Abstract
Background and aim: Water retention is a major clinical problem in patients with liver cirrhosis. Recent research suggests that renal aquaporins may be pathophysiologically involved in this condition. The aim of the present cross sectional study of patients with liver cirrhosis was to determine if 24 hour urinary excretion of renal aquaporin 2 (AQP2) differed from that of healthy control subjects and if such excretion was related to the severity of liver disease and to the patient’s water balance.
Results: Twenty four hour urinary excretion of AQP2 and free water clearance were measured in 33 stable cirrhosis patients on usual medication and in eight healthy subjects. AQP2 excretion, quantitated by immunoblotting, was eight times higher in cirrhosis patients than in controls (0.167 (0.270) U/day v 0.021 (0.017); p<0.05). Stratification according to clinical manifestations (Child- Pugh classes) revealed that it increased with the clinical severity of cirrhosis (class A 0.04 (0.04); class B 0.09 (0.16); class C 0.31 (0.35); p<0.05) but was not related to liver function, as measured by galactose elimination capacity. Excretion correlated inversely with free water clearance (rho=−0.57, p<0.01). It was higher in patients with oesophagogastric varices but not in those with ascites. Plasma vasopressin concentrations were not related to AQP2 excretion and there was no relation to dose or type of diuretic treatment.
Conclusions: Urinary AQP2 excretion was increased in patients with cirrhosis. Moreover, urinary AQP2 excretion increased with severity of cirrhosis in parallel with impairment of free water clearance. This suggests a functional association between increased AQP2 excretion and increased renal reabsorption of water in cirrhosis.
Keywords: aquaporin 2, cirrhosis, free water clearance
In patients with liver cirrhosis, changes in renal handling of water with decreased free water clearance1 has clinical consequences, such as ascites and the hepatorenal syndrome, with impact on quality of life, rate of complications, and survival. Recent research suggests that renal aquaporins may be involved, indicating a molecular explanation for the water retention of these patients. Aquaporins are membrane proteins serving the primary function of facilitating water transport. At least six of the 10 known mammalian aquaporins are expressed in the kidney (AQP1, -2, -3, -4, -6, and -7).2 Water is reabsorbed both in the proximal nephron and in the collecting duct. The latter is vasopressin regulated and at least three aquaporins are active. AQP2 acts on the apical part of the principal cells of the collecting duct under regulation by vasopressin.3 AQP3 and AQP4 are exit channels of water taken up by cells by AQP2. The abundance of AQP2 seems to be the determinant of epithelial water transport capacity. AQP2 is excreted in urine4,5 and varies with osmotic challenge.5,6
As to the involvement of AQP2 in water retention in cirrhosis, animal models show conflicting results. CCl4 induced cirrhosis in rats results in more AQP2 mRNA and protein7,8 but bile duct ligation in less.9,10 A recent study found no difference in the membrane fraction of AQP211 but more intracellular AQP2 containing vesicles, indicating increased AQP2 trafficking to the apical plasma membrane. This higher AQP2 turnover is expected to result in increased water permeability in the distal part of the nephron12 which may explain the water retention. No study in humans with cirrhosis is available.
The aim of the present cross sectional study in patients with liver cirrhosis was to quantitate 24 hour urinary excretion of renal AQP2 compared with that of healthy control subjects under daily living conditions. The findings were related to the severity of liver disease and to spontaneous free water clearance.
METHODS
Study population
Patient eligibility criteria were liver cirrhosis, age greater than 18 years, stable body weight, and no change in diuretic treatment for at least five days before entering the study. Exclusion criteria were malignant or infectious disease, congestive heart failure, diabetes insipidus, lithium treatment, and obstructive kidney disease. The total number of patients recruited was 37, of whom 33 were included. Three patients were excluded because of paracentesis three days before the study and one because of a recent increase in diuretic treatment. The 33 patients (21 males and 12 females) had a median age of 50 years (range 33–81).
The diagnosis of liver cirrhosis was based on liver biopsy (n=23) or classical ultrasonographic, clinical, and biochemical characteristics. The aetiology of liver cirrhosis was alcohol in 29, primary biliary cirrhosis in one (Child-Pugh A), viral hepatitis C in one (Child-Pugh B), and cryptogenic in two patients (Child-Pugh A and B).
