Abstract
Background: Covered metallic oesophageal stents offer effective palliation of malignant oesophageal strictures. However, first generation devices were associated with a high rate of migration, particularly when used in the lower oesophagus.
Aim: To compare the rate of complications and palliative effect of two newer covered metallic oesophageal stents.
Patients and methods: We performed a prospective randomised study using two of these newer stent designs in the treatment of malignant lower third oesophageal tumours. Fifty three patients with dysphagia due to inoperable oesophageal carcinoma involving the lower third of the oesophagus were randomly selected to receive either a Flamingo covered Wallstent (Boston Scientific Inc., Watertown, Massachusetts, USA) or an Ultraflex covered stent (Boston Scientific Inc.). Dysphagia was scored on a five point scale, recorded before stent insertion, the day after, and at least one month later at follow up. Technical success, early and late complications (perforation, migration, severe gastro-oesophageal reflux, haematemesis, and reobstruction due to tumour overgrowth) were also recorded.
Results: In both stent groups, a significant improvement in dysphagia score was seen both the next day post stenting and at late follow up (p<0.05). No significant difference was seen in the improvement in dysphagia between the two groups (p>0.1). The frequency of complications encountered in the two groups was similar. Three patients in the Ultraflex group required two stents at primary stenting.
Conclusion: The two types of stent are equally effective in the palliation of dysphagia associated with lower third oesophageal malignancy and the complication rates associated with their use are comparable.
Keywords: oesophageal carcinoma, metallic stents, dysphagia, palliation
The use of self expanding metallic stents (SEMS) in the palliation of dysphagia in patients with inoperable carcinoma of the oesophagus is well established. The main indication for placement of these stents is alleviation of dysphagia. Covered stents are superior at resisting tumour ingrowth than uncovered types but at the price of increased rates of migration.1–7 This complication is particularly frequent when the stents are used for the treatment of malignant strictures in the lower third of the oesophagus.1 The choice of available metallic stents is now large and includes the oesophageal Wallstent and Flamingo stent (Boston Scientific Inc., Watertown, Massachusetts, USA), the Ultraflex stent (Boston Scientific Inc.), the Gianturco-Rosch Z stent (William Cook, Bloomington, Indiana, USA), and the Esophacoil (Instent, Eden Prairie). The Flamingo and Ultraflex devices are both effective but have different migration-resistant characteristics.2 We have performed a prospective randomised comparison of these two stent designs in patients with primary lower third oesophageal carcinoma.
METHODS AND MATERIALS
Study design
This was a prospective randomised comparison between the Ultraflex and Flamingo Wallstent endoprostheses in patients with primary lower third oesophageal carcinoma.
The procedure was deemed a technical success when one or more stents were placed across the stricture, and when the stents were shown to be patent following injection of contrast medium. The degree of dysphagia experienced by each patient was scored using a standard five point scale: grade 0, no dysphagia; grade 1, some solid food; grade 2, swallow liquids only; grade 3, difficulty with liquids and saliva; grade 4, complete dysphagia.1 Dysphagia was scored before stent insertion, the day after stent deployment, and later during the follow up period. The length of follow up was variable because of the frail condition of some of the patients. Five patients (all in the Ultraflex group) who were lost to follow up after two weeks were not included in the late follow up data. The relevant dysphagia information was obtained during attendance at outpatient clinics and from telephone interviews with the patients, their relatives, primary care physicians, or community nursing practitioners. Pre and post stent dysphagia scores in each group were compared. The tumour site (involving the distal third of the oesophagus, the gastro-oesophageal junction, or both), mean tumour length, and number of primary stents used were recorded (table 1 ▶). Information on tumour location and length was obtained from the endoscopy records and from fluoroscopic contrast studies using a radio-opaque ruler at the time of stent insertion. Complications were recorded and classified into early (before 30 days) and late. “Early” complications included perforation, severe gastro-oesophageal reflux, and migration. “Late” complications included haematemesis, described as significant only if the patient became compromised haemodynamically, and tumour ingrowth or overgrowth. If patients complained of increased dysphagia, a further oesophagogram and/or endoscopic examination was performed.
Table 1.
