Endoscopic ultrasonography (EUS), introduced into gastroenterological diagnostics more than 20 years ago, has undergone extensive evaluation of its diagnostic capability, probably to a larger extent than most other endoscopic and other imaging techniques in gastroenterology. Almost necessarily, as to be expected with an imaging method being assessed with continuing interest for two decades, initial enthusiasm has waned and questions about its influence on management and outcome have been dealt with, yielding mixed results. Other imaging techniques are usually not burdened by such a self critical approach, mostly due to the fact that technical progress has hindered proper evaluation, and techniques are marketed with only little good evidence of their real value. Methodological questions about study quality in gastrointestinal imaging have attracted limited interest, and only rarely are factors looked into, which may be responsible for divergent study results.1 However, it happened a few years ago that EUS was revitalised, mainly due to the advent of EUS guided fine needle aspiration (FNA),2 and even more recently, several emerging techniques of EUS guided therapy.3 The following does not aim at giving a full overview on the ever rising body of literature on the accuracy of diagnostic EUS, including FNA. For this purpose textbooks and review articles in various journals2,4 are recommended. Some current trends and possible future tendencies will be outlined.
TECHNICAL FACTORS
Echoendoscopes—with some recent exceptions—are oblique viewing endoscopes which carry a rigid ultrasound transducer at their tip, which either generates a 360° round view perpendicular to the shaft axis or a linear image of variable width parallel to the endoscope axis. Radial scanners have been mechanical scanners but recently electronic scanning—the principle of linear scanners—is being developed for radial scanning also. EUS utilises high ultrasound frequencies (5–20 MHz, 7.5 MHz being the most frequently used ultrasound frequency) which generate a high resolution image in the near field with limited penetration depth, ranging from 1–2 to 5–6 cm, depending of the ultrasound frequency used. EUS is usually done with the patient in the left lateral position, mostly under conscious sedation, and is associated with very low complication rates5 with very few exceptions.6 Details of the examination technique for the various organs in focus—gastrointestinal tract and immediate surroundings, mainly the pancreatobiliary tract—are described elsewhere.7,8 Miniprobes are a further development which mirror the miniaturisation of the technique (they are referred to below).
Linear echoendoscopes are necessary for the performance of EUS guided FNA as only with these instruments can the course of the puncture needle be followed. An average of 2–4 passes is necessary to obtain adequate tissue for cytological smears, and the presence of an in- room cytopathologist seems to improve the yield5; some examiners try to obtain small core specimens for histopathological analysis but the relative yield and accuracy of cytological and histological analysis, as well as the best needle diameter (19 or 22 gauge), are still unclear.
ENDOSONOGRAPHIC IMAGING: TUMOUR STAGING STILL THE MAIN INDICATION?
After primary diagnosis of gastrointestinal malignancies by endoscopy and biopsy, EUS is used secondarily for locoregional tumour staging; this applies to oesophageal, gastric, and rectal cancer as well as to gastric lymphoma9–11 (figs 1 ▶, 2 ▶); the value of EUS in suprarectal colonic cancer has not yet been established. The use of EUS is limited to patients in whom surgery is considered, either primarily or after neoadjuvant therapy, as EUS is not believed to be useful in inoperable patients or in those with known unresectable disease or distant metastases. The value of EUS in restaging after such neoadjuvant treatment has been burdened with poor results12 but more elaborate assessment methods such as two dimensional13 or even three dimensional volume measurement may fare better. In gastric lymphoma, EUS is clinically useful in selecting patients for Helocobactor pylori eradication treatment,14,15 and restaging after chemotherapy seems to be more successful than in upper gastrointestinal cancer16
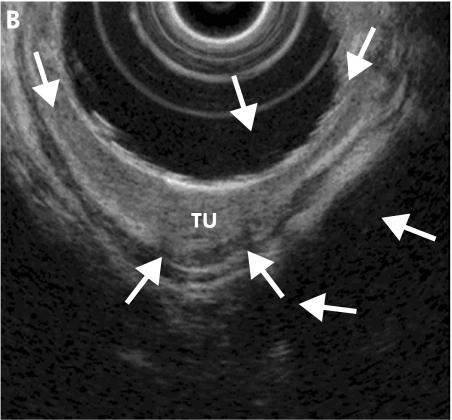
Figure 1.
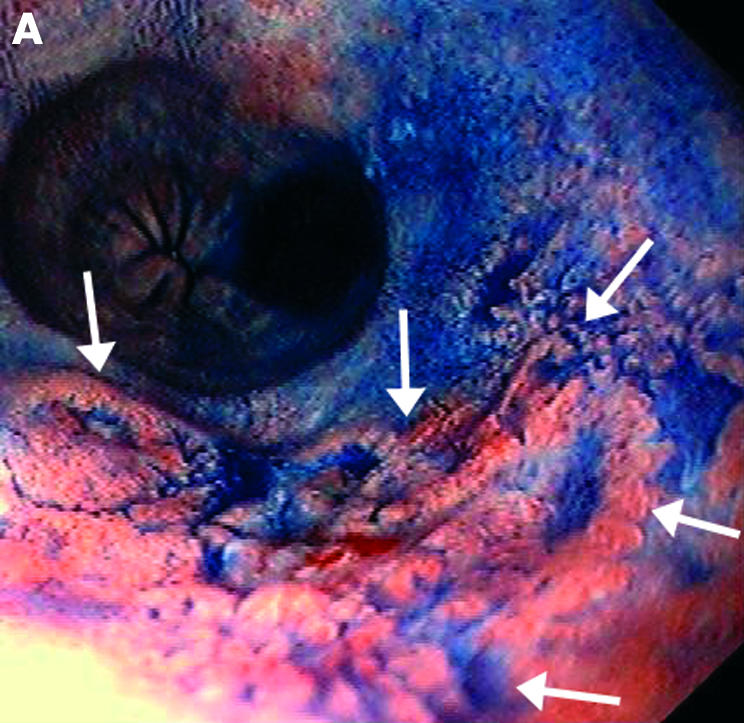
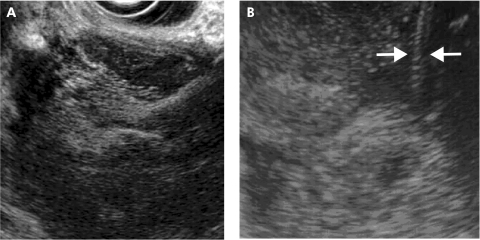
Barrett cancer in stage T1sm; endoscopic view of a large flat tumour with the margins indicated by arrows (A). Endoscopic ultrasonography shows a focal echo poor thickening (TU) of the mucosa with thinned submucosal layer (arrows) due to tumour infiltration (B).
