only ≈10% of total basal cells but 100% of label-retaining populations adhere to the substrate. We tested whether a similar assay could be used to further differentiate between p21−/− and p21+/+ keratinocytes. When primary cells from mice of either genotype were plated onto specific substrates, the two types of cells did not differ significantly in their attachment properties, except that at higher laminin concentrations, p21 knockout cells attached less efficiently than the controls (Fig. 3A). Rather than the bulk population, increased cell adhesion may be limited to the subpopulations of p21 knockout keratinocytes with an enhanced growth potential and high attachment properties (corresponding to the stem cell/label-retaining populations; ref. 9). To test this possibility, freshly prepared keratinocytes from p21−/− and p21+/+ mice were plated in large numbers (106 per dish) and allowed to attach to the dish for either 5 min or 24 h. In all cases, unattached cells were removed, and cultures were further incubated for 2 weeks. Wild-type keratinocytes that attached for 24 h were able to grow and populate the dish, whereas no growth was detectable on dishes where cells were left to attach for only 5 min. In contrast, the p21 knockout keratinocytes showed similar active growth irrespective of the amount of time they were given to attach (Fig. 3B). Thus, p21-deficient keratinocytes contain an increased number of cells with high growth potential, as revealed by cultivation under sparse conditions and/or selection for high rate of attachment.
Figure 3.
Cell adhesive properties of p21-deficient versus wild-type keratinocytes. (A) Primary keratinocytes were plated onto 96-well dishes coated with increasing amounts (0–10 μg/ml) of collagen IV, laminin, and fibronectin. After 90 min, adherent cells were fixed and stained with 0.1% crystal violet. Staining intensity was determined with an ELISA reader at 570 nm. Each point represents the average of triplicate wells, and similar results were obtained in a second independent experiment. (B) Primary keratinocytes from p21−/− and p21+/+ mice were plated on triplicate dishes in relatively large numbers (106 per 60-mm dish) and allowed to attach for either 24 h or 5 min. After 2 weeks of cultivation, dishes were fixed and stained with 0.1% crystal violet. Similar results were obtained in a second independent experiment.
p21 Plays an Essential Function in the Irreversible Commitment to Differentiation.
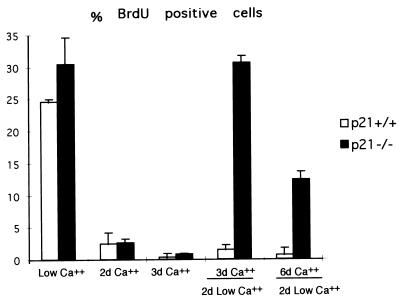
Besides populations with an increased growth potential, the lack of p21 may result in cells with a lesser commitment to differentiation. This possibility was initially tested with keratinocytes under well defined in vitro conditions. Increased extracellular calcium functions as a potent inducer of keratinocyte differentiation and induces growth arrest in >95% of cells by 24 h of treatment (28). However, the irreversible commitment to differentiation takes a longer period of time in that, after 1 day of calcium exposure, >50% of cells resume proliferation if they are switched back to medium having low calcium concentrations (unpublished observations). p21 function is not essential for differentiation-associated growth arrest (17) but may be required for the irreversible commitment to differentiation. To test this possibility, p21+/+ and p21−/− keratinocytes were cultured in medium at high calcium concentrations for an increasing number of days. The number of DNA-synthesizing cells was then determined by BrdUrd labeling of cultures that were either maintained in high-calcium conditions or were switched to low-calcium medium for additional 48 h (Fig. 4). As previously reported, there was a similar block of DNA synthesis in the calcium-treated p21+/+ and p21−/− cultures. Very few of the wild-type cells cultured in high-calcium medium for either 3 or 6 days resumed proliferation when switched back to low-calcium conditions. By contrast, a subfraction of p21−/− keratinocytes was capable of resuming DNA synthesis under these conditions. Thus, loss of p21 function is not essential for differentiation-associated growth arrest but results in cell populations with a lesser commitment toward differentiation.
Figure 4.
Reversible versus irreversible commitment to differentiation of primary keratinocytes in culture. BrdUrd labeling index of p21−/− versus p21+/+ keratinocytes under growing conditions in low-calcium medium, after induction of differentiation by calcium (2 mM) for the indicated number of days (d) and after induction of differentiation by calcium and subsequent incubation in low-calcium medium for an additional 2 days. The percentage of BrdUrd-positive nuclei was determined as described in Materials and Methods. In each case, four independent fields were counted (a minimum of 100 cells per field), and the SD among values from the various fields was calculated. Similar results were obtained in two independent experiments.