Patient characteristics, tumour site, mean tumour length, and number of primary stents used
| Ultraflex | Flamingo | |
| No of patients | 31 | 22 |
| Males | 25 | 15 |
| Mean (SD) age (y) | 71.6 (9.7) | 61.2 (9.6) |
| No of primary stents | ||
| 1 | 28 | 22 |
| 2 | 3 | 1 |
| Mean tumour length (cm) | 5.3 | 5.5 |
| No of tumours involving distal third | 24 | 16 |
| No of tumours involving the GOJ | 11 | 9 |
GOJ, gastro-oesophageal junction.
Patients
During the period 1997 to 2001, 53 patients (39 male) were recruited into the study. Mean age of the patients in the Ultraflex group was 71.6 years and 61.2 years in the Flamingo group. All had histologically proven carcinoma involving predominantly the lower one third of the oesophagus. All tumours were staged with computed tomography and endoscopic ultrasound, and were considered unresectable. Patients had been referred to the radiology department for insertion of a metallic stent for palliation of dysphagia. Patients were randomised into two groups to receive either the Flamingo Wallstent or the Ultraflex stent. This was done by randomly selecting sealed envelopes (100) with the label Ultraflex (50) or Flamingo (50) enclosed inside. Table 2 ▶ lists patient age and sex, tumour length and site, dysphagia scores, and types of stents used.
Table 2.
Patient demographics and dysphagia scores
| Patient No | Sex | Age (y) | Stent | TU L (cm) | TU site | DS0 | DS1 | DS2 |
| 1 | M | 48 | UL | 7 | D1/3 | 3 | 1 | 1 |
| 2 | M | 74 | US | 5 | D1/3 | 3 | 1 | 1 |
| 3 | M | 68 | UL | 6 | D1/3 | 2 | 0 | 1 |
| 4 | M | 70 | US×2 | 8 | D1/3 | 3 | 1 | 1 |
| 5 | F | 64 | US | 8 | D1/3 | 2 | 0 | 0 |
| 6 | F | 80 | US | 4 | GOJ | 2 | 0 | — |
| 7 | F | 90 | UL | 7 | D1/3 | 4 | 1 | 2 |
| 8 | M | 80 | US | 6 | D1/3+GOJ | 2 | 0 | — |
| 9 | M | 74 | UL | 7 | D1/3+GOJ | 3 | 1 | 2 |
| 10 | F | 77 | US | 6 | GOJ | 2 | 0 | 1 |
| 11 | M | 88 | US×2 | 9 | D1/3 | 3 | 1 | 1 |
| 12 | M | 56 | UL | 6 | D1/3 | 3 | 1 | 1 |
| 13 | M | 62 | US | 4 | D1/3+GOJ | 3 | 1 | 2 |
| 14 | M | 74 | US | 4 | GOJ | 2 | 0 | 1 |
| 15 | M | 73 | US | 4 | D1/3+GOJ | 2 | 0 | 1 |
| 16 | F | 73 | US | 4 | D1/3 | 3 | 1 | 1 |
| 17 | M | 77 | US | 4 | D1/3 | 2 | 0 | 1 |
| 18 | M | 78 | UL | 5 | D1/3 | 2 | 0 | 1 |
| 19 | M | 76 | US | 4 | D1/3 | 2 | 0 | 1 |
| 20 | M | 77 | US | 5 | D1/3 | 3 | 1 | 1 |
| 21 | F | 77 | US | 4 | GOJ | 3 | 1 | 1 |
| 22 | M | 58 | US | 4 | D1/3 | 3 | 1 | 1 |
| 23 | M | 55 | US | 5 | D1/3 | 3 | 1 | 1 |