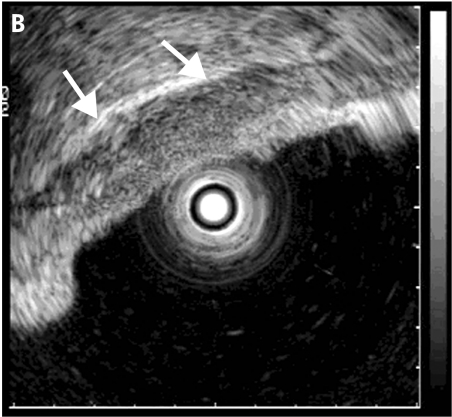
Figure 2.
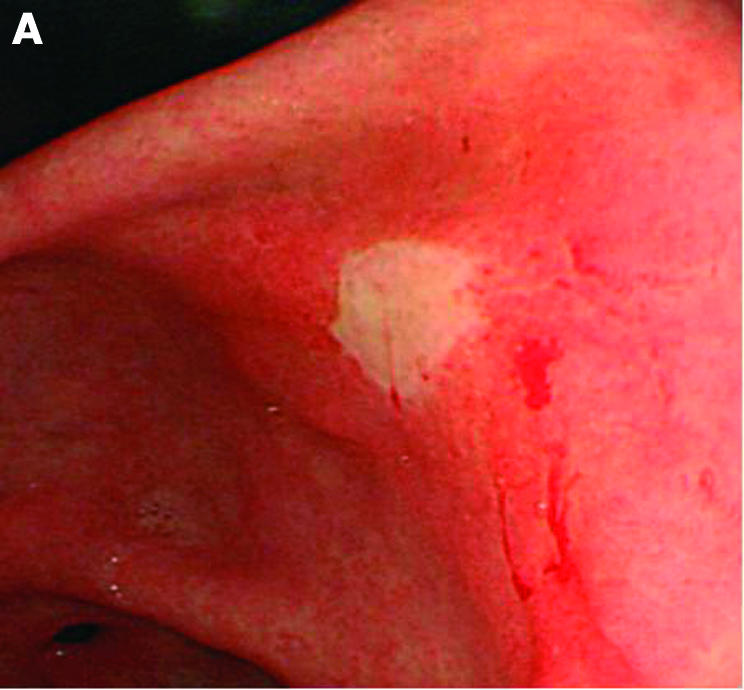
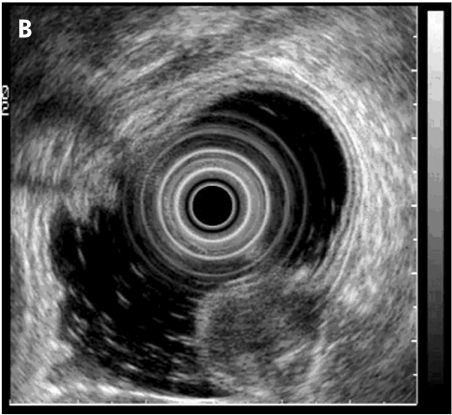
Malignant gastric ulcer (A: endoscopic view). Endoscopic ultrasonography using a miniprobe shows an echo poor wall thickening around the ulcer with smooth outer margins (arrows) (B) indicating stage T2.
In the pancreatobiliary tract the situation is less clear. EUS was repeatedly reported to be the most accurate method for diagnosing small cancers17 but this was flawed by study designs with a very high disease prevalence, and has not consistently been confirmed by other studies.18 The value of EUS in “screening” for pancreatic tumours in patients with only a vague suspicion is therefore not established. As with all other imaging tests including, most recently, positron emission tomography (PET),19 EUS is not useful for differentiating focal chronic pancreatitis from cancer,20 and its accuracy in locoregional staging is seen both enthusiastically21–23 as well as more sceptically.24 For pancreatic and biliary cancer staging (fig 3 ▶), helical computed tomography (CT) is probably at present the method of choice, merely due to its widespread existence—comparative studies between EUS and helical CT revealing greatly divergent results25–27—and EUS might be used as a secondline test in case of uncertainty on CT, or for additional information (FNA) or treatment (plexus neurolysis); these possibilities are discussed below.
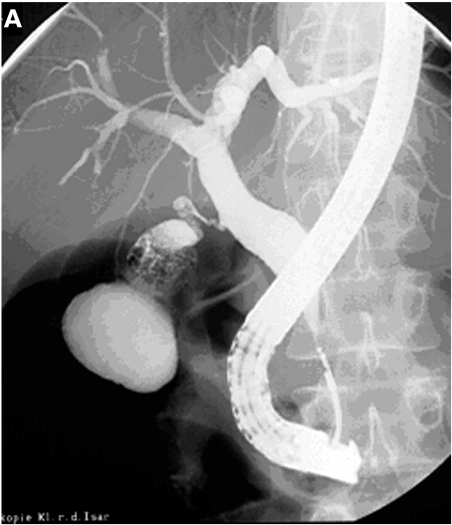
Figure 3.
