Abstract
Background and aims: We tested if host gastric Lewis antigens and the babA2 genotype of Helicobacter pylori correlated with clinicohistological outcome.
Methods: We enrolled 188 dyspeptic patients (45 with duodenal ulcer, 45 with gastric ulcer, and 98 with chronic gastritis) with H pylori infection, proved by culture and gastric histology, reviewed by the updated Sydney system. Gastric expression of Lewis (Le) antigens Lea, Leb, Lex, and Ley was determined immunochemically to determine intensity (range 0–3). The corresponding 188 H pylori isolates were screened for babA2 genotype by polymerase chain reaction.
Results: All H pylori isolates had a positive babA2 genotype. We identified Lea in 33.5%, Leb in 72.9%, Lex in 86.2%, and Ley in 97.4% of biopsies from these 188 patients. Patients who expressed Leb had a higher H pylori density than those who did not express Leb (p<0.001). Among 139 patients who expressed Leb, H pylori density increased with a higher Leb intensity (p<0.05). Gastric atrophy decreased with Leb intensity and thus resulted in lower H pylori density in the antrum (p<0.05). For the 49 patients without gastric Leb expression, H pylori density was positively related with Lex and Lea expression (p<0.05).
Conclusions: Taiwanese H pylori isolates are 100% babA2 genopositive. Gastric Leb as well as Lex intensity may be major determinants of H pylori density. While lacking gastric Leb expression, Lex and Lea were closely related to H pylori colonisation.
Keywords: Helicobacter pylori, babA2, Lewis antigen expression, gastric histology
Helicobacter pylori is a well recognised gastric pathogen in humans.1,2 The ability of H pylori to achieve persistent colonisation in the human stomach has become the focus of intense research.3 Several studies have proposed that the molecular mimicry of H pylori lipopolysaccharide antigens to human Lewis (Le) antigens may help H pylori to evade the immune response and enhance bacterial adherence to gastric epithelium.3–6 As Le antigens are also found on the gastric epithelium in humans,7 Le antigen expression may mediate the attachment of H pylori to the gastric mucosa.8 Strong evidence was provided by Ilver et al who purified the blood group antigen binding adhesin (BabA) of H pylori and found that BabA selectively adheres to the Leb antigen of the host.9 Their findings suggest that gastric Leb antigens selectively interact with the products of the babA2 (blood group associated binding gene) allele of H pylori and thus may possibly facilitate a more dense colonisation in the stomach. However, contradictory data focused on the role of the babA2 genotype in terms of clinicohistological outcome without analysing the host status for Leb expression in the stomach.10–12 Therefore, we conducted this study to elucidate if the interaction of the babA2 genotype of H pylori and gastric Leb antigen expression of the host are correlated with different clinical outcomes.
As gastric Leb antigens cannot be found in all humans, some other pathways must exist to facilitate adherence of H pylori. In contrast with the rare expression of Leb, Lex and Ley antigens are commonly expressed.3–5,13 As the adhesion pedestal formation contained Lex on both H pylori and gastric epithelium, these Lewis antigens may be required to establish or maintain infection.3,13 Thus we tested if these Lewis antigens have a role in bacterial adherence, when the host has weak or no gastric Leb expression, interacting with the BabA of H pylori.
MATERIALS AND METHODS
Patients and study design
A total of 188 dyspeptic patients (112 men and 76 women; mean age 44.8 years) gave informed consent and were consecutively enrolled after they were proved to have H pylori infection, defined as a positive culture. None had a previous history of anti-H pylori therapy. Each patient had undergone panendoscopy to obtain a gastric biopsy for culture and histology of H pylori infection. The endoscopic diagnosis of these 188 study patients included uncomplicated chronic active gastritis (n=98), duodenal ulcer (n=45), and gastric ulcer (n=45).