| 24 | M | 59 | US | 4 | GOJ | 3 | 1 | 1 |
| 25 | M | 64 | US | 5 | D1/3 | 3 | 1 | 1 |
| 26 | M | 75 | US | 5 | D1/3 | 2 | 0 | - |
| 27 | M | 75 | US | 4 | D1/3 | 3 | 1 | 1 |
| 28 | M | 77 | US | 5 | D1/3+GOJ | 3 | 1 | 1 |
| 29 | M | 80 | US | 6 | D1/3 | 4 | 1 | - |
| 30 | M | 69 | US×2 | 4 | D1/3 | 3 | 1 | 1 |
| 31 | M | 74 | US | 5 | GOJ | 2 | 0 | - |
| 32 | M | 61 | FS | 4 | D1/3 | 3 | 1 | 1 |
| 33 | M | 75 | FS | 5 | D1/3 | 3 | 0 | 1 |
| 34 | F | 62 | FS | 8 | D1/3 | 3 | 0 | 1 |
| 35 | F | 73 | FS | 4 | GOJ | 4 | 1 | 1 |
| 36 | F | 51 | FL | 7 | GOJ | 3 | 1 | 1 |
| 37 | M | 54 | FS×2 | 5 | GOJ | 3 | 2 | 3 |
| 38 | M | 52 | FS | 4 | D1/3 | 3 | 0 | 1 |
| 39 | M | 62 | FL | 7 | D1/3 | 2 | 0 | 0 |
| 40 | F | 53 | FS | 4 | D1/3 | 2 | 0 | 1 |
| 41 | M | 61 | FS | 6 | D1/3+GOJ | 3 | 0 | 1 |
| 42 | F | 62 | FS | 6 | D1/3+GOJ | 3 | 1 | 1 |
| 43 | M | 60 | FL | 6 | D1/3 | 3 | 1 | 1 |
| 44 | F | 88 | FL | 8 | D1/3 | 3 | 1 | 1 |
| 45 | M | 61 | FS | 5 | D1/3 | 3 | 1 | 1 |
| 46 | M | 60 | FL | 5 | GOJ | 3 | 1 | 1 |
| 47 | M | 54 | FL | 5 | GOJ | 4 | 1 | 2 |
| 48 | M | 50 | FS | 4 | D1/3 | 3 | 1 | 1 |
| 49 | M | 53 | FS | 5 | D1/3 | 3 | 1 | 1 |
| 50 | M | 51 | FS | 6 | D1/3 | 4 | 1 | 1 |
| 51 | M | 74 | FS | 7 | D1/3+GOJ | 2 | 0 | 0 |
| 52 | F | 62 | FS | 4 | D1/3 | 2 | 0 | 0 |
| 53 | M | 68 | FL | 6 | GOJ | 2 | 0 | 0 |
TU L, tumour length; DS0,dysphagia score pre stenting; DS1, dysphagia score (day 1); DS2, dysphagia score on late follow up; US, short Ultraflex stent; UL, long Ultraflex stent; FS, short Flamingo stent; FL, long Flamingo stent; D1/3, distal oesophageal one third; GOJ, gastro-oesophageal junction.
Stent insertion technique
The characteristics of the Flamingo Wallstent and Ultraflex stent are listed in table 3 ▶.
Table 3.
Comparison between the Flamingo stent and the Ultraflex stent
| Flamingo stent | Ultraflex stent | |
| Shape | Conical (proximal flaring) | Proximal flaring |
| Covering | Internal | External |
| Rigidity | Rigid | Flexible |
| Length and diameter | ||
| Short | 12 cm; 24 mm×16 mm | 12 cm; 28 mm×22 mm |
| Long | 14 cm; 30 mm×20 mm | 15 cm; 23 mm×17 mm |
| The end first released | Distal | Proximal or distal |
| Re-sheath | Yes | No |
The Flamingo Wallstent
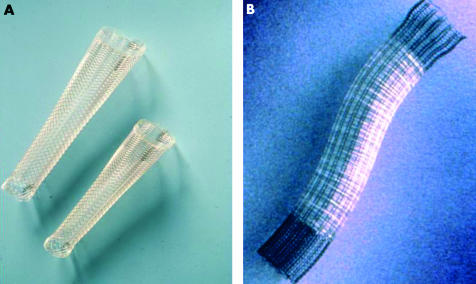
The Flamingo Wallstent has three important features designed to resist distal migration (fig 1A ▶):
Figure 1.
(A) The Flamingo Wallstent; (B) the Ultraflex stent.
It is conical in shape with proximal flaring.