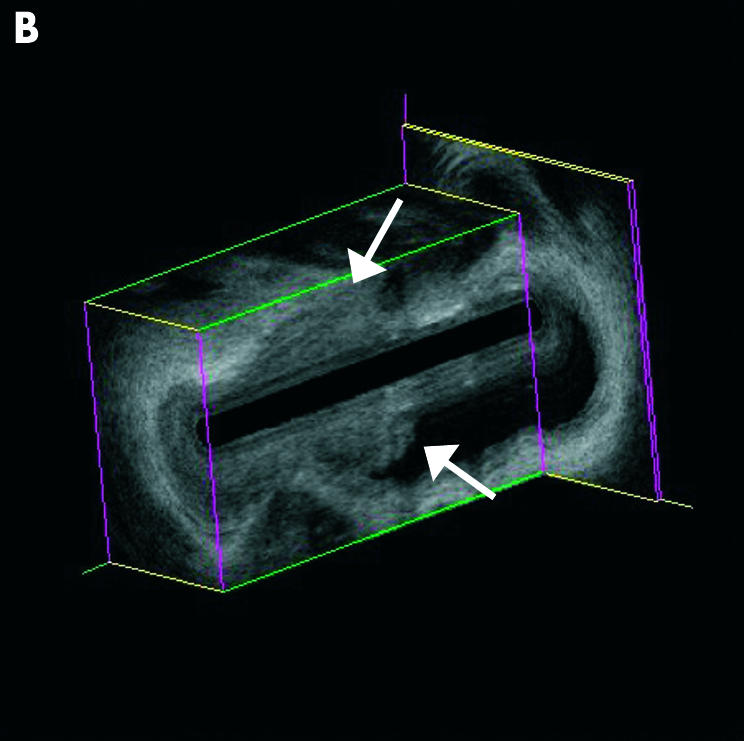
Distal periampullary cancer infiltrating the distal common bile duct (CBD). (A) Endoscopic retrograde cholangiopancreatography image with a 3 cm distal tumour stricture (arrow) and the miniprobe being introduced. (B) Three dimensional reconstruction of the miniprobe picture showing the proximal tumour end (arrows); the full tumour delineation in relation to the pancreatic head is seen on the conventional endoscopic ultrasonography image in (C); the arrows delineate the wall thickening extending from the area of the papilla/duodenal wall up to the CBD.
In summary, in the year 2003, EUS is still the standard in locoregional staging of oesophagogastric and rectal cancer; the situation in pancreatic cancer being less clear, and EUS may be used as a secondary step in cases with indeterminate CT and/or for FNA or treatment in pancreatic tumours. Recent studies showing less impressive results for EUS in gastrointestinal and pancreatic cancer staging24,28,29 have to be viewed in the perspective of a routine test usually doing less well under routine circumstances—a fact that is mostly not assessed with other imaging methods but probably applies to them all.
ENDOSONOGRAPHIC IMAGING: USE IN PRIMARY DIAGNOSIS
In the diagnosis of gastrointestinal disorders, EUS plays a substantial role in some areas but has yielded poor results in the differential diagnosis between benign and malignant conditions such as indeterminate ulcers, oesophageal strictures, and pancreatic tumours (both solid and cystic).
In submucosal lesions, EUS is crucial for distinguishing intramural tumours/lesions from extramural compressions30,31 (fig 4 ▶), and in the latter case management is quite different (most impressions are due to normal organs). EUS is also the most important tool to assess the most likely tumour nature and the risk of malignancy30; again a reliable histological diagnosis cannot be expected. The diagnosis of common bile duct stones is another good indication for EUS, as confirmed by a large number of fairly homogeneous studies from all over the world,32–35 yielding accuracy rates of well over 90%. Direct comparisons with magnetic resonance cholangiopancreatography (MRCP) showed EUS to be superior or equal.36 EUS could therefore be used in patients with low or intermediate risk for common bile duct stones; a negative EUS examination has a very high negative predictive value.37 The diagnosis of pancreatic endocrine tumours is another good indication for EUS, and other tests have repeatedly shown to be inferior to EUS.38
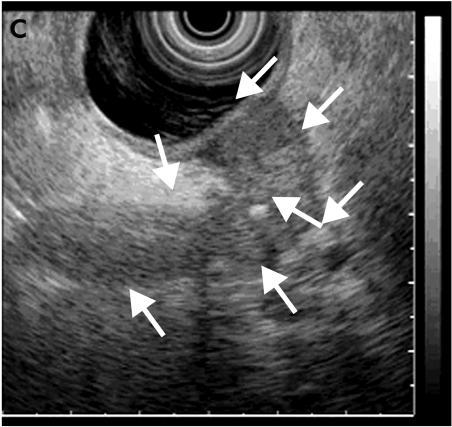
Figure 4.
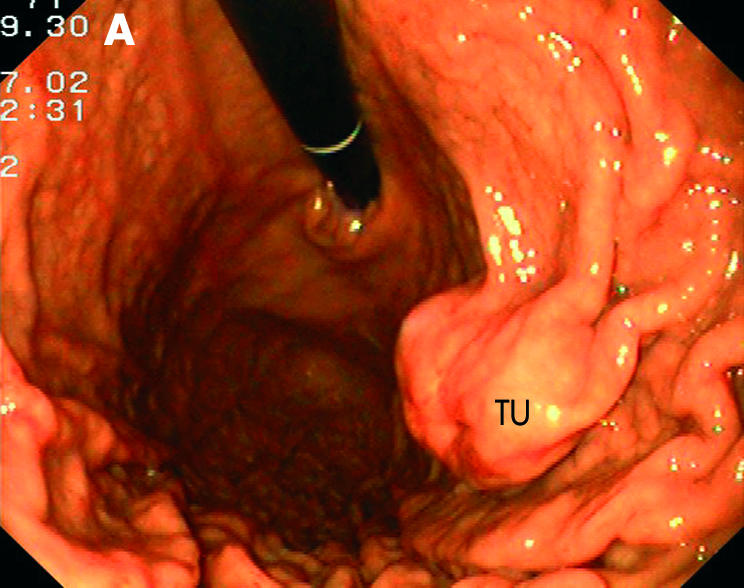
Submucosal tumour (TU) in the gastric body with endoscopic (A) and endosonographic (B) views.