Loss of p21 Function Increases the Number of Pluripotent Keratinocyte Populations with Unrestricted Differentiation Potential.
In vivo, keratinocytes undergo a much more complex differentiation program than under in vitro conditions. In fact, whereas the interfollicular epidermis contains a single type of terminally differentiated keratinocyte, hair follicles are composed of a minimum of six or seven types of differentiated keratinocytes organized in three concentric regions (shaft and inner and outer root sheaths) (29). By use of a skin/hair-reconstitution assay onto nude mice, we previously showed that primary keratinocyte cultures contain progenitor cells able to reconstitute on their own columnar units of the interfollicular epidermis as well as one or the other of the three concentric regions of the hair follicle (23). Pluripotent progenitor cells capable of reconstituting on their own entire hair follicles were <1% in these assays.
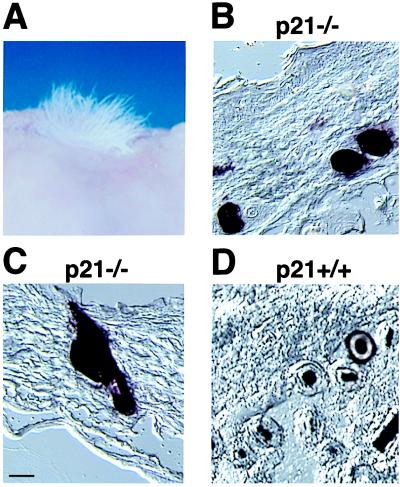
To test whether loss of p21 results in an increased number of pluripotent keratinocyte progenitor cells, skin/hair-reconstitution assays were performed with p21 knockout keratinocytes versus wild-type controls. As in our previous studies (23), primary keratinocytes were infected with a retrovirus carrying a human AP gene 2 days after plating (25) under conditions where only ≈10% of cells expressed this marker. Four days after infection, cells were trypsinized, admixed with a hair-inducing dermal-cell preparation, and grafted onto nude mice. Hair formation was detected by 3–4 weeks of grafting, and mice were sacrificed at 6 weeks, at a time when reconstituted hair follicles have undergone at least two full hair cycles and have entered a third (22). Grafts were analyzed histologically and after staining for AP production. As previously reported (23), the great majority of reconstituted hair follicles that contained AP-expressing cells were homogeneously AP-positive in either their external, central, or internal regions. However, in the grafts of p21−/− keratinocytes, ≈20% of the AP-positive follicles that were observed in cross section were uniformly composed of AP-positive cells, whereas <1% of these follicles were found in the controls (Fig. 5). Thus, loss of p21 results in populations of cells with an increased differentiation potential and that are capable of reconstituting on their own not only distinct tract of the epidermis but also entire hair follicles.
Figure 5.
Pluripotent populations of p21−/− keratinocytes as assessed in vivo by a skin/hair-reconstitution assay. Primary keratinocytes derived from p21−/− and p21+/+ mice were infected with an AP-transducing retrovirus under conditions that resulted in 10–12.5% of cells expressing the AP marker. Cells were grafted onto nude mice together with a hair-inducing dermal cell preparation, as described in Materials and Methods. (A) Macroscopical view of hair formation on the back of a nude mouse transplanted with p21-deficient keratinocytes. Similar hair formation was observed after transplantation of wild-type cells (data not shown). (B and D) Cross section of reconstituted follicles formed by p21-knockout keratinocytes (B) and their wild-type counterparts (D). Note the uniformly labeled follicles formed by AP-positive p21−/− cells and the restricted labeling of follicles formed by AP-positive p21+/+ cells. (C) A longitudinal section of a hair follicle formed by p21-deficient keratinocytes, with uniform AP staining, extending to the attached sebaceous gland. Two independent grafting experiments were performed. Results were quantified by counting only follicles in cross section, where the various concentric regions can be distinctly visualized. Of the ≈200 AP-positive follicles that were counted in grafts of p21−/− keratinocytes, 80% showed restricted labeling to either the outer or inner root sheath and/or shaft, whereas 20% showed uniform staining of all three regions. In contrast, <1% of uniformly stained follicles were found in the p21+/+ keratinocyte grafts. [Bar = 230 μm (B and D) and 250 μm (C).]