Twenty five patients were treated with aldosterone antagonists, 22 with loop diuretics, seven with beta blockers, six with H2 antagonists, two with selective serotonin reuptake inhibitors, three with neuroleptics, three with antiepileptics, two with digoxin, one with angiotensin II receptor inhibitor, one with calcium antagonists, two with insulin, two with antabuse, 11 with vitamin K, and two with slow release nitrates. None took cyclooxygenase inhibitors.
A 24 hour urine was collected the day before blood sampling, which was done during the morning.
The latest endoscopy identified patients with oesophagogastric varices or portal hypertensive gastropathy.
Ascites was graded clinically as: none, slight, moderate, or tense. Peripheral oedema was either present or not present.
The Child-Pugh score was used to stratify patients according to clinical severity of their liver disease (table 1 ▶).13
Table 1.
Descriptive data for healthy control subjects and patients with cirrhosis stratified according to Child-Pugh class
| Controls | Child A | Child B | Child C | |
| n | 8 | 6 | 13 | 14 |
| Age (y) (median (range)) | 25 (22–39)§ | 52 (41–64) | 51 (40–81) | 50 (33–67) |
| Sex (M/F) | 8/0§ | 4/2 | 6/7 | 11/3 |
| Varices (+/−) | — | 4/2 | 9/4 | 12/2 |
| Ascites (0/1/2/3) | — | 5/1/0/0 | 5/2/5/1 | 1/1/2/8 |
| GEC (μmol/min)¶ (>2.3) | — | 2.0 (0.6)‡ | 1.3 (0.2) | 1.5 (0.3) |
| P-sodium (mmol/l) (136–146) | — | 140 (6) | 135 (4)† | 128 (8)** |
| P-albumin (μmol/l) (596–769) | — | 449 (126) | 433 (65) | 382 (64) |
| P-bilirubin (μmol/l) (<22) | — | 29 (26) | 29 (25)† | 94 (92)* |
| Prothrombin time (s) (0.8–1.2) | — | 0.72 (0.25) | 0.51 (0.13)†† | 0.37 (0.12)** |
| P-potassium (mmol/l) (3.2–5.0) | — | 3.9 (0.3) | 3.8 (0.3) | 4.1 (0.8) |
| P-creatinine (μmol/l) (55–120) | — | 65 (7) | 104 (66) | 78 (30) |
| CrCl (ml/min/1.73 m2) (>80) | — | 72 (19) | 57 (35) | 62 (34) |
Data are mean (SD).
Degree of ascites was graded as 0=none, 1=slight, 2=moderate, and 3=tense.
P, plasma; GEC, galactose elimation capacity; CrCl, creatinine clearance (values in parentheses are normal values).
*p<0.05, **p<0.01 for Child Pugh class C versus A.
†p<0.05, ††p<0.01 for Child Pugh class C versus B+A.
‡p<0.05 for Child Pugh class B versus A.
§p< 0.05 for controls versus Child Pugh class C, B, and A, respectively.
¶Class B, n=8; class C, n=8.
Control subjects
Eight healthy men (median age 25 years (range 21–39)) with normal blood pressure (115/80 mm Hg (120–110/85–75)) and with no signs or symptoms of disease volunteered to participate (table 1 ▶). None received drugs for a fortnight before each study day. Seven were studied twice.
Ethics
Informed consent was obtained from all participants. The study was approved by the local committee of ethics and carried out in accordance with the Second Helsinki Declaration.
Analysis
Urine AQP2
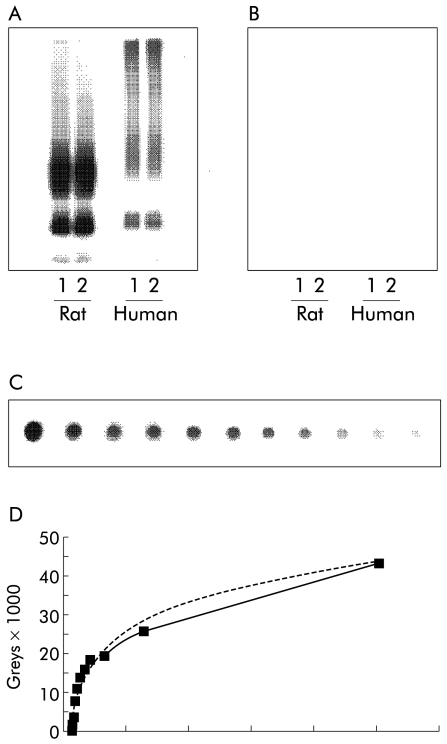
Semiquantitative AQP2 excretion in urine was determined by a dot blot technique. Urine samples from each patient were centrifuged at 3000 g for 10 minutes at 4°C to remove whole cells and sediment. A pre-wetted nitrocellulose membrane was placed on a sealing gasket in a Bio-Dot apparatus (BioRad, Hercules, California, USA). By adjusting the flow valve, the vacuum chamber was opened to the atmosphere. To obtain a standard, 12 wells were filled with a serially diluted AQP2 peptide solution, the concentration of which was reduced by 50% in each successive well (fig 1 ▶).