Due to its good accuracy in detecting common bile duct dilatation, common bile duct stones, pancreatic tumours and, although disputed, chronic pancreatitis,39 EUS has been suggested as a primary tool in patients with a clinical suspicion of pancreatobiliary disease. However, data are mainly from tertiary referral centres which see preselected patients and some had a rather high rate of chronic pancreatitis cases.40 Large and good outcome studies in patient populations with a low disease prevalence are still lacking. The use of the echoendoscope as an endoscope for upper gastrointestinal tract endoscopic screening, including an endosonographic view of the pancreas, is intriguing but has to be assessed properly. First, data on patients with dyspepsia are appearing.41
In other areas such as portal hypertension, achalasia, and inflammatory bowel disease, the clinical value of EUS has been less well established. Good results have been described for rectal and anal EUS in detecting fistulas, abscesses, and anal sphincter defects in incontinence.42,43
DIAGNOSTIC EUS WITH MINIPROBES
Simplification of EUS by using small probes (MP, miniprobes) introduced through the working channel of conventional gastroscopes, duodenoscopes, and colonoscopes for use in the gastrointestinal and pancreatobiliary tract has been welcomed but results of these probes have been ambiguous. Good staging results have been shown by one group for oesophageal cancer44 but others have limited MP use to small and flat gastrointestinal lesions, again with varying accuracy rates45–48 (see fig 2 ▶). The advantage of miniprobes over conventional echoendoscopes is their precise placement onto a lesion which is otherwise difficult to localise by conventional EUS. The disadvantage of the gastrointestinal use of MP is that water filling is necessary (although there are balloon types now available) which gives rise to some aspiration risk, at least in the oesophagus.
By and large, MP are mainly used in clinical routine (if available) for staging early cancer prior to planned endoscopic resection using mucosal resection (EMR) techniques. Results in the staging of these early cancers, differentiating between mucosal and submucosal invasion, have however been somewhat variable and not consistently over 70–75%.49 It is therefore still a matter of debate whether pre-EMR EUS is indispensable or whether the endoscopic impression together with the histopathological examination of the resection specimen is sufficient. According to this approach, EMR starts out as diagnostic EMR and can be regarded as therapeutic only after histopathological review of the resected specimen.
Intrabiliary use of MP has been published in a fair number of studies,50–52 but their real value in the diagnosis of biliary strictures and staging of biliary tumours is still limited in clinical practice due to limited durability, costs, expertise, and uncertain accuracy.
DIAGNOSTIC EUS AND OUTCOME
A growing body of evidence deals with the impact of EUS on outcome and management,53–57 although it might be difficult to ascribe outcome in complex situations such as gastroenterological tumours to one single imaging test. EUS prediction of advanced tumours has been linked to very poor prognosis in oesophageal and pancreatic cancer.58–63 In submucosal tumours, EUS saves a large number of other tests,64 with better accuracy, and may save money, but not consistently, depending on the medical reimbursement system.65 Calculations of cost effectiveness in cancer staging,66–71 submucosal tumours,72 and biliary pancreatitis73 have mostly revealed encouraging results but not all of these outcome studies showed huge differences induced by EUS; this however is not to be expected from a merely diagnostic tests, and it has to be mentioned that outcome studies are usually avoided in the field of research dealing with gastrointestinal imaging (endoscopy, radiology, nuclear medicine). A broader application of EUS in patients with abdominal pain/dyspepsia has therefore to be evaluated further.
Application of EUS (echoendoscopes or high frequency MP) prior to endoscopic resection of gastrointestinal tumours has been advocated for early cancer (see above) as well as for submucosal tumours74–76; in submucosal tumours however, endoscopic resection techniques have not yet gained widespread acceptance. In the latter case, EUS would be useful to delineate the layer of origin of the lesion. On the other hand, transabdominal ultrasound may do almost as well in the follow up of endosonographically diagnosed submucosal gastric tumours.77 EUS has not been shown to be useful in predicting post- polypectomy bleeding in the colon.78
Prior to transluminal endoscopic drainage of pancreatic pseudocysts, EUS was shown to change management in almost 40% of cases.79 EUS can diagnose intervening vessels, possibly preventing the blind transluminal approach, and it can guide the way to the best approach and non-bulging cysts. It is however not backed up by study data, whether in pseudocysts with clear bulging, the endoscopic drainage attempt should only be performed after EUS. It is nevertheless only logical that nowadays cyst drainage can be performed under direct EUS guidance (see below)
ENDOSONOGRAPHIC TISSUE ACQUISITION
The addition of guided needle aspiration has clearly widened the spectrum of diagnostic EUS (fig 5 ▶). Generally, specificity is close to 100% in all indications but the diagnostic sensitivity somewhat depends on the indications5,80,81: the highest sensitivity (80–90%) is achieved in mediastinal tumours and lymph node metastases82–84 as well as paramural lymph nodes somewhere else, mostly around the coeliac trunk.85 In this area, FNA results may replace more invasive tests such as mediastinoscopy and they have some important impact on outcome—for example, when proving distant metastases in oesophageal cancer and advanced malignancy with contralateral lymph nodes in non-small cell lung cancer. Pancreatic malignancy can be proved with a lower sensitivity, between 70% and 85%, and the influence on outcome is less clear. In irresectable tumours, EUS-FNA is necessary when radiochemotherapy regimens are applied, and can be performed in one step with staging and perhaps coeliac plexus blockade in case of severe pain. In resectable tumours, most would go straight to surgery and a negative FNA result would not change this approach; the minority opinion relies on the fact that resectable tumours may be of different histology than adenocarcinoma and then treated by limited surgery.86 In pancreatic cystic lesions, the situation is less clear, and sensitivity of EUS imaging can be improved by FNA results, using cytology and tumour markers, but specificity may be negatively affected.87,88 The accuracy in puncturing submucosal tumours has initially been shown to be low but some (not all) recent papers have shown better results, also involving antibodies to diagnose gastrointestinal stroma cell tumours.89 In the application of FNA in cystic lesions, infection seems to be a risk5 but may have been overestimated.88
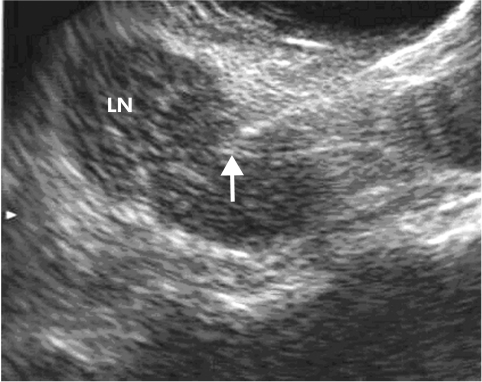
Figure 5.