At gastric biopsy, five samples, including two from the antrum, two from the corpus, and one from the cardia, were obtained during endoscopy. Three gastric specimens, each one from antrum, corpus, and cardia, were stained with haematoxylin and eosin as well as with modified Giemsa stains. Apart from analysis of H pylori related gastric histology, these three gastric specimens were stained immunochemically for expression of Lewis antigens Lea, Leb, Lex, and Ley. The remaining two gastric specimens were used for H pylori culture.14 Genomic DNA of these H pylori isolates were then extracted by polymerase chain reaction (PCR) to detect the babA2 genotype. Extraction of DNA was performed using the same method as reported in our previous publication.15
PCR and primers for babA2 genotypes
Extracted DNA from each strain was subjected to PCR for amplification of the babA2 genes, applying one pair of primers (babo-F: CTT AAA TAT CTC CCT ATC CC, corresponding to bp 1 to 20 of AF033654; babo-R: CGA TTT GAT AGC CTA CGC TTA TG, corresponding to bp 369 to 391 of AF033654) designed by Ilver and colleagues9 or another self designed primer (bab7-F: CCA AAC GAA ACA AAA AGC GT, corresponding to bp 105 to 124 of AF033654; bab7-R: GCT TGT GTA AAA GCC GTC GT, corresponding to bp 357 to 375 of AF033654).
The PCR mixtures were performed in a volume of 50 μl containing 0.2 μM of each primer, 0.2 mM each of deoxynucleoside triphosphates, reaction buffer with MgCl2, and 1 unit of DyNAzyme II DNA polymerase (Finnzymes OY, Espoo, Finland). Amplification was carried out over 30 cycles consisting of 94°C for one minute, 45°C for one minute, and 72°C for one minute in a thermal cycler (Perkins-Elmer Cooperation, Norwalk, Connecticut, USA). The two primers achieved a 391 bp product (by primers designed by Ilver et al) and a 271 bp product (using the self designed primers in this study), respectively. The sequences of these two PCR products were determined using an ABI PRISM 377 DNA Sequencer (Applied Biosystems, Foster, California, USA).
In addition, we randomly selected 30 babA2 genopositive strains (proven by the presence of the 271 bp PCR product) to test their BabA producing phenotype by western blotting using BabA specific antiserum. BabA specific antiserum was obtained from Drs Thomas Boren and Stefan Odenbreit.8,9,16 Each selected H pylori extract was analysed on a sodium dodecyl sulphate-10% polyacrylamide gel. The blot was then subjected to a 1:500 dilution of the anti- BabA antibody and detected with goat antirabbit antibody conjugated to horseradish peroxidase (Chemicon International Inc., Temecula, California, USA).
Analysis of H pylori related histology
The same pathologist, unaware of the endoscopic and culture results, analysed the gastric histology. H pylori density for each specimen was scored according to Yang and colleagues17: score 0, no bacteria; score 1, one or two small clusters with less than 10 bacteria; score 2, less than half the superficial crypt area with less than 10 bacteria in each crypt; score 3, less than half the area but with more than 10 bacteria, or more than half the area with less than 10 bacteria in each crypt; score 4, >10 bacteria in forvelae with some free area; and score 5, >10 bacteria without a free area. Total H pylori density (HPD) was defined as the sum of the densities from the three biopsy samples, obtained from the antrum, corpus, and cardia. Thus the HPD score ranged from 1 to 15. The acute inflammatory score (range 0–3), chronic inflammation score (range 0–3), atrophic change (absent, 0; present, 1), and intestinal metaplasia (IM) (absent, 0; presence, 1) were graded using the updated Sydney system.18 The total acute (AIS) and chronic (CIS) inflammatory scores were also a sum of the three specimens (range 0–9).