Figure 1.
Immunoblots and standard curve for determination of aquaporin 2 (AQP2) expression levels in urine. (A) Immunoblot of membrane fractions of inner medulla prepared from rat and human kidneys. The immunoblot was exposed to affinity purified anti-AQP2, diluted 1:2000, revealing 29 and 35–50 kDa bands for both rat and human kidneys. (B) Preabsorption control performed with anti-AQP2 previously incubated with immunising peptide. (C) Dot blot of serial peptide dilution, which was exposed to affinity purified anti- AQP2, diluted 1:800. (D) Standard curve obtained from serial peptide dilution including the best logarithmic equation of the relationship between AQP2 and signal intensity.
Samples of centrifuged urine were pipetted to an appropriate number of wells. All samples were filtered through the membrane by gravity flow and washed by adding Tris buffered saline (TBS) buffer (100:l) to each well, ensuring that all protein in the applied samples was filtered through the membrane. Samples were washed twice by adding TBS buffer and Tween (T-TBS) (200:l) to each well. This solution was vacuumed through the membrane by adjusting the flow valve to the vacuum position. The membranes were removed from the apparatus and placed in a blocking solution (5% milk in T-TBS) on a shaking table for 60 minutes and washed in T-TBS. Membranes were incubated overnight on a shaking table at 5°C with a 1:2000 dilution of our primary rabbit antihuman AQP2 solution (AN-368AP) which had been raised against the 15 C terminal amino acids of human AQP2.14 The antibody has been characterised by immunoblotting using membrane fractions of human kidney inner medulla which revealed 27 and 35–55 kDa bands corresponding to unglycosylated and glycosylated forms of AQP2. Membranes were then washed in T-TBS and a secondary antibody (P448) solution was added to the membrane and incubated on a shaking table for another 60 minutes at room temperature (20°C). Membranes were finally washed in T-TBS.
The dot blot was developed using ECL PLUS and scanned. The detection limit was 0.0035 units/day (U/day). Any sample in which the level was undetectable was assigned this value.
AQP2 was undetectable in eight patients and in two controls. The day to day variation (n=5) in urinary AQP2 excretion (day 1: 0.018 (0.011) U/day; day 2: 0.023 (0.012) U/day; NS) in controls was not statistically significant.
Vasopressin
Plasma arginine vasopressin was measured by an antibody (AB3096, generously supplied by Dr Peter Bie15) at a final dilution of 1:175 000. Plasma was extracted by Oasis HLB extraction cartridge (Waters A/S), and otherwise the assay was performed according to an already published method.16 Cross reactivity was determined for a number of analogous peptides: [Lys8]-vasopressin, oxytocin, and pressinoic acid, all <0.001%, [deamino-Cys1,D- Arg8]-vasopressin <0.07 %, and [Arg8]-vasotocin <0.25%. The detection limit was 0.26 pg/ml and mean recovery of unlabeled arginine vasopressin was 69%. Intra-assay and interassay coefficients of variation were 6.8% and 9.3%, respectively. A sample in which the level was undetectable was assigned a value of 0.26 pg/ml. Vasopressin was undetectable in 13 patients.
Galactose elimination capacity
The galactose elimination capacity (GEC) was determined from blood concentration decay curves corrected for urinary excretion after an intravenous load of galactose, as described by Tygstrup.17
Osmolality
Serum and urine osmolality were measured by freezing point depression. Free water clearance (ClH2O) was calculated as:
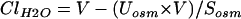 |
where V is urine volume, Uosm is urine osmolality, and Sosm is serum osmolality.
The day to day variations in free water clearance (day 1: −1.31 (0.63) ml/min; day 2: −1.36 (0.56) ml/min; NS), Uosm (day 1: 638 (238) mmol/l; day 2: 676 (223) mmol/l; NS), and Sosm (day 1: 285 (8) mmol/l; day 2: 282 (11); NS) in controls were not statistically significant.