Endosonographic puncture of a large lymph node (LN) in the mediastinum; the needle tip is marked by an arrow.
Other indications are less frequent and include FNA of liver lesions,90 ascites,91 and visible adrenal lesions,92 without clear evidence of clinical impact.
TRAINING AND COMPETENCE
EUS is commonly regarded as the most difficult diagnostic technique in gastrointestinal endoscopy (although high quality diagnostic endoscopic retrograde cholangiopancreatography (ERCP) is by no means easier). Data on acquisition of competence are nevertheless sparse. The respective American and European societies recommend EUS numbers between 50 (oesophageal) and 200 (pancreatobiliary)93,94 with actual data usually showing somewhat higher numbers to be necessary.95,96 Training courses are held throughout the world, ranging from live demonstrations during larger meetings, small tutorials with the presence of trainees during examinations, and hands on training in biomodels including FNA.97 More data however are clearly needed on which to base reliable figures to decide on numbers needed to confirm competence in EUS and FNA.
ENDOSONOGRAPHIC THERAPY: CURRENT POSSIBILITIES AND FUTURE AREAS OF RESEARCH
A variety of therapeutic possibilities have either been partially explored or are evolving, with some animal data presented. Transmural drainage of pancreatic and peripancreatic fluid collections under direct EUS guidance is one of the most logical applications of therapeutic use, and in a recent series of 35 cases, 20 of whom had infected cysts/absecces, a 89% initial success rate with three recurrences was reported. Notably, almost all lesions (n=32) did not cause any bulging and would not have been amenable by conventional endoscopic drainage.98 EUS may open the way to more aggressive therapy, such as direct endoscopic removal of pancreatic necroses99 (fig 6 ▶). EUS guided coeliac plexus blockade has also been reported in a variety of studies on pancreatic cancer and chronic pancreatitis pain, with better results in cancer (78%)100 than in chronic pancreatitis (55%)101; a small randomised study showed the EUS guided technique to be superior to the CT technique.102 Other indications such as EUS guided botulinum toxin injection, injection treatment for tumours, suprapapillary bile duct drainage, and transgastric approach to the left biliary system have been reported in case reports whereas other techniques such as creation of gastroenterostomy and antireflux techniques are still in the experimental stage.2,103 Nevertheless, some indications will remain and some new ones will evolve which will turn out to be clinically useful.
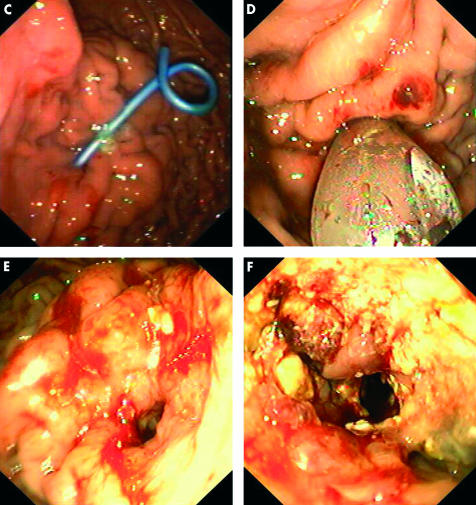
Figure 6.
Endosonographic access to an infected and necrotic pancreatic pseudocyst, opening the way to further endoscopic treatment (necrosectomy). After endosonographic visualisation of the cyst containing pus and necrotic material, as seen by the echo rich material in the large cyst (A), a catheter (arrows) is introduced after endoscopic ultrasonography guided puncture over a guidewire under endosonographic (B) and radiological control, followed by stenting (C). A few days later, access is dilatated with a large balloon (D), creating a large orifice (E) for introduction of a conventional gastroscope (F) and performance of endoscopic necrosectomy; this view is taken through the gastroscope which is introduced through the large orifice transgastrally into the retroperitoneal space with necrotic material.
CONCLUSION
EUS has been used for imaging with clinical impact in gastrointestinal tumour staging and the diagnosis of submucosal tumours and common bile duct stones, based on a large number of prospective controlled studies. In diagnosis and staging of pancreatic lesions, EUS should be used in conjunction with other methods, and most probably after an adequate helical CT. Other potential indications such as search for pancreatobiliary disease (mainly benign) are not yet fully established and require further studies.
REFERENCES
- 1.Meining A, Dittler HJ, Wolf A, et al. You get what you expect? A critical appraisal of imaging methodology in endosonographic cancer staging. Gut 2002;50:599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fusaroli P, Caletti G. Endoscopic ultrasonography. Endoscopy 2003;35:127–35. [DOI] [PubMed] [Google Scholar]
- 3.Waxman I, Dye CE. Interventional endosonography. Cancer J 2002;8(suppl 1):S113–23. [PubMed] [Google Scholar]
- 4.Byrne MF, Jowell PS. Gastrointestinal imaging: endoscopic ultrasound. Gastroenterology 2002;122:1631–48. [DOI] [PubMed] [Google Scholar]
- 5.Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology 1997;112:1087–95. [DOI] [PubMed] [Google Scholar]
- 6.Ryan AG, Zamvar V, Roberts SA. Iatrogenic candidal infection of a mediastinal foregut cyst following endoscopic ultrasound-guided fine-needle aspiration. Endoscopy 2002;34:838–9. [DOI] [PubMed] [Google Scholar]
- 7.Rösch T, Classen M. Gastroenterologic Endosonograpy. New York: Thieme Stuttgart, 1992.