Immunochemical staining for gastric Lewis expression
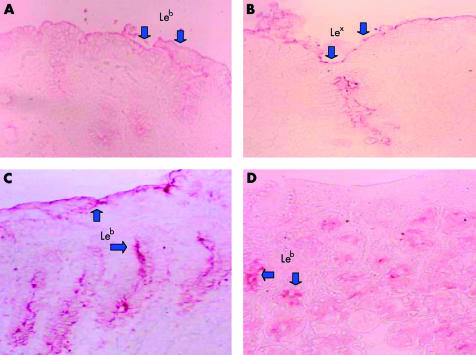
Immunostaining of biopsy specimens for Lewis antigens was performed using the standard avidin-biotin-peroxidase technique. Formalin fixed paraffin embedded tissue sections, including topographical specimens from the antrum, corpus, and cardia from each patient, were deparaffinised through xylene and hydrated with ethanol. Slides were washed with distilled water and then placed in 1× phosphate buffered saline (PBS) for five minutes. Incubation with 3% hydrogen peroxide for three minutes blocked the endogenous peroxidase activities of these sections. After incubation with 2% bovine serum albumin for two hours and washing with PBS, the primary monoclonal antibodies for detection of gastric Lewis antigens were used (anti-Lewis Lea, Leb, Lex, and Ley ; Signet Laboratories, Inc., Dedham, Massachusetts, USA). The reaction time for the primary monoclonal antibodies (anti-Lea, Leb, Lex, and Ley) was three hours at 25°C. These slides were again washed with PBS and incubated with the secondary antibody to achieve a 1:2000 dilution of antimouse IgG and IgM conjugated to horseradish peroxidase (Chemicon International Inc., Temecula, California, USA) for two hours at 25°C. These slides were finally washed with PBS, and the AEC kit (Sigma, St Louis, USA) was used as substrate to illustrate the stain. All slides were evaluated blindly by the same pathologist. For each gastric site, the intensity of Lea, Leb, Lex, and Ley was scored from 0 to 3 (0, no staining; 1, staining of either surface mucous cells or deep gastric glands; 2, staining of surface cells, intercryptal epithelium, and deep glands but expressed in ⩽50% of the analysed specimens; 3, diffuse staining of ⩾50% of the analysed specimens on surface cells, intercryptal epithelium, and deep glands). Examples of intensity 1 and intensity 2 gastric Leb expression are shown in fig 1A ▶ and 1B ▶, respectively. Total gastric Lewis antigen intensity (TLI) for Lea, Leb, Lex, and Ley was the sum of three biopsy samples from the antrum, corpus and cardia (range 0–9).
Figure 1.
(A, B) Gastric immunohistochemical stains of Lewis antigen Leb expression. (A) Positive staining over the surface epithelium only. (B) Diffuse staining over the intercryptal epithelium. (C, D) Gastric immunohistochemical stains of Lex expression. (C) Positive staining over the surface epithelium only. (D) Diffuse staining over the deep glands.
Statistics
The Student’s t test and paired t test were used as appropriate for parametric differences. One way ANOVA with Bonferroni’s method was used for multiple testing of data. Pearson’s χ2 test was used for non-parametric proportions. All significance tests were two tailed and a p value <0.05 was taken as significant.
RESULTS
Prevalence of babA2 genotypes of H pylori infection in Taiwan
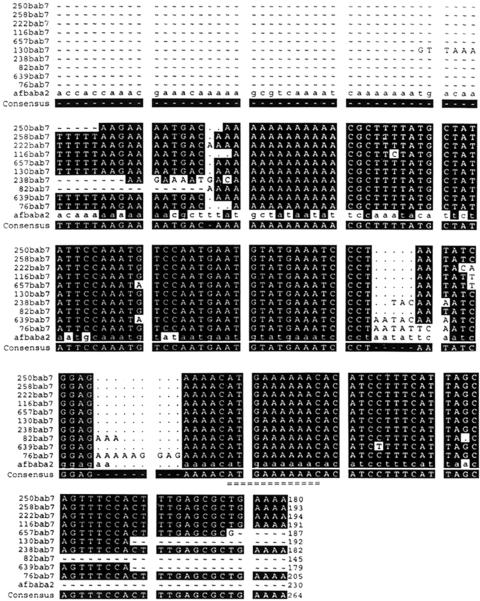
Fifty per cent (94/188) of H pylori isolates had a positive babA2 genotype by PCR, applying the primers used by Ilver et al to obtain a band of 371 bp. However, the nucleotide sequence of this 391 bp PCR product from the Taiwanese isolates was confirmed as not being babA2 in origin but had >90% homology with the published sequence of adenine specific DNA methyltransferase in H pylori 26695. To detect the babA2 genotype for the domestic strains, we self designed a pair of primers and achieved a 271 bp PCR product whose nucleotide sequence was confirmed with >95% homology to the babA2 gene of CCUG 17875 (fig 2 ▶). Based on PCR using these primers to obtain a 271 bp band, the prevalence rate of the babA2 genotype was 100% in all 188 Taiwanese H pylori isolates. Western blotting also confirmed that the 30 randomly selected 271 bp genopositive strains had a uniformly positive phenotype.
Figure 2.