Other biochemical variables were measured at the Departments of Clinical Chemistry, Aarhus University Hospital and Hvidovre University Hospital.
Statistics
Data are presented as mean (SD), unless otherwise stated. Analyses were performed using paired Student’s t tests or ANOVA. Log transformation was performed if data were not normally distributed. Controls were included only with the values of day 1 for further statistical analysis. A p value <0.05 was considered statistically significant. Spearman’s rank correlation test was used to determine the relation between two variables.
RESULTS
Table 1 ▶ shows descriptive data for healthy control subjects and cirrhotic patients stratified by Child-Pugh class. Creatinine clearance did not differ among patient groups.
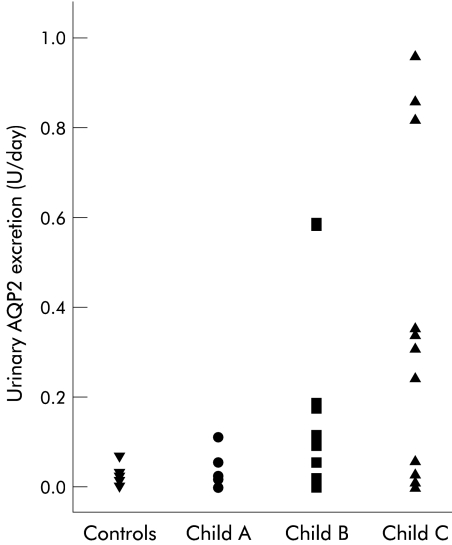
Urinary AQP2 excretion was overall more than eight times higher in cirrhotic patients (cirrhosis: 0.167 (0.270) U/day) compared with controls (0.021 (0.017) U/day; p<0.05) and excretion increased with Child-Pugh class (fig 2 ▶, table 2 ▶). Excretion of AQP2 was not related to GEC.
Figure 2.
Twenty four hour urinary excretion of aquaporin 2 (AQP2) in control subjects and in patients with cirrhosis stratified according to Child-Pugh class (Child A, Child B, and Child C).
Table 2.
Twenty four hour urine excretion of aquaporin 2 (AQP2), free water clearance (ClH20), osmolar clearance, urine volume, 24 hour osmolality, urine and serum osmolality, and plasma vasopressin in control subjects and patients with cirrhosis stratified according to their Child-Pugh score
| Controls | Child A | Child B | Child C | |
| AQP2 (U/24 h) | 0.021 (0.017) | 0.040 (0.039) | 0.092 (0.160) | 0.315 (0.348)† |
| ClH20 (ml/min) | −1.3 (0.56) | 0.19 (0.24)††† | −0.17 (0.37)*††† | −0.38 (0.39)*††† |
| U-volume (l/24 h) | 1.8 (0.7) | 2.0 (0.6) | 1.8 (1.2) | 1.6 (0.8) |
| Closm (mmol/min) | 3.8 (0.7) | 1.7 (0.8)††† | 2.0 (1.3)†† | 2.2 (1.0)†† |
| 24 h osmol (mosmol/24 h) | 1046 (190) | 478 (244)††† | 559 (369)††† | 572 (264)††† |
| U-osmolality (mosmol/l) | 643 (220) | 239 (50)††† | 335 (86)*† | 395 (154)*†† |
| S-osmolality (mosmol/l) | 282 (11) | 281 (7) | 280 (10) | 266 (20) |
| P-vasopressin (pg/ml)§ | — | 0.52 (0.80) | 0.71 (0.51) | 0.64 (0.48) |
Data are mean (SD).
*p<0.05 Child Pugh class C or B versus A.
†p<0.05, ††p<0.01, †††p<0.005 versus controls.
§Child Pugh class A, n=1; class B, n=10; class C, n=9.
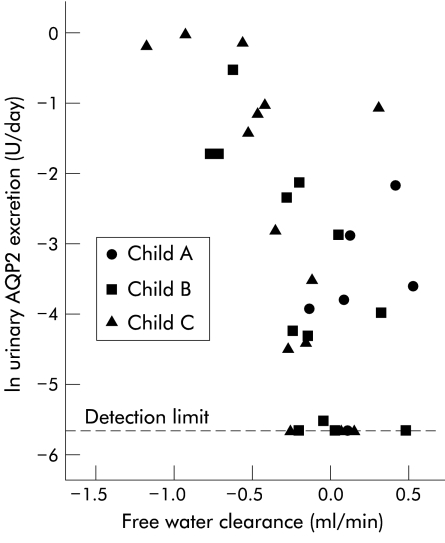
Spontaneous free water clearance was positive in class A patients and decreased and became negative with higher Child-Pugh class (table 2 ▶). There was a significant relation between spontaneous free water clearance and AQP2 excretion in cirrhotic patients (rho=−0.57, p<0.01) (fig 3 ▶).