It has a polyethylene cover on the inside of the stent. However, the distal and proximal 1.5 cm segments of the device remain uncovered.
The braiding angle is large in the upper part of the stent and small in the lower part. When oesophageal peristalsis propels the stent towards the stomach, the upper end becomes trapped above the stricture whereas the lower end becomes stretched.2
The Flamingo stent can be recovered and repositioned, provided less then 50% of the endoprosthesis has been uncovered.
The Ultraflex stent
The Ultraflex stent (fig 1B ▶) is also flared proximally and has uncovered ends. It is made of nitinol and is more flexible both in the longitudinal and axial plane. A long fabric thread holds the compressed stent. The thread is pulled during deployment, thus releasing the stent. Once deployment has started the endoprosthesis cannot be recovered into the delivery catheter. The stent comes as “proximal release” or “distal release”, terms indicating which end of the device is released first. Maximum shortening of the stent during deployment occurs at the end first release.
The introducer systems of both the Flamingo and Ultraflex stents have four radio-opaque markers. The inner two markers represent the final position of the covered part of the deployed stent and the outer two the position of the uncovered portion.
Informed consent was obtained from all patients. All procedures were carried out in the interventional suite using fluoroscopic guidance and sedation. General anaesthesia was not required for any patient. The technique for stent insertion has been previously described.2 The stricture was dilated with a 15 mm diameter balloon before stent deployment in all cases.
Statistical analysis
Statistical analysis was performed using the Mann-Whitney U test. Sample sizes were sufficient to assume approximation with normal distribution, and probability values were calculated using the two tailed Student’s t distribution. Standard deviation values were also obtained.
RESULTS
Technical success was obtained in all patients in the study. Mean duration of follow up for the Ultraflex and Flamingo stent groups was 96.5 days and 97.1 days, respectively. Three patients in the Ultraflex stent group required two primary stents whereas one patient in the Flamingo group required two primary stents. Mean dysphagia scores before stenting, the day after stenting, and at late follow up are shown in table 4 ▶. Both stent groups had a significant improvement in their dysphagia score both on the next day (p<0.05) and at late follow up (p<0.05). There was no significant difference in improvement of dysphagia scores between the two stent groups (p>0.1). Early and late complications are shown in table 4 ▶. One case of immediate perforation following proximal migration of the stent was seen in the Flamingo Wallstent group. A second covered Wallstent was deployed to cover the site of perforation but this was inadvertently released in too distal a position, resulting in its lower end coming into apposition with the greater curve of the stomach. Consequently, the stent was partially obstructed and there was no change in this patient’s dysphagia score. Despite this the patient survived for a further three months before dying from progression of his malignant disease. In two patients, Flamingo Wallstents came into apposition with the greater curve of the stomach. No patient who received the more flexible Ultraflex stent suffered this problem. There was one case of substantial distal stent migration in the Ultraflex group in which the first stent migrated almost completely into the stomach within two weeks of initial deployment. A second Ultraflex stent was deployed to anchor the first and maintain patency of the lower oesophagus. This patient continued to experience palliation of dysphagia until his death from progressive disease five weeks later. There was only one other case of distal stent migration (also in the Ultraflex group) but this had no clinical consequences.
Table 4.
Improvement in dysphagia scores and complications
| Ultraflex (n=31) | Flamingo (n=22) | |
| Mean (SD) dysphagia score | ||
| Before stenting | 2.7 (0.6) | 2.9 (0.6) |
| Day 1 post stenting | 0.6 (0.5) | 0.6 (0.6) |
| Late follow up | 1.0 (0.4)* | 0.9 (0.5)* |
| All deaths | 22 | 14 |
| Deaths within 30 days | 5 | 4 |
| Complications | ||
| Early (<30 days) | ||
| Migration | 2 (6.4%) | 1 (4.54%) |
| Perforation | 0 | 1 (4.54%) |
| Severe reflux | 2 (6.4%) | 1 (4.54%) |
| Unrelated | 1 (3.2%) | 0 |
| Late (>30 days) | ||
| Haematemesis | 1 (3.2%) | 1 (4.54%) |
| Tumour overgrowth | 1 (3.2%) | 1 (4.54%) |
*p>0.1.