The nucleotide sequence of the 271 bp polymerase chain reaction product gained from the self designed babA2 primers. The nucleotide sequence of the 10 randomly selected domestic strains (hp250, hp258, hp222, hp116, hp657, hp130, hp238, hp82, hp 639, and hp76) was confirmed to be babA2 in origin with >90% homology to the published sequence of the babA2 gene (afbabA2) of CCUG 17875. The 10 nucleotides (ATG AAA AAA C), representative of the babA2 gene of Helicobacter pylori, are indicated by “=”.
Topographic gastric Lewis antigen expression in H pylori infected Taiwanese
Based on the presence of staining of any one of the three gastric specimens, we identified Lea in 33.5%, Leb in 72.9%, Lex in 86.2%, and Ley in 97.4% of gastric biopsies in these 188 patients. As shown in table 1 ▶, the topographic intensity of gastric Ley expression was higher in the corpus than in the antrum or cardia (1.76 v 1.61 and 1.68; paired t test, p<0.05). The intensity of Leb expression was also higher in the corpus and cardia than in the antrum (1.74 v 1.37, and 1.59 v 1.37; paired t test, p<0.05). In contrast, the topographic intensity of Lea or Lex was higher in the antrum than in the corpus and cardia (Lea: 0.56 v 0.41 and 0.45, p<0.05; Lex: 1.19 v 0.88 and 0.75, p<0.05).
Table 1.
Topographic distribution of the intensity of gastric Lewis antigen expression in 188 patients with Helicobacter pylori infection
| Lewis antigen | Antrum | Body | Cardia | Significance* |
| Intensity (range 0–3) | ||||
| Lea | 0.56 (0.77) | 0.41 (0.65) | 0.45 (0.81) | A>B; A>C |
| Leb | 1.37 (1.24) | 1.74 (0.95) | 1.59 (1.03) | B>A; C>A |
| Lex | 1.19 (0.72) | 0.88 (0.71) | 0.75 (0.73) | A>B; A>C |
| Ley | 1.61 (0.95) | 1.76 (1.07) | 1.68 (0.88) | B>A; C>A |
Values are mean (SD).
*Significant difference by paired t test with two tailed analysis (p<0.05).
A, antrum; B, body; C, cardia.
Lewis antigen expression and clinicohistological features of H pylori infection
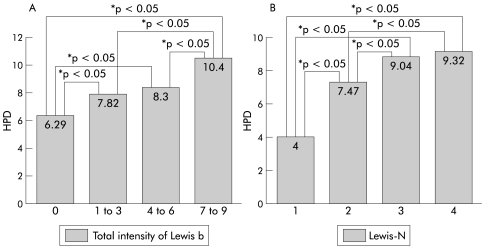
There was no difference in ulcer rate between patients with or without Lewis antigen expression in the stomach (table 2 ▶). Patients with gastric Leb expression had significantly higher HPD and CIS than those without Leb expressions (HPD: 9.29 v 6.35, p<0.001; CIS: 7.59 v 6.15, p<0.05). We also found that mean HPD of 12 Lea+b− patients was significantly lower than that of either 88 Lea−b+ patients or 51 Lea+b+ patients (7.42 v 9.22 and 9.41; p<0.05 by one way ANOVA). In fig 3A ▶, TLI of Leb was found to be positively correlated with HPD (one way ANOVA, p<0.05). In table 2 ▶, although the statistical significance was limited, HPD was evidently higher in those patients who expressed Lea, Lex, and Ley in the stomach. Furthermore, patients who expressed Lea and Lex had a higher bacterial density in biopsies (p<0.05). HPD was even elevated when the total number of gastric Lewis expression (Lewis-N) of each study patient increased (fig 3B ▶). Multivariate logistic regression disclosed that the intensity of Leb and Lex expression, rather than Lewis-N, was an independent factor correlated with HPD in H pylori infected patients (table 3 ▶).
Table 2.