Figure 3.
Relation between 24 hour spontaneous free water clearance (ml/min) and 24 hour urinary excretion of aquaporin 2 (U/day) in cirrhosis patients (rho=−0.57, p<0.01). Both were calculated for the same 24 hour period, and the abscissa is given as natural log.
Urine osmolality and 24 hour urinary osmolyte excretion was lower in patients with cirrhosis compared with controls (cirrhosis: 549 (303) mosmol/day; controls: 1046 (190) mosmol/day; p<0.001) Osmolar clearance was decreased in all three Child-Pugh classes but there was no relation to urinary AQP2 excretion. There was no difference in Sosm and urinary output between patients and controls. AQP2 excretion was not related to either creatinine clearance or morning vasopressin concentrations.
None of the patients without oesophagogastric varices or portal hypertensive gastropathy had increased urinary AQP2 excretion (no varices: 0.04 (0.04) U/day; with varices: 0.19 (0.26) U/day; p=0.08). In Child-Pugh class C patients, AQP2 excretion was significantly elevated in the presence of varices (Child C: no varices 0.02 (0.03); with varices 0.33 (0.32); p=0.04). Presence or absence of ascites was not related to AQP2 excretion.
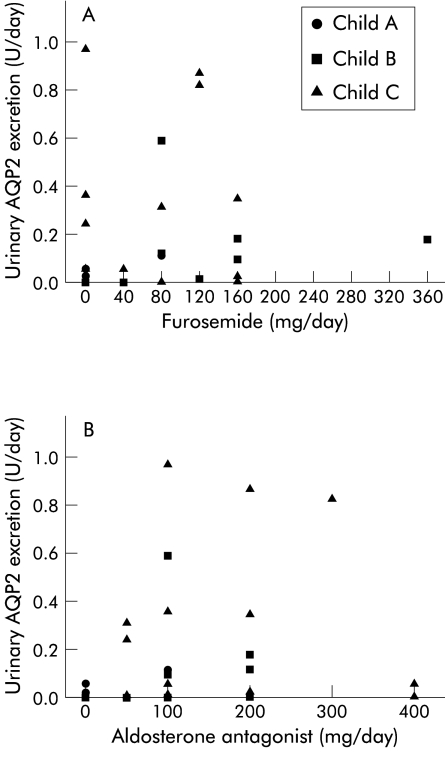
There was no relation between the dose or type of diuretic (furosemide or spironolactone) and urinary AQP2 excretion (fig 4 ▶).
Figure 4.
Daily dose of (A) furosemide and (B) aldosterone antagonist and 24 hour urinary excretion of aquaporin 2 (AQP2) in cirrhosis patients. No relation was found between dose or type of diuretic and AQP2 excretion.
DISCUSSION
We found markedly increased urine AQP2 excretion in patients with liver cirrhosis. The highest values were in those patients with advanced disease (Child-Pugh C patients). Furthermore, we found an inverse relation between urine AQP2 excretion and spontaneous free water clearance.
Urine AQP2 excretion is increased in cirrhosis
This is the first report to describe AQP2 excretion in human cirrhosis. A recent rat cirrhosis study11 showed increased cellular trafficking of AQP2, which would be expected to increase urinary AQP2, in accordance with the present results.10 Age and sex match to controls was not optimal but AQP2 excretion seems to be unaffected by age and sex.5,18 AQP2 was below the assay detection limit in eight patients (one class A, four class B, and three class C) and in two controls. These subjects did not exhibit any clinical or biochemical peculiarities. In the two controls a second 24 hour urine collection showed a very low AQP2 value, just above the detection limit (0.0046 and 0.0064 U/day). This and similar experience from our previous use of the same assay leads us to believe that the values below the detection limit represent a biological phenomenon rather than an assay failure. More sensitive assays are awaited.