Three patients continued to complain of severe symptoms of gastro-oesophageal reflux despite proton pump inhibitor therapy and advice on sleeping semi erect. One patient in each group had episodes of severe haematemesis during follow up. In both of these patients haematemesis was found to be due to tumour ingrowth, and as the extent of the disease had increased substantially no further active intervention was considered necessary. Two patients in the Ultraflex stent group and one in the Flamingo stent group experienced recurrent dysphagia. Subsequent endoscopy and oesophagography revealed tumour overgrowth around the proximal ends of the stent in two patients (one in each group) and around the distal end in one patient in the Ultraflex group. In all cases further palliation of dysphagia was obtained with either laser therapy (Flamingo) or deployment of a second stent (Ultraflex). One patient in the Ultraflex group died five days after stent insertion from complications of acute arterial lower limb ischaemia, presumed to be unrelated to the stenting procedure. In total, nine patients died within 30 days of stent insertion (9/53, 7%) (table 4 ▶). In no case was the death directly attributable to the procedure.
DISCUSSION
The use of self expanding metallic stents (SEMS) in the management of oesophageal cancer is well established. Almost half of patients benefit from palliative therapy rather than definitive surgery due to late presentation and advanced disease.8,9 Dysphagia is usually the first symptom experienced and without surgery it increases relentlessly. SEMS have been shown to be superior to radiotherapy, laser therapy, and plastic endoprostheses in the palliation of dysphagia.10–13 Covered metallic stents are more resistant to tumour ingrowth and remain patent for longer than uncovered devices.3–6,14 However, covered stents migrate more frequently than uncovered endoprostheses.3,4,7,10 Migrated stents pose a problem because they can cause distal obstruction or perforation and are difficult to remove endoscopically, especially in the presence of a tight stricture. If a stent migrates completely into the stomach it may be left in place and a new stent inserted. If the displaced stent causes symptoms, it should be removed surgically.7 The position of a partially migrated endoprosthesis that still covers the stricture can be secured by coaxial insertion of a second endoprosthesis overlapping the first.1 Alternatively, the stent can be “pulled” back at endoscopy using forceps. From anecdotal reports and our experience with oesophageal stents outside this trial, it is easier to retrieve the Ultraflex stent than the Flamingo Wallstent. Patients in whom the malignant stricture involves the lower third of the oesophagus, the lower end of the stent has to cross the gastro-oesophageal junction and protrude into the gastric lumen, thus reducing its anchoring properties.2 Placement of stents across the lower third oesophageal tumours can result in the lower end of the stent lying in apposition with the greater curve of the stomach leading to partial obstruction of the endoprosthesis.15–17 Several operators have advocated the use of uncovered SEMS for lower third tumours in order to reduce the problem of migration.4,18 However, the newer designs of covered stent are much more resistant to migration,19,20 making it unnecessary to dispense with the advantage of the plastic covering, which is an important barrier to tumour ingrowth.
The results of the dysphagia scores for each of the two groups in this study confirms that insertion of SEMS significantly improves the dysphagia associated with oesophageal malignancy and that this improvement lasts for several months. The two types of stent were equally effective in this respect, both immediately and at later follow up.
The 6% rate (3/53) of significant stent migration is much lower than that previously observed with covered stents deployed across the cardia, which has been as high as 50%.1 This compares favourably with the migration rates for previous stent designs when placed across the gastro-oesophageal junction, which have been quoted, indicating that the conical or flared stent design works well in preventing migration. Interestingly, the single case of Flamingo Wallstent migration in our study was proximal in nature whereas that of the Ultraflex stent was distal. The direction of migration may be related to the degree of proximal flaring which is greater with the Flamingo Wallstent. In the case of proximal migration with the Flamingo Wallstent, we found an underlying perforation which was adequately dealt with by deployment of a second covered Flamingo stent.
Three patients in the Ultraflex stent group required two primary stents. The reason for this was that positioning of the initial stent was suboptimal and failed to completely cover the stricture. The ripcord type deployment of the Ultraflex stent does not offer any mechanism for recovering a partly deployed stent, unlike the Flamingo Wallstent. Therefore, the Flamingo Wallstent has an advantage in allowing more accurate positioning. The position of the stent markers on the introducer system and the final position of the stents correlated equally in both groups when the stents fully expanded. However, in the absence of full expansion, we found the Ultraflex stent markers to be less reliable.