- 8.Rösch T, Will U, Chang K, et al. Longitudinal Endosonography. New York: Springer Heidelberg, 1992.
- 9.Kelly S, Harris KM, Berry E, et al. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut 2001;49:534–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puspok A, Raderer M, Chott A, et al. Endoscopic ultrasound in the follow up and response assessment of patients with primary gastric lymphoma. Gut 2002;51:691–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harewood GC, Wiersema MJ, Nelson H, et al. A prospective, blinded assessment of the impact of preoperative staging on the management of rectal cancer. Gastroenterology 2002;123:24–32. [DOI] [PubMed] [Google Scholar]
- 12.Hordijk ML. Restaging after radiotherapy and chemotherapy: value of endoscopic ultrasonography. Gastrointest Endosc Clin N Am 1995;5:601–8. [PubMed] [Google Scholar]
- 13.Isenberg G, Chak A, Canto MI, et al. Endoscopic ultrasound in restaging of esophageal cancer after neoadjuvant chemoradiation. Gastrointest Endosc 1998;48:158–63. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura S, Matsumoto T, Suekane H, et al. Predictive value of endoscopic ultrasonography for regression of gastric low grade and high grade MALT lymphomas after eradication of Helicobacter pylori. Gut 2001;48:454–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruskone-Fourmestraux A, Lavergne A, Aegerter PH, et al. Predictive factors for regression of gastric MALT lymphoma after anti-Helicobacter pylori treatment. Gut 2001;48:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caletti G, Fusaroli P, Togliani T. EUS in MALT lymphoma. Gastrointest Endosc 2002;56(suppl 4):S21–6. [DOI] [PubMed] [Google Scholar]
- 17.Rösch T, Lorenz R, Braig C, et al. Endoscopic ultrasound in pancreatic tumor diagnosis. Gastrointest Endosc 1991;37:347–52. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa H, Okada S, Saisho H, et al. Clinicopathologic features of small pancreatic adenocarcinoma. A collective study. Cancer 1996;78:986–90. [DOI] [PubMed] [Google Scholar]
- 19.Sendler A, Avril N, Helmberger H, et al. Preoperative evaluation of pancreatic masses with positron emission tomography using 18F-fluorodeoxyglucose: diagnostic limitations. World J Surg 2000;24:1121–9. [DOI] [PubMed] [Google Scholar]
- 20.Rösch T, Schusdziarra V, Born P, et al. Modern imaging methods versus clinical assessment in the evaluation of hospital in-patients with suspected pancreatic disease. Am J Gastroenterol 2000;95:2261–70. [DOI] [PubMed] [Google Scholar]
- 21.Rösch T, Braig C, Gain T, et al. Staging of pancreatic and ampullary carcinoma by endoscopic ultrasonography. Comparison with conventional sonography, computed tomography, and angiography. Gastroenterology 1992;102:188–99. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad NA, Lewis JD, Ginsberg GG, et al. EUS in preoperative staging of pancreatic cancer. Gastrointest Endosc 2000;52:463–8. [DOI] [PubMed] [Google Scholar]
- 23.Gress FG, Hawes RH, Savides TJ, et al. Role of EUS in the preoperative staging of pancreatic cancer: a large single-center experience. Gastrointest Endosc 1999;50:786–91. [DOI] [PubMed] [Google Scholar]
- 24.Rösch T, Dittler HJ, Strobel K, et al. Endoscopic ultrasound criteria for vascular invasion in the staging of cancer of the head of the pancreas: a blind reevaluation of videotapes. Gastrointest Endosc 2000;52:469–77. [DOI] [PubMed] [Google Scholar]
- 25.Mertz HR, Sechopoulos P, Delbeke D, et al. EUS, PET, and CT scanning for evaluation of pancreatic adenocarcinoma. Gastrointest Endosc 2000;52:367–71. [DOI] [PubMed] [Google Scholar]
- 26.Tierney WM, Francis IR, Eckhauser F, et al. The accuracy of EUS and helical CT in the assessment of vascular invasion by peripapillary malignancy. Gastrointest Endosc 2001;53:182–8. [DOI] [PubMed] [Google Scholar]
- 27.Howard TJ, Chin AC, Streib EW, et al. Value of helical computed tomography, angiography, and endoscopic ultrasound in determining resectability of periampullary carcinoma. Am J Surg 1997;174:237–41. [DOI] [PubMed] [Google Scholar]
- 28.Willis S, Truong S, Gribnitz S, et al. Endoscopic ultrasonography in the preoperative staging of gastric cancer: accuracy and impact on surgical therapy. Surg Endosc 2000;14:951–4. [DOI] [PubMed] [Google Scholar]
- 29.Marusch F, Koch A, Schmidt U, et al. Routine use of transrectal ultrasound in rectal carcinoma: results of a prospective multicenter study. Endoscopy 2002;34:385–90. [DOI] [PubMed] [Google Scholar]
- 30.Rösch T, Kapfer B, Will U, et al. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol 2002;37:856–62. [PubMed] [Google Scholar]
- 31.Chak A. EUS in submucosal tumors. Gastrointest Endosc 2002;56(suppl 4):S43–8. [DOI] [PubMed] [Google Scholar]
- 32.Rösch T, Mayr P, Kassem MA. Endoscopic ultrasonography in acute biliary pancreatitis. J Gastrointest Surg 2001;5:223–8. [DOI] [PubMed] [Google Scholar]
- 33.Kohut M, Nowakowska-Dulawa E, Marek T, et al. Accuracy of linear endoscopic ultrasonography in the evaluation of patients with suspected common bile duct stones. Endoscopy 2002;34:299–303. [DOI] [PubMed] [Google Scholar]
- 34.Palazzo L, O’Toole D. EUS in common bile duct stones. Gastrointest Endosc 2002;56(suppl 4):S49–57. [DOI] [PubMed] [Google Scholar]
- 35.Berdah SV, Orsoni P, Bege T, et al. Follow-up of selective endoscopic ultrasonography and/or endoscopic retrograde cholangiography prior to laparoscopic cholecystectomy: a prospective study of 300 patients. Endoscopy 2001;33:216–20. [DOI] [PubMed] [Google Scholar]
- 36.Fulcher AS. MRCP and ERCP in the diagnosis of common bile duct stones. Gastrointest Endosc 2002;56(suppl 6):S178–82. [DOI] [PubMed] [Google Scholar]