A recent study reported a relation between excretion of AQP2 and plasma vasopressin concentration during hypertonic changes in healthy subjects19; thus AQP2 excretion might reflect the action of vasopressin on the collecting duct. We found no relation between AQP2 excretion and morning vasopressin. A single concentration of vasopressin however does not adequately reflect 24 hour vasopressin levels in quantitative terms as vasopressin has a circadian rhythm, even in cirrhosis.20 Furthermore, AQP2 excretion and free water clearance also depend on several other factors (for example, prostaglandins). In rats, cyclooxygenase inhibitors increase AQP2 trafficking to the plasma membrane, increasing the collecting duct water permeability.21,22 This may be related to the known untoward effect of cyclooxygenase inhibitory drugs on kidney function in human cirrhosis,23 although the effect of such inhibition on AQP2 excretion is not known.
Our patients were maintained on their usual treatment, including diuretics. In some cirrhosis patients the natriuretic response to both furosemide and aldosterone antagonists is dependent on prostaglandins.24–26 Rat studies have not shown any relation between AQP2 excretion and short or long term treatment with diuretics.27,28 Correspondingly, stratification of our data according to treatment with furosemide or spironolactone did not result in differences in AQP2 excretion (fig 4 ▶).
Our findings were not related to functional kidney impairment as AQP2 excretion was not related to creatinine clearance. The increase in urinary AQP2 excretion was also not related to functional liver mass as there was no relation to GEC. Excretion was also not related to the clinical presence of ascites. This may be due in part to difficulties in estimating ascites, and to the fact that nearly all patients were treated with diuretics. In contrast, only patients with varices or gastropathy had increased excretion. Also, this secondary finding is of uncertain significance but if true may indicate a relation to the degree of portal-systemic shunting.
Functional association between urine AQP2 and free water clearance
We studied 24 hour spontaneous free water clearance in stable patients. The ability to excrete free water depends both on the load of solutes to the distal tubule and on reabsorption of water in the collecting duct (that is, its permeability) which again depends on the amount of AQP2 on the luminal side. As also reported in earlier studies, we found that spontaneous free water clearance was positive in Child Pugh class A in contrast with the negative value in healthy controls.23,29 This reflects the larger osmolyte load to the kidneys of controls. The level found in Child Pugh class B/C was in the same range as reported by Guyader and colleagues.30 As urine volume changed little among classes, the fall in free water clearance with increasing Child Pugh classes mostly reflects increased solute excretion. Thus a more negative value indicates compromised water elimination, balanced by increased osmolyte excretion, to some extent related to increasing use of diuretics in class B and C patients. One difference between our study and those quoted is that our patients were maintained on their diuretic treatment. The reason for doing so was that we wished to study our patients in the clinical setting and also because withdrawal of diuretics would probably cause acute water dyshomeostasis during which unforeseeable changes in AQP2 might occur. We presume that this did not change our results as the diuretics used depend on their ability to excrete solutes and would not be expected to appreciably influence free water clearance.
In both healthy and diseased subjects, solute and AQP2 excretion may be dissociated and osmolyte excretion not related to urinary AQP2 excretion.6,19 In our study, the reasonably good relationship between the ability to excrete water and excretion of AQP2 indicates a physiological role of aquaporins for water balance of patients with cirrhosis.
Dehydration decreases fractional urinary AQP2 excretion.18,31 Extrapolated to our finding of increased excretion this would imply that patients were overhydrated from the point of view of collecting ducts.
The mechanism of excretion of AQP2 into urine remains unknown. Vasopressin analogues increase the release of AQP219,32 while the effects of a hypertonic challenge are conflicting.6,19 Hypertonicity of the renal tubular fluid has been hypothesised to increase excretion of AQP2 although this did not in fact occur during rapid osmotic changes.19
The inverse relation between AQP2 excretion and free water clearance supports the notion that increased AQP2 trafficking may play a role in the regulation of water balance in liver cirrhosis patients. Recently, it was demonstrated that a non-peptide selective V2 vasopressin receptor antagonist decreased urinary aquaporin excretion, correlated to an increase in solute free water clearance.33 The same drug has been shown to increase free water clearance in decompensated cirrhotic patients.30,34 In summary, we interpret the data to indicate that increased water permeability in the collecting duct is involved in impairment of water excretion in human cirrhosis.