Lewis antigen expression and clinicohistological features of Helicobacter pylori infection
| Lea | Leb | Lex | Ley | |||||
| Parameter (mean) | (+) (n=63) | (−) (n=125) | (+) (n=139) | (−) (n=49) | (+) (n=162) | (−) (n=26) | (+) (n=183) | (−) (n=5) |
| AIS (0–9)§ | 2.31 | 2.61 | 2.62 | 2.21 | 2.81 | 2.45 | 2.55 | 1.18 |
| CIS (0–9)† | 6.89 | 6.97 | 7.59 | 6.15 | 6.95 | 6.88 | 6.96 | 6.40 |
| AT (%) | 57.1 | 57.6 | 57.6 | 57.1 | 54.9 | 73 | 56.3 | 100 |
| IM (%)ठ| 28.6 | 29.6 | 28.1 | 32.7 | 26.7 | 57.7 | 25.1 | 80 |
| Ulcer rate (%) | 49 | 45.6 | 47.5 | 44.9 | 47.5 | 42.3 | 46.9 | 40 |
| HPD (1–15)† | 9.03 | 8.29 | 9.29 | 6.35 | 8.59 | 8.03 | 8.57 | 7.41 |
| Antrum (1–5)† | 2.89 | 2.69 | 2.87 | 2.41 | 2.73 | 2.71 | 2.77 | 2.14 |
| Body (1–5)*†‡ | 3.46 | 3.08 | 3.52 | 2.31 | 3.31 | 2.69 | 3.21 | 2.85 |
| Cardia (1–5)† | 2.67 | 2.51 | 2.89 | 1.63 | 2.66 | 2.53 | 2.57 | 2.41 |
AIS, acute inflammatory score; CIS, chronic inflammatory score; AT, antral atrophy; IM, intestinal metaplasia; HPD, total density of H pylori.
Significant difference (p<0.05): *between Lea (+) and Lea (−) patients; †between Leb (+) and Leb (−) patients; ‡between Lex (+) and Lex (−) patients; §between Ley (+) and Ley (−) patients.
Figure 3.
(A) Total Helicobacter pylori density (HPD) was positively correlated with an increase in Leb intensity (*p<0.05, significant difference analysed using one way ANOVA). (B) Total Lewis number (Lewis-N) of each patient was also positively correlated with higher HPD in the stomach (one way ANOVA, p<0.05).
Table 3.
Multivariate logistic regression for independent factors relevant to total Helicobacter pylori density of the stomach
| Parameter | Coefficient | Standard error | p Value | 95% confidence interval |
| Total 188 patients | ||||
| Total Leb intensity | 0.491 | 0.052 | 0.000* | (0.389~0.593) |
| Lewis-N | −0.327 | 0.294 | 0.267 | (−0.906~0.253) |
| Total Lex intensity | 0.230 | 0.075 | 0.002* | (0.083~0.378) |
| Total Ley intensity | 0.092 | 0.056 | 0.116 | (−0.022~0.199) |
| Total Lea intensity | 0.123 | 0.076 | 0.221 | (−0.057~0.244) |
| In 139 patients with Leb | ||||
| Total Leb intensity | 0.518 | 0.066 | 0.000* | (0.386~0.649) |
| Total Lex intensity | 0.183 | 0.074 | 0.015* | (0.037~0.329) |
| Total Ley intensity | 0.061 | 0.055 | 0.269 | (−0.048~0.169) |
| Total Lea intensity | −0.044 | 0.073 | 0.546 | (−0.187~0.099) |
| In 49 patients without Leb | ||||
| Total Lex intensity | 0.594 | 0.136 | 0.000* | (0.318~0.868) |
| Total Lea intensity | 0.242 | 0.105 | 0.025* | (0.031~0.456) |
| Total Ley intensity | 0.111 | 0.093 | 0.241 | (−0.077~0.298) |
Lewis-N, total number of four Lewis antigen expressions in the stomach of each patient.
*Significant difference.
As also shown in table 2 ▶, patients with no expression of Lex or Ley had higher rates of IM in the stomach (p<0.01). Among those patients who expressed Lex, TLI of Lex was significantly lower in the presence of IM (2.68 v 3.83; p< 0.01) but higher in the presence of antral atrophy (4.05 v 3.01; p< 0.01). There was no decrease in TLI of Lea, Leb, or Ley, despite the presence of IM or antral atrophy.
Factors correlating with HPD in non-Leb patients
Of the 49 H pylori infected patients without Leb expression, HPD was higher in patients who expressed gastric Lea and Lex (Lea: 7.41 v 6.05, p<0.05; Lex: 6.81 v 4.25, p<0.005). In table 3 ▶, multivariate logistic regression confirmed that Lea and Lex antigens were both independently correlated with HPD in the 49 patients without Leb expression. In contrast, for the 139 patients who expressed Leb, only Lex but not Lea antigens were independently correlated with HPD (table 3 ▶).