- 37.Napoleon B, Dumortier J, Keriven-Souquet O, et al. Do normal findings at biliaryendoscopic ultrasonography obviate the need for endoscopic retrograde cholangiography in patients with suspicion of common bile duct stone? A prospective follow- up study of 238 patients. Endoscopy 2003;35:411–15. [DOI] [PubMed] [Google Scholar]
- 38.Kahl S, Glasbrenner B, Leodolter A, et al. EUS in the diagnosis of early chronic pancreatitis: a prospective follow-up study. Gastrointest Endosc 2002;55:507–11. [DOI] [PubMed] [Google Scholar]
- 39.Coyle WJ, Pineau BC, Tarnasky PR, et al. Evaluation of unexplained acute and acute recurrent pancreatitis using endoscopic retrograde cholangiopancreatography, sphincter of Oddi manometry and endoscopic ultrasound. Endoscopy 2002;34:617–23. [DOI] [PubMed] [Google Scholar]
- 40.Sahai AV, Penman ID, Mishra G, et al. An assessment of the potential value of endoscopic ultrasound as a cost-minimizing tool in dyspeptic patients with persistent symptoms. Endoscopy 2001;33:662–7. [DOI] [PubMed] [Google Scholar]
- 41.Lee YT, Lai AC, Hui Y, et al. S in the management of uninvestigated dyspepsia. Gastrointest Endosc 2002;56:842–8. [DOI] [PubMed] [Google Scholar]
- 42.Van Outryve SM, Van Outryve MJ, De Winter BY, et al. Is anorectal endosonography valuable in dyschesia? Gut 2002;51:695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rottenberg GT, Williams AB. Endoanal ultrasound. Br J Radiol 2002;75:482–8. [DOI] [PubMed] [Google Scholar]
- 44.Menzel J, Hoepffner N, Nottberg H, et al. Preoperative staging of esophageal carcinoma: miniprobe sonography versus conventional endoscopic ultrasound in a prospective histopathologically verified study. Endoscopy 1999;31:291–7. [DOI] [PubMed] [Google Scholar]
- 45.Hünerbein M, Ulmer C, Handke T, et al. Endosonography of upper gastrointestinal tract cancer on demand using miniprobes or endoscopic ultrasound. Surg Endosc 2003;17:615–9. [DOI] [PubMed] [Google Scholar]
- 46.Tseng LJ, Jao YT, Mo LR. Preoperative staging of colorectal cancer with a balloon- sheathed miniprobe. Endoscopy 2002;34:564–8. [DOI] [PubMed] [Google Scholar]
- 47.Hizawa K, Iwai K, Esaki M, et al. Is endoscopic ultrasonography indispensable in assessing the appropriateness of endoscopic resection for gastric cancer? Endoscopy 2002;34:973–8. [DOI] [PubMed] [Google Scholar]
- 48.Ohashi S, Segawa K, Okamura S, et al. The utility of endoscopic ultrasonography and endoscopy in the endoscopic mucosal resection of early gastric cancer. Gut 1999;45:599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waxman I, Saitoh Y. Outcome of endoscopic mucosal resection for superficial GI lesions and the role of high-frequency US probe sonography in an American population. Gastrointest Endosc 2000;52:322–7. [DOI] [PubMed] [Google Scholar]
- 50.Domagk D, Poremba C, Dietl KH, et al. Endoscopic transpapillary biopsies and intraductal ultrasonography in the diagnostics of bile duct strictures: a prospective study. Gut 2002;51:240–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamada K, Tomiyama T, Wada S, et al. Endoscopic transpapillary bile duct biopsy with the combination of intraductal ultrasonography in the diagnosis of biliary strictures. Gut 2002;50:326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gress F, Chen YK, Sherman S, et al. Experience with a catheter-based ultrasound probe in the bile duct and pancreas. Endoscopy 1995;27:178–84. [DOI] [PubMed] [Google Scholar]
- 53.Fusaroli P, Caletti G. EUS and disease management. Endoscopy 2002;34:492–4. [DOI] [PubMed] [Google Scholar]
- 54.Ainsworth AP, Mortensen MB, Durup J, et al. Clinical impact of endoscopic ultrasonography at a county hospital. Endoscopy 2002;34:447–50. [DOI] [PubMed] [Google Scholar]
- 55.Allescher HD, Rosch T, Willkomm G, et al. Performance, patient acceptance, appropriateness of indications and potential influence on outcome of EUS: a prospective study in 397 consecutive patients. Gastrointest Endosc 1999;50:737–45. [DOI] [PubMed] [Google Scholar]
- 56.Jafri IH, Saltzman JR, Colby JM, et al. Evaluation of the clinical impact of endoscopic ultrasonography in gastrointestinal disease. Gastrointest Endosc 1996;44:367–70. [DOI] [PubMed] [Google Scholar]
- 57.Nickl NJ, Bhutani MS, Catalano M, et al. Clinical implications of endoscopic ultrasound: the American Endosonography Club Study. Gastrointest Endosc 1996;44:371–7. [DOI] [PubMed] [Google Scholar]
- 58.Natsugoe S, Yoshinaka H, Shimada M, et al. Number of lymph node metastases determined by presurgical ultrasound and endoscopic ultrasound is related to prognosis in patients with esophageal carcinoma. Ann Surg 2001;234:613–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eloubeidi MA, Wallace MB, Hoffman BJ, et al. Predictors of survival for esophageal cancer patients with and without celiac axis lymphadenopathy: impact of staging endosonography. Ann Thorac Surg 2001;72:212–9. [DOI] [PubMed] [Google Scholar]
- 60.Shinkai M, Niwa Y, Arisawa T, et al. Evaluation of prognosis of squamous cell carcinoma of the oesophagus by endoscopic ultrasonography. Gut 2000;47:120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chak A, Canto MI, Cooper GS, et al. Endosonographic assessment of multimodality therapy predicts survival of esophageal carcinoma patients. Cancer 2000;88:1788–95. [PubMed] [Google Scholar]
- 62.Fockens P, Kisman K, Merkus MP, et al. The prognosis of esophageal carcinoma staged irresectable (T4) by endosonography. J Am Coll Surg 1998;186:17–23. [DOI] [PubMed] [Google Scholar]
- 63.Buscail L, Pages P, Berthelemy P, et al. Role of EUS in the management of pancreatic and ampullary carcinoma: a prospective study assessing resectability and prognosis. Gastrointest Endosc 1999;50:34–40. [DOI] [PubMed] [Google Scholar]
- 64.Rösch T, Kapfer B, Will U, et al. Influence of endoscopic ultrasonography on the further management of patients with upper gastrointestinal submucosal lesions, part 1: clinical outcome analysis. Am J Gastroenterol (in press).