In conclusion, we showed that cirrhosis patients had increased urinary excretion of AQP2 compared with healthy controls and that excretion was augmented with the clinical severity of the disease. There was an inverse relation between urinary AQP2 excretion and free water clearance but no relation to vasopressin. Thus the data suggest that AQP2 may be involved in water retention of cirrhosis patients but the mechanism remains unclear and it is still not known whether increased AQP2 excretion in urine is a primary or secondary phenomenon.
Acknowledgments
The authors thank Gitte Kall for expert technical assistance. The study was supported by the Danish National Research Foundation (Danmarks Grundforskningsfond), and the Danish Medical Research Council.
Abbreviations
AQP2, aquaporin 2
GEC, galactose elimination capacity
ClH2O, free water clearance
TBS, Tris buffered saline
T-TBS, TBS buffer and Tween
V, urine volume
Uosm, urine osmolality
Sosm
serum osmolality
REFERENCES
- 1.Gines P, Berl T, Bernardi M, et al. Hyponatremia in cirrhosis: from pathogenesis to treatment. Hepatology 1998;28:851–64. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen S, Frokiaer J, Marples D, et al. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 2002;82:205–44. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen S, Chou CL, Marples D, et al. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci U S A 1995;92:1013–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanno K, Sasaki S, Hirata Y, et al. Urinary excretion of aquaporin-2 in patients with diabetes insipidus. N Engl J Med 1995;332:1540–5. [DOI] [PubMed] [Google Scholar]
- 5.Elliot S, Goldsmith P, Knepper M, et al. Urinary excretion of aquaporin-2 in humans: A potential marker of collecting duct responsiveness to vasopressin. J Am Soc Nephrol 1996;7:403–9. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen RS, Bentzen H, Bech JN, et al. Effect of water deprivation and hypertonic saline infusion on urinary AQP2 excretion in healthy humans. Am J Physiol Renal Physiol 2001;280:F860–7. [DOI] [PubMed] [Google Scholar]
- 7.Fujita N, Ishikawa SE, Sasaki S, et al. Role of water channel AQP-CD in water retention in SIADH and cirrhotic rats. Am J Physiol 1995;269:F926–31. [DOI] [PubMed] [Google Scholar]
- 8.Asahina Y, Izumi N, Enomoto N, et al. Increased gene expression of water channel in cirrhotic rat kidneys. Hepatology 1995;21:169–73. [PubMed] [Google Scholar]
- 9.Fernandez-Llama P., Turner R, et al. Renal expression of aquaporins in liver cirrhosis induced by chronic common bile duct ligation in rats. J Am Soc Nephrol 1999;10:1950–7. [DOI] [PubMed] [Google Scholar]
- 10.Jonassen TE, Nielsen S, Christensen S, et al. Decreased vasopressin-mediated renal water reabsorption in rats with compensated liver cirrhosis. Am J Physiol 1998;275:F216–25. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Llama P, Jimenez W, et al. Dysregulation of renal aquaporins and Na-Cl cotransporter in CCl4-induced cirrhosis. Kidney Int 2000;58:216–28. [DOI] [PubMed] [Google Scholar]
- 12.Kishore BK, Mandon B, Oza NB, et al. Rat renal arcade segment expresses vasopressin-regulated water channel and vasopressin V2 receptor. J Clin Invest 1996;97:2763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pugh RN, Murray-Lyon IM, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646–9. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki S, Fushimi K, Saito H, et al. Cloning, characterization, and chromosomal mapping of human aquaporin of collecting duct. J Clin Invest 1994;93:1250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bie P, Sandgaard NC. Determinants of the natriuresis after acute, slow sodium loading in conscious dogs. Am J Physiol Regul Integr Comp Physiol 2000;278:R1–10. [DOI] [PubMed] [Google Scholar]
- 16.Emmeluth C, Drummer C, Gerzer R, et al. Natriuresis in conscious dogs caused by increased carotid [Na+] during angiotensin II and aldosterone blockade. Acta Physiol Scand 1994;151:403–11. [DOI] [PubMed] [Google Scholar]