Furthermore, the choice of proximal and distal release and the preferential shortening of the Ultraflex stent means that familiarity with the Ultraflex stent system is essential to minimise malposition. Malposition and the necessity for multiple primary stents have cost implications, which should not be ignored. We recommend the proximal release version for lower third tumours. Firstly, the introducer system will not have to be advanced so far into the stomach or pylorus. Secondly, if a distal release Ultraflex stent is placed over a lower third stricture, the operator risks snagging the distal end of the stent in the stomach so that once stent shortening has occurred, the upper end of the stricture is left uncovered.
The other complications in our study were comparable between the two groups and are in keeping with the general rate of complications associated with these devices.7,9,18,21 No cases of stent deformation due to extrinsic disease resulting in increased dysphagia were encountered in our study, as others have experienced.18,22
Oesophageal pain following stenting is common but usually resolves after a few days. Persistent severe pain is uncommon and seems to occur more frequently when stents are placed in the upper oesophagus. Pain is commonly believed to be more common with rigid endoprostheses such as the Gianturco stent than with softer stents such as the Ultraflex device.2 However, the incidence and severity of pain in relation to oesophageal stenting has never been properly assessed, nor has the relationship of pain following stenting been correlated with the type of device used.20
In the past, we have often used uncovered endoprostheses in patients with low oesophageal strictures because the 20% obstruction rate of such devices from tumour ingrowth1 is lower than the rate of migration of first generation covered endoprostheses deployed across malignant strictures in the lower oesophagus. In the light of the results of this study, we now use covered second generation devices for tumours affecting any part of the oesophagus.
We reserve the use of uncovered SEMS for patients who have marked proximal oesophageal dilatation above a tight malignant stricture. In these patients fluid may still pass though the stent mesh at the shoulder of the stricture below the upper end of the stent whereas the use of a covered device would result in accumulation of food in the gap between the stent and the wall of the oesophagus. In patients in whom the stent projects into the stomach, we routinely prescribe a proton pump inhibitor to protect against the symptoms of gastro- oesophageal reflux. There are now stents that have an antireflux valve.23 However, it still remains to be seen whether such devices are as effective as conventional stents at palliating dysphagia, and whether they have a comparable rate of complications.
CONCLUSION
The rate of migration of covered Ultraflex and Flamingo stents is much lower than that reported with first generation oesophageal metallic endoprostheses. There is no significant difference in the rate of migration of these devices, or in their effectiveness at palliating malignant dysphagia. The use of uncovered SEMS for tumours of the lower third of the oesophagus is no longer justified on the grounds of stent migration.
Abbreviations
SEMS, self expanding metallic stents
REFERENCES
- 1.Adam A, Ellul J, Watkinson A, et al. Palliation of inoperable esophageal carcinoma: a prospective randomized trial of laser therapy and stent placement. Radiology 1997;202:344–8. [DOI] [PubMed] [Google Scholar]
- 2.Morgan R, Adam A. Use of metallic stents and balloons in the esophagus and gastrointestinal tract. J Vasc Interv Radiol 2001;12:283–97. [DOI] [PubMed] [Google Scholar]
- 3.Tan B, Mason R, Adam A. Minimally invasive therapy for advanced oesophageal malignancy. Clin Radiol 1996;51:828–36. [DOI] [PubMed] [Google Scholar]