DISCUSSION
p21WAF1/Cip1 is one of the best characterized downstream targets of p53, and the growth-suppressing function of this cyclin-dependent kinase inhibitor is well established. However, it is still not known to what extent the tumor-suppressing function of p53 is mediated by p21, nor is it clear whether p21 can exert a tumor-suppressing function of its own. Against this possibility stand the facts that the p21 gene is very rarely mutated in human tumors, and mice with a homozygous disruption of the p21 gene develop no spontaneous tumors (30). However, even in p53-deficient mice, spontaneous tumor formation occurs only in selected tissues (31). Thus, in other tissues and/or specific cell types, p53 and/or p21 may still exert a significant tumor-suppressing function, which is unmasked under conditions of altered tissue homeostasis. Our findings show that, in chemically induced skin carcinogenesis, p21 does indeed play a significant tumor-suppressing function and that this function overlaps to a partial extent with that of p53.
The carcinomas arising in the p53-deficient mice were found to be significantly anaplastic (27), and their origin was related, among other causes, to increased genomic instability (32, 33). By contrast, carcinomas of p21−/− mice exhibited a relatively high degree of squamous-cell differentiation, consistent with the more restricted function of the p21 gene (34). Besides the higher number and shorter latency, p21-deficient mice showed a multiplicity of carcinoma formation per mouse, which was rarely seen with the controls. This suggested that the number of target cells for carcinoma formation is increased in the p21-deficient animals. Considerable evidence points to keratinocyte stem cells as the cells of origin for chemically induced mouse skin tumors (11), and the stem-cell concept has been previously proposed as a general basis for tumorigenesis (35, 36). Relative to wild-type mouse keratinocytes, keratinocytes derived from p21−/− mice contain a significantly increased number of cells with clonogenic potential and high rates of attachment, two interrelated properties which have been directly connected with label-retaining stem-cell populations (9). Besides increased growth potential, p21-deficient keratinocytes contain subpopulations with a lesser commitment to differentiation and the capability to generate all types of terminally differentiated keratinocytes that are present in vivo, not only in the interfollicular epidermis but also in the hair follicles. This situation may be analogous to that reported for the p21 homologue in Drosophila, where the presence or absence of the p21 homologue determines the number of cell divisions that progenitor cells undergo before becoming committed to a more restricted differentiation lineage (37, 38).
In contrast to increased susceptibility to carcinoma formation, loss of the p53 gene results in a significantly reduced number of papillomas (27, 39). Direct experimental evidence indicates that, in a conventional initiation–promotion protocol, most papillomas grow to a large size but do not progress to carcinomas, whereas the carcinomas arise from a distinct subset of papillomas with a high risk of malignant conversion (12, 13). Relative to the intrinsically benign papillomas, the “high-risk” papillomas and associated carcinomas are likely to originate from cells with a greater growth potential and decreased commitment toward differentiation (14). The present studies were based on a chemical carcinogenesis protocol and a strain of mice that generate a high number of papillomas per mouse. Under these conditions, p21 deficiency exerted a suppressive effect on papilloma development similar to that associated with lack of p53. Interestingly, whereas p21 expression is reduced in chemically induced skin carcinomas, it appears to be increased rather than decreased in the benign papillomas (40). As reported in other systems (41), p21 expression in papillomas may exert a significant protective function against apoptosis, and the absence of this molecule may lead to an increase in apoptosis, thereby explaining the observed decrease in papilloma yield. Although TUNEL assays revealed no significant differences in p21−/− versus p21+/+ papillomas, these assays were confined to papillomas at a relatively late stage of development, whereas significant differences may exist at earlier stages, possibly before macroscopic outgrowth is detectable. A second attractive explanation is suggested by our recent demonstration that elevated p21 expression results in the unexpected suppression of late aspects of keratinocyte differentiation independently from effects on the cell cycle (20). Thus, in parallel with a dual function on differentiation (20), p21 may exert opposite roles in keratinocyte tumor growth or suppression, depending on the target cells in which this molecule is expressed and/or their level of commitment to differentiation.