Compensatory effect of Lex to maintain HPD for weak Leb intensity in antral atrophy
Among the 139 Leb positive patients, the topographic distribution of H pylori density and the intensity of Lea, Leb, and Lex were compared between patients with and without antral atrophy (table 4 ▶). There was no decrease in either Lea or Lex intensity over the antrum despite the presence of atrophy. In contrast, patients with antral atrophy had a lower Leb intensity over the antrum (p<0.05) and thus had a significantly lower bacterial density (p<0.05).
Table 4.
Topographic distribution of Helicobacter pylori density and gastric Lewis antigen intensity in 139 Leb positive patients with and without antral atrophy
| Parameter (mean) | Antrum | Body | Cardia | Body+cardia | Total |
| Lea intensity | |||||
| AT (n=80) | 0.54 | 0.39 | 0.54 | 0.93 | 1.36 |
| Non-AT (n=59) | 0.59 | 0.51 | 0.49 | 1.03 | 1.64 |
| Leb intensity | |||||
| AT (n=80) | 1.63* | 2.43 | 2.23 | 4.66 | 6.05 |
| Non-AT (n=59) | 2.15 | 2.26 | 2.25 | 4.51 | 6.44 |
| Lex intensity | |||||
| AT (n=80) | 1.21 | 1.31* | 0.94* | 2.24 * | 2.49 |
| Non-AT (n=59) | 1.28 | 0.88 | 0.77 | 1.66 | 2.24 |
| H pylori density | |||||
| AT (n=80) | 2.52* | 3.65* | 3.05* | 6.71* | 9.23 |
| Non-AT (n=59) | 3.35 | 3.25 | 2.67 | 5.98 | 9.38 |
Body+cardia, the summation of the data from the body and cardia; Total, the sum of antrum, body, and cardia,
*Significant difference between patients with (AT) and without (non-AT) antral atrophy, analysed by the two tailed Student’s t test (p<0.05).
Although both bacterial density and the intensity of Leb over the antrum were lower, HPD was not decreased by the presence of antral atrophy (table 4 ▶). The paradoxical increase in bacterial density on the body and cardia were found to maintain HPD under the presence of antral atrophy (p<0.05). However, there was no significant increase in Leb intensity elsewhere in the body or cardia (table 4 ▶). In contrast, a significant increase in the intensity of Lex over the gastric body and cardia was found in those patients with antral atrophy compared with those without antral atrophy (p<0.05).
DISCUSSION
Identification of specific receptors for H pylori on the gastric mucosa may explain why the organism can only adhere to those cells in humans. Ilver et al disclosed that the babA2 gene of H pylori is a putative determinant allowing it to adhere to Leb of the gastric epithelium and thus could promote bacterial invasion of the human stomach.9 Our prospective study enrolled 188 H pylori infected patients and is the first to analyse both bacterial babA2 genotype and gastric antigen expression (including Leb), thus further elucidating the impact of any interactions between BabA and Leb on the clinicohistological outcome after H pylori infection.
In the present study, after applying the primer of Ilver et al to obtain a 391 bp PCR product, we discovered it was non-babA2 in origin. By applying our self designed primers, a 271 bp PCR product was found and was confirmed to have >95% homology to the published sequence of babA2. The nucleotide sequence data confirmed that our self designed pair of primers were suitable for babA2 genotyping in Taiwan and all 188 isolates in this study were uniformly proven to have a babA2 positive genotype. The prevalence was higher than in previous reports (38–85%).10–12,19 Moreover, such an extremely high prevalence of babA2 in Taiwan suggests this could be an ideal country in which to study whether babA2 is a good target for preventive vaccination if BabA interacts strongly with Leb to impact on H pylori colonisation of patients.
The prevalence rates of the different Lewis antigens in our study were compatible with Kobayashi et al, who reported that Lea had the lowest incidence and that gastric Lex or Ley may disappear when H pylori infection is induced by IM.7 Such a finding was indirectly supported by our data (table 1 ▶) which showed that patients without expression of Lex or Ley had higher rates of IM than those with Lex and Ley (p<0.05).