- 17.Tygstrup N. Determination of the hepatic elimination capacity (Lm) of galactose by single injection. Scand J Clin Lab Invest Suppl 1966;18:118–25. [PubMed] [Google Scholar]
- 18.Rai T, Sekine K, Kanno K, et al. Urinary excretion of aquaporin-2 water channel protein in human and rat. J Am Soc Nephrol 1997;8:1357–62. [DOI] [PubMed] [Google Scholar]
- 19.Baumgarten R, van de Pol MH, Deen PM, et al. Dissociation between urine osmolality and urinary excretion of aquaporin-2 in healthy volunteers. Nephrol Dial Transplant 2000;15:1155–61. [DOI] [PubMed] [Google Scholar]
- 20.Pasqualetti P, Festuccia V, Collacciani A, et al. Circadian rhythm of arginine vasopressin in hepatorenal syndrome. Nephron 1998;78:33–7. [DOI] [PubMed] [Google Scholar]
- 21.Ecelbarger CA, Fernandez-Llama P, Lee AJ, et al. Enhancement of urinary concentration ability by cyclooxygenase inhibitors is associated with increased expression of Na-K-2Cl cotransporter and increased trafficking of aquaproin-2 water channel. J Am Soc Nephrol 1997;8: 413A. [Google Scholar]
- 22.Zelenina M, Christensen BM, Palmer J, et al. Prostaglandin E(2) interaction with AVP: effects on AQP2 phosphorylation and distribution. Am J Physiol Renal Physiol 2000;278:F388–94. [DOI] [PubMed] [Google Scholar]
- 23.Jespersen B, Pedersen EB, Madsen M, et al. Disturbed relationship between urinary prostaglandin E2 excretion, plasma arginine vasopressin and renal water excretion after oral water loading in early hepatic cirrhosis. Eur J Clin Invest 1988;18:202–6. [DOI] [PubMed] [Google Scholar]
- 24.Mirouze D, Zipser RD, Reynolds TB. Effect of inhibitors of prostaglandin synthesis on induced diuresis in cirrhosis. Hepatology 1983;3:50–5. [DOI] [PubMed] [Google Scholar]
- 25.Pinzani M, Laffi G, Meacci E, et al. Intrarenal thromboxane A2 generation reduces the furosemide-induced sodium and water diuresis in cirrhosis with ascites. Gastroenterology 1988;95:1081–7. [DOI] [PubMed] [Google Scholar]
- 26.Planas R, Arroyo V, Rimola A, et al. Acetylsalicylic acid suppresses the renal hemodynamic effect and reduces the diuretic action of furosemide in cirrhosis with ascites. Gastroenterology 1983;84:247–52. [PubMed] [Google Scholar]
- 27.Marples D, Christensen BM, Frokiaer J, et al. Dehydration reverses vasopressin antagonist-induced diuresis and aquaporin-2 downregulation in rats. Am J Physiol 1998;275:F400–9. [DOI] [PubMed] [Google Scholar]
- 28.Marples D, Frokiaer J, Dorup J, et al. Hypokalemia-induced downregulation of aquaporin-2 water channel expression in rat kidney medulla and cortex. J Clin Invest 1996;97:1960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madsen M, Pedersen EB, Danielsen H, et al. Impaired renal water excretion in early hepatic cirrhosis. Lack of relationship between renal water excretion and plasma levels of arginine vasopressin, angiotensin II, and aldosterone after water loading. Scand J Gastroenterol 1986;21:749–55. [DOI] [PubMed] [Google Scholar]
- 30.Guyader D, Patat A, Ellis-Grosse EJ, et al. Pharmacodynamic effect of a nonpeptide antidiuretic hormone V2 antagonist in cirrhotic patients with ascites. Hepatology 2002;36:1197–205. [DOI] [PubMed] [Google Scholar]
- 31.Wen H, Frokiaer J, Kwon TH, et al. Urinary excretion of aquaporin-2 in rat is mediated by a vasopressin-dependent apical pathway. J Am Soc Nephrol 1999;10:1416–29. [DOI] [PubMed] [Google Scholar]
- 32.Saito T, Ishikawa SE, Sasaki S, et al. Urinary excretion of aquaporin-2 in the diagnosis of central diabetes insipidus. J Clin Endocrinol Metab 1997;82:1823–7. [DOI] [PubMed] [Google Scholar]
- 33.Martin Py, Abraham WT, Lieming X, et al. Selective V2-receptor vasopressin antagonism decreases urinary aquaporin-2 excretion in patients with chronic heart failure. J Am Soc Nephrol 1999;10:2165–70. [DOI] [PubMed] [Google Scholar]
- 34.Wong F, Blei AT, Blendis LM, et al. A vasopressin receptor antagonist (VPA-985) improves serum sodium concentration in patients with hyponatremia: a multicenter, randomized palcebo-controlled trial. Hepatology 2003;37:182–19. [DOI] [PubMed] [Google Scholar]