Acknowledgments
We are thankful to Drs. Cathrin Brisken, Caterina Missero, and Enzo Calautti for critical reading of the manuscript. We also thank D. A. Johnston, from the Biostatistic Core of University of Texas M.D. Anderson Cancer Center, for statistical analysis, and the histology core from the National Institute on Environmental Health Sciences Center Grant ES 07784 for histological analysis. This work was supported by National Institutes of Health Grants AR39190, CA16038, and CA73796 to G.P.D., and, in part, by the Cutaneous Biology Research Center through the Massachusetts General Hospital/Shiseido Co. Ltd. Agreement.
ABBREVIATION
- AP
alkaline phosphatase
Note Added in Proof
While this work was being considered for publication, a similar skin carcinogenesis study appeared, which reported an increase of both papilloma and carcinoma formation in p21−/− vs. p21+/+ mice (42). The protocol and strain of mice used for this other study resulted only in a very low number of papillomas per mouse, which correspond conceivably to the subset of papillomas with “high risk” of malignant conversion discussed above.
References
- 1.Dover R, Wright N. In: Physiology, Biochemistry, and Molecular Biology of the Skin. Goldsmith L, editor. New York: Oxford Univ. Press; 1991. pp. 239–265. [Google Scholar]
- 2.Cotsarelis G, Sun T T, Lavker R M. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 3.Jones P H, Watt F M. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 4.Jones P H, Harper S, Watt F M. Cell. 1995;80:83–93. doi: 10.1016/0092-8674(95)90453-0. [DOI] [PubMed] [Google Scholar]
- 5.Moll I. J Invest Dermatol. 1995;105:14–21. doi: 10.1111/1523-1747.ep12312406. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds A J, Jahoda C A. Skin Pharmacol. 1994;7:16–19. doi: 10.1159/000211268. [DOI] [PubMed] [Google Scholar]
- 7.Rochat A, Kobayashi K, Barrandon Y. Cell. 1994;76:1063–1073. doi: 10.1016/0092-8674(94)90383-2. [DOI] [PubMed] [Google Scholar]
- 8.Yang J S, Lavker R M, Sun T T. J Invest Dermatol. 1993;101:652–659. doi: 10.1111/1523-1747.ep12371671. [DOI] [PubMed] [Google Scholar]
- 9.Bickenbach J R, Chism E. Exp Cell Res. 1998;244:184–195. doi: 10.1006/excr.1998.4163. [DOI] [PubMed] [Google Scholar]
- 10.Binder R L, Gallagher P M, Johnson G R, Stockman S L, Smith B J, Sundberg J P, Conti C J. Mol Carcinog. 1997;20:151–158. doi: 10.1002/(sici)1098-2744(199709)20:1<151::aid-mc17>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Morris R J, Coulter K, Tryson K, Steinberg S R. Cancer Res. 1997;57:3436–3443. [PubMed] [Google Scholar]
- 12.DuBowski A, Johnston D A, Rupp T, Beltran L, Conti C J, DiGiovanni J. Carcinogenesis. 1998;19:1141–1147. doi: 10.1093/carcin/19.6.1141. [DOI] [PubMed] [Google Scholar]
- 13.Hennings H, Shores R, Mitchell P, Spangler E F, Yuspa S H. Carcinogenesis. 1985;6:1607–1610. doi: 10.1093/carcin/6.11.1607. [DOI] [PubMed] [Google Scholar]
- 14.Brown K, Strathdee D, Bryson S, Lambie W, Balmain A. Curr Biol. 1998;8:516–524. doi: 10.1016/s0960-9822(98)70203-9. [DOI] [PubMed] [Google Scholar]
- 15.Foulds L. Neoplastic Development. London: Academic; 1969. [Google Scholar]
- 16.Missero C, Calautti E, Eckner R, Chin J, Tsai L H, Livingston D M, Dotto G P. Proc Natl Acad Sci USA. 1995;92:5451–5455. doi: 10.1073/pnas.92.12.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Missero C, Di Cunto F, Kiyokawa H, Koff A, Dotto G P. Genes Dev. 1996;10:3065–3075. doi: 10.1101/gad.10.23.3065. [DOI] [PubMed] [Google Scholar]