Patients with gastric Leb expression had a higher bacterial density of H pylori than those without Leb expression (p<0.05) (table 2 ▶). TLI of Leb expression was also positively correlated with HPD (fig 3A ▶). Moreover, HPD was higher in Lea−b+ weak and Lea+b+ strong secretors than in Lea−b− non-secretors (p<0.05). Accordingly, the intensity of Leb was proved to be an independent factor in determining HPD (table 3 ▶). As all domestic strains were babA2 positive, our study from Taiwan may be the most rational in elucidating the fact that gastric Leb really serves as an important receptor for H pylori adherence.
There were 49 patients with H pylori infection but no expression of Leb in the stomach. Bacterial densities of the body remained higher in patients with positive expression of Lea and Lex (p<0.05) (table 2 ▶). An increasing trend for HPD was found in patients whose Lewis-N ranked high (p<0.05, by one way ANOVA) (fig 3B ▶). These data imply that there may be some additive effect of expression of other Lewis antigens, apart from Leb, serving as adherent receptors for H pylori. This is compatible with the finding of Clyne and Drumm who confirmed that blocking with a monoclonal antibody for the Leb antigen on the gastric epithelium could not totally abolish adherence of H pylori.20 Thus we tested if other gastric Lewis types also enhanced bacterial adherence in the 49 patients without Leb expression. Our study found that patients who expressed Lex and Lea had higher HPD than those who did not express Lex and Lea (Lea: 7.41 v 6.05, p<0.05; Lex: 6.81 v 4.25, p<0.005). By multiple logistic regression, Lex and Lea were further confirmed to be independent factors in enhancing colonisation of H pylori (table 3 ▶). These clinical data thus support the laboratory findings of Taylor et al which found adhesion pedestal formations stained with Lex on both H pylori and gastric epithelium. Accordingly, our study confirmed that Lewis antigens other than Leb can be used to establish or maintain H pylori infection in the stomach.3,10,16
Expression of Leb was stronger in the body, in contrast with the antral dominant distribution of Lea and Lex (table 1 ▶). Therefore, we tested whether Lex and Lea had additive effects when present with Leb for enhancement of H pylori colonisation in the 139 patients with Leb expression. Patients with antral atrophy had different topographic distributions of bacterial density but the total density of H pylori did not differ (table 4 ▶). The presence of antral atrophy decreased the intensity of Leb, which was expected, as Leb usually stained the superficial glands.7,9,19 When the intensity of Leb was lower, bacterial density here decreased. However, overall HPD was maintained by the paradoxical increased density over the body and corpus. As the intensity of Lex over the body and cardia were higher in the presence of antral atrophy, increased bacterial densities here could be mediated by Lex expression. These clinical data supported the finding that gastric Lex antigen can enhance H pylori adherence.16 Moreover, Lex may have compensatory or additive effects with Leb to maintain bacterial loads during ongoing atrophy changes.
Among those patients who expressed Lex, TLI of Lex in the presence of IM was 2.68 versus 3.83 (p<0.01) but was higher in the presence of antral atrophy (4.05 v 3.01; p< 0.01). These data confirm that IM and antral atrophy may change Lex expression and thus alter the H pylori colonisation pattern.
In summary, Taiwanese H pylori isolates are 100% babA2 positive. Gastric Leb intensity as well as Lex intensity appear to be major determinants of bacterial density of H pylori. When lacking gastric Leb expression, Lex and Lea are closely related to H pylori colonisation. To overcome H pylori adherence, genomic targets such as babA2 (or others interacting with gastric Lewis antigens) may be promising.
Acknowledgments
The study was supported by grants NHRI-EX91-9041SC from the National Health Research Institute, and NSC90-2320-B-006-048 and NSC90-2320-B-006-091 from the National Science Council, Taiwan. The authors also appreciate the assistance of Ms Hunt-Wei Wu, and the anti-BabA serum from Drs Thomas Boren and Stefan Odenbreit.