- 4.Watkinson A, Mason R, Adam A. The role of self-expanding metallic endoprostheses in esophageal strictures. Semin Interv Radiol 1996;13:17–26. [Google Scholar]
- 5.Winkelbauer F, Schofl R, Niederle B,et al. Palliative treatment of obstructing esophageal cancer with nitinol stents. Am J Roentgenol 1996;166:79–84. [DOI] [PubMed] [Google Scholar]
- 6.Watson A. Self-expanding metal oesophageal endoprostheses: which is best? Eur J Gastroenterol Hepatol 1998;10:363–5. [DOI] [PubMed] [Google Scholar]
- 7.Watkinson A, Ellul J, Entwistle K, et al. Esophageal carcinoma: initial results of palliative treatment with covered self-expanding endoprostheses. Radiology 1995;195:821–7. [DOI] [PubMed] [Google Scholar]
- 8.Earlam R, Cunha-Melo JR. Oesophageal squamous cell carcinoma: 1. A Critical Review of Surgery. Br J Surg 1980;67:381–90. [DOI] [PubMed] [Google Scholar]
- 9.Muller JM, Ersami H, Stelzner M, et al. Surgical therapy of oesophageal carcinoma. Br J Surg 1990;77:845–57. [DOI] [PubMed] [Google Scholar]
- 10.Mellow MH, Pinkas H. Endoscopic laser therapy for malignancies affecting the oesophagus and gastrooesophageal junction: analysis of technical and functional efficacy. Arch Intern Med 1985;145:1443–6. [PubMed] [Google Scholar]
- 11.Cwikiel W, Tranberg KG, Cwikiel M, et al. Malignant dysphagia: palliation with esophageal stents—long-term results in 100 patients. Radiology 1998;207:513–18. [DOI] [PubMed] [Google Scholar]
- 12.Mason R, Bright N, McColl I. Palliation of malignant dysphagia with laser therapy: predictability of results. Br J Surg 1991;78:1346–7. [DOI] [PubMed] [Google Scholar]
- 13.Knyrim K, Wagner HJ, Bethge N, et al. A controlled trial of an expansile metal stent for palliation of esophageal obstruction due to inoperable cancer. N Engl J Med 1993;329:1302–7. [DOI] [PubMed] [Google Scholar]
- 14.Hills S, Chopra K, Pal A, et al. Self-expanding metal oesophageal endoprostheses, covered and uncovered: a review of 30 cases. Eur J Gastroenterol Hepatol 1998;10:371–4. [DOI] [PubMed] [Google Scholar]
- 15.Ell C, Hochberger J, May A, et al. Coated and uncoated self-expanding metal stents for malignant stenosis in the upper GI tract: preliminary clinical experiences with Wallstents. Am J Gastroenterol 1994;89:1496–500. [PubMed] [Google Scholar]
- 16.Spinelli P, Cerral F, Ciuffi M, et al. Endoscopic stent placement for cancer of the lower third and gastric cardia. Gastointest Endosc 1994;40:455–7. [DOI] [PubMed] [Google Scholar]
- 17.Park HS, Do YS, Suh SW, et al. Upper gastrointestinal tract malignant obstruction: initial results of palliation with a flexible covered stent. Radiology 1999;210:865–70. [DOI] [PubMed] [Google Scholar]
- 18.O’Sullivan G, Grundy A. Palliation of malignant dysphagia with expanding metallic stents. J Vasc Interv Radiol 1999;10:346–51. [DOI] [PubMed] [Google Scholar]
- 19.Adam A, Morgan R, Ellul J, et al. A new design of the esophageal wallstent endoprosthesis resistant to distal migration. Am J Roentgenol 1997;170:1477–81. [DOI] [PubMed] [Google Scholar]
- 20.Siersema PD, Hop WC, van Blankenstein M, et al. A new design metal stent (Flamingo stent) for palliation of malignant dysphagia: a prospective study. The Rotterdam Esophageal Tumor Study Group. Gastrointest Endosc 2000;51:139–45. [DOI] [PubMed] [Google Scholar]
- 21.Song HY, Do YS, Han YM, et al. Covered, expandable esophageal metallic stent tubes: experiences in 119 patients. Radiology 1994;193:689–95. [DOI] [PubMed] [Google Scholar]
- 22.Cowling M, Hale H, Grundy A. Management of malignant oesophageal obstruction with self-expanding metallic stents. Br J Surg 1998;85:264–6. [DOI] [PubMed] [Google Scholar]
- 23.Kocher M, Dlouhy M, Neoral C, et al. Esophageal stent with anti-reflux valve for tumors involving the cardia: work in progress. J Vasc Interv Radiol 1998;9:1007–10. [DOI] [PubMed] [Google Scholar]