- 18.Gartel A L, Serfas M S, Gartel M, Goufman E, Wu G S, el-Deiry W S, Tyner A L. Exp Cell Res. 1996;227:171–181. doi: 10.1006/excr.1996.0264. [DOI] [PubMed] [Google Scholar]
- 19.Ponten F, Berne B, Ren Z P, Nister M, Ponten J. J Invest Dermatol. 1995;105:402–406. doi: 10.1111/1523-1747.ep12321071. [DOI] [PubMed] [Google Scholar]
- 20.Di Cunto F, Topley G, Calautti E, Hsiao J, Ong L, Seth P K, Dotto G P. Science. 1998;280:1069–1072. doi: 10.1126/science.280.5366.1069. [DOI] [PubMed] [Google Scholar]
- 21.Rousselle P, Aumailley M. J Cell Biol. 1994;125:205–214. doi: 10.1083/jcb.125.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichti U, Weinberg W C, Goodman L, Ledbetter S, Dooley T, Morgan D, Yuspa S H. J Invest Dermatol. 1993;101:124S–129S. doi: 10.1111/1523-1747.ep12363165. [DOI] [PubMed] [Google Scholar]
- 23.Kamimura J, Lee D, Baden H P, Brissette J, Dotto G P. J Invest Dermatol. 1997;109:534–540. doi: 10.1111/1523-1747.ep12336704. [DOI] [PubMed] [Google Scholar]
- 24.Prouty S M, Lawrence L, Stenn K S. Am J Pathol. 1996;148:1871–1885. [PMC free article] [PubMed] [Google Scholar]
- 25.Cepko C, Ryder E F, Austin C P, Walsh C, Fekete D M. Methods Enzymol. 1995;254:387–419. doi: 10.1016/0076-6879(95)54027-x. [DOI] [PubMed] [Google Scholar]
- 26.Zar J H. Biostatistical Analysis. New York: McGraw–Hill; 1996. [Google Scholar]
- 27.Kemp C J, Donehower L A, Bradley A, Balmain A. Cell. 1993;74:813–822. doi: 10.1016/0092-8674(93)90461-x. [DOI] [PubMed] [Google Scholar]
- 28.Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa S H. Cell. 1980;19:245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- 29.Holbrook K A. Structure and Development of the Skin. New York: McGraw–Hill; 1984. [Google Scholar]
- 30.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 31.Donehower L A, Harvey M, Slagle B L, McArthur M J, Montgomery C A, Jr, Butel J S, Bradley A. Nature (London) 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 32.Wang X J, Greenhalgh D A, Jiang A, He D, Zhong L, Medina D, Brinkley B R, Roop D R. Oncogene. 1998;17:35–45. doi: 10.1038/sj.onc.1201890. [DOI] [PubMed] [Google Scholar]
- 33.Wang X J, Greenhalgh D A, Jiang A, He D, Zhong L, Brinkley B R, Roop D R. Mol Carcinog. 1998;23:185–192. doi: 10.1002/(sici)1098-2744(199811)23:3<185::aid-mc7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 34.Levine A J. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 35.Till, J. E. (1982) J. Cell Physiol., Suppl. 1, 3–11. [DOI] [PubMed]
- 36.Greaves M F. Science. 1986;234:697–704. doi: 10.1126/science.3535067. [DOI] [PubMed] [Google Scholar]
- 37.de Nooij J C, Letendre M A, Hariharan I K. Cell. 1996;87:1237–1247. doi: 10.1016/s0092-8674(00)81819-x. [DOI] [PubMed] [Google Scholar]
- 38.Lane M E, Sauer K, Wallace K, Jan Y N, Lehner C F, Vaessin H. Cell. 1996;87:1225–1235. doi: 10.1016/s0092-8674(00)81818-8. [DOI] [PubMed] [Google Scholar]
- 39.Greenhalgh D A, Wang X J, Donehower L A, Roop D R. Cancer Res. 1996;56:4413–4423. [PubMed] [Google Scholar]
- 40.Rodriguez-Puebla M L, LaCava M, Gimenez-Conti I B, Johnson D G, Conti C J. Oncogene. 1998;17:2251–2258. doi: 10.1038/sj.onc.1202131. [DOI] [PubMed] [Google Scholar]
- 41.Asada M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumuro K, Mizutani S. EMBO J. 1999;18:1223–1234. doi: 10.1093/emboj/18.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinberg W C, Fernandez-Salas E, Morgan D L, Shalizi A, Mirosh E, Stanulis E, Deng C, Hennings H, Yuspa S H. Cancer Res. 1999;59:2050–2054. [PubMed] [Google Scholar]