Abbreviations
Le, Lewis
Lewis-N, total Lewis number
BabA, blood group antigen binding adhesin
babA2, blood group associated binding gene
PCR, polymerase chain reaction
TLI, total gastric Lewis antigen expression intensity
HPD, Helicobacter pylori density
IM, intestinal metaplasia
CIS, chronic inflammatory score
PBS, phosphate buffered saline
REFERENCES
- 1.Cover TL, Blaser MJ. Helicobacter pylori factors associated with disease. Gastroenterology 1999;117:257–61. [DOI] [PubMed] [Google Scholar]
- 2.Sherburne R, Taylor DE. Helicobacter pylori expresses a complex surface carbohydrate, Lewis x. Infect Immun 1995;63:4564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor DE, Rasko DA, Sherburne R, et al. Lack of correlation between Lewis antigen expression by Helicobacter pylori and gastric epithelial cells in infected patients. Gastroenterology 1998;115:1113–22. [DOI] [PubMed] [Google Scholar]
- 4.Monteiro MA, Chan KH, Rasko DA, et al. Simultaneous expression of type 1 and type 2 Lewis blood group antigens by Helicobacter pylori lipopolysaccharides: molecular mimicry between H. pylori lipopolysaccharides and human gastric epithelial cell surface glycoforms. J Biol Chem 1998;273:11533–43. [DOI] [PubMed] [Google Scholar]
- 5.Appelmelk BJ, Simoons-Smit I, Negrini R, et al. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect Immun 1996;64:2031–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wirth HP, Yang M, Peek RM Jr, et al. Helicobacter pylori Lewis expression is related to the host Lewis phenotype. Gastroenterology 1997;113:1091–8. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi K, Sakamoto J, Kito T, et al. Lewis blood group-related antigen expression in normal gastric epithelium, intestinal metaplasia, gastric adenoma and gastric carcinoma. Am J Gastroenterol 1993;88:919–24. [PubMed] [Google Scholar]
- 8.Boren T, Falk P, Roth KA, et al. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 1993;262:1892–5. [DOI] [PubMed] [Google Scholar]
- 9.Ilver D, Arnqvist A, Ogren J, et al. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science 1998;279:373–7. [DOI] [PubMed] [Google Scholar]
- 10.Prinz C, Schoniger M, Rad R, et al. Key importance of the Helicobacter pylori adherence factor blood group antigen binding adhesin during chronic gastric inflammation. Cancer Res 2001;61:1903–9. [PubMed] [Google Scholar]
- 11.Gerhard M, Lehn N, Neumayer N, et al. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci U S A 1999;96:12778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizushima T, Sugiyama T, Komatsu Y, et al. Clinical relevance of the babA2 genotype of Helicobacter pylori in Japanese clinical isolates. J Clin Microbiol 2001;39:2463–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor DE, Fedorak RN, Sherburne R. Antigenic mimicry between Helicobacter pylori and gastric mucosa: failure to implicate heat-shock protein Hsp60 using immunoelectron microscopy. Helicobacter 1999;4:148–53. [DOI] [PubMed] [Google Scholar]
- 14.Huang AH, Sheu BS, Yang HB, et al. Antimicrobial resistance of H. pylori to the outcome of one-week lansoprazole-based triple therapy. J Formos Med Assoc 2000;90:704–9. [PubMed] [Google Scholar]
- 15.Sheu SM, Sheu BS, Yang HB, et al. The Presence of iceA1 but not cagA, cagC, cagE, cagF, cagN, cagT, or orf13 genes of Helicobacter pylori is associated with a more severe gastric inflammation in Taiwanese. J Formos Med Assoc 2002;101:18–23. [PubMed] [Google Scholar]
- 16.Mahdavi J, Sonden B, Hurtig M, et al. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 2002;297:573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang SB, Sheu BS, Su IJ, et al. Clinical application of gastric histology to monitor treatment of dual therapy in H. pylori eradication. Dig Dis Sci 1997;42:1835–40. [DOI] [PubMed] [Google Scholar]
- 18.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis: the updated Sydney system: International Workshop on the Histology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161–81. [DOI] [PubMed] [Google Scholar]
- 19.Yamaoka Y, Kwon DH, Graham DY. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc Natl Acad Sci U S A 2000;97:7533–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clyne M, Drumm B. Absence of effect of Lewis A and Lewis B expression on adherence of Helicobacter pylori to human gastric cells. Gastroenterology 1997;113:72–80. [DOI] [PubMed] [Google Scholar]