- 65.Sahai AV, Siess M, Kapfer B, et al. Endoscopic ultrasonography for upper gastrointestinal submucosal lesions: a cost minimization analysis with an international perspective. Am J Gastroenterol (in press). [DOI] [PubMed]
- 66.Wallace MB, Nietert PJ, Earle C, et al. An analysis of multiple staging management strategies for carcinoma of the esophagus: computed tomography, endoscopic ultrasound, positron emission tomography, and thoracoscopy/laparoscopy. Ann Thorac Surg 2002;74:1026–32. [DOI] [PubMed] [Google Scholar]
- 67.Harewood GC, Wiersema MJ. Cost-effectiveness of endoscopic ultrasonography in the evaluation of proximal rectal cancer. Am J Gastroenterol 2002;97:874–82. [DOI] [PubMed] [Google Scholar]
- 68.Harewood GC, Wiersema MJ. A cost analysis of endoscopic ultrasound in the evaluation of esophageal cancer. Am J Gastroenterol 2002;97:452–8. [DOI] [PubMed] [Google Scholar]
- 69.Hadzijahic N, Wallace MB, Hawes RH, et al. CT or EUS for the initial staging of esophageal cancer? A cost minimization analysis. Gastrointest Endosc 2000;52:715–20. [DOI] [PubMed] [Google Scholar]
- 70.Mortensen MB, Ainsworth AP, Langkilde LK, et al. Cost-effectiveness of different diagnostic strategies in patients with nonresectable upper gastrointestinal tract malignancies. Surg Endosc 2000;14:278–81. [DOI] [PubMed] [Google Scholar]
- 71.Aabakken L, Silvestri GA, Hawes R, et al. Cost-efficacy of endoscopic ultrasonography with fine-needle aspiration vs. mediastinotomy in patients with lung cancer and suspected mediastinal adenopathy. Endoscopy 1999;31:707–11. [DOI] [PubMed] [Google Scholar]
- 72.Bansal R, Tierney W, Carpenter S, et al. Cost effectiveness of EUS for preoperative localization of pancreatic endocrine tumors. Gastrointest Endosc 1999;49:19–25. [DOI] [PubMed] [Google Scholar]
- 73.Arguedas MR, Dupont AW, Wilcox CM. Where do ERCP, endoscopic ultrasound, magnetic resonance cholangiopancreatography, and intraoperative cholangiography fit in the management of acute biliary pancreatitis? A decision analysis model. Am J Gastroenterol 2001;96:2892–9. [DOI] [PubMed] [Google Scholar]
- 74.Takada N, Higashino M, Osugi H, et al. Utility of endoscopic ultrasonography in assessing the indications for endoscopic surgery of submucosal esophageal tumors. Surg Endosc 1999;13:228–30. [DOI] [PubMed] [Google Scholar]
- 75.Waxman I, Saitoh Y, Raju GS, et al. High-frequency probe EUS-assisted endoscopic mucosal resection: a therapeutic strategy for submucosal tumors of the GI tract. Gastrointest Endosc 2002;55:44–9. [DOI] [PubMed] [Google Scholar]
- 76.Sun S, Wang M, Sun S. Use of endoscopic ultrasound-guided injection in endoscopic resection of solid submucosal tumors. Endoscopy 2002;34:82–5. [DOI] [PubMed] [Google Scholar]
- 77.Polkowski M, Palucki J, Butruk E. Transabdominal ultrasound for visualizing gastric submucosal tumors diagnosed by endosonography: can surveillance be simplified? Endoscopy 2002;34:979–83. [DOI] [PubMed] [Google Scholar]
- 78.Polkowski M, Regula J, Wronska E, et al. Endoscopic ultrasonography for prediction of postpolypectomy bleeding in patients with large nonpedunculated rectosigmoid adenomas. Endoscopy 2003;35: 343–7. [DOI] [PubMed] [Google Scholar]
- 79.Fockens P, Johnson TG, van Dullemen HM, et al. Endosonographic imaging of pancreatic pseudocysts before endoscopic transmural drainage. Gastrointest Endosc 1997;46:412–16. [DOI] [PubMed] [Google Scholar]
- 80.Giovannini M, Seitz JF, Monges G, et al. Fine-needle aspiration cytology guided by endoscopic ultrasonography: results in 141 patients. Endoscopy 1995;27:171–7. [DOI] [PubMed] [Google Scholar]
- 81.Williams DB, Sahai AV, Aabakken L, et al. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut 1999;44:720–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fritscher-Ravens A, Bohuslavizki KH, Brandt L, et al. Mediastinal lymph node involvement in potentially resectable lung cancer: comparison of CT, positron emission tomography, and endoscopic ultrasonography with and without fine-needle aspiration. Chest 2003;123:442–51. [DOI] [PubMed] [Google Scholar]
- 83.Catalano MF, Nayar R, Gress F, et al. EUS-guided fine needle aspiration in mediastinal lymphadenopathy of unknown etiology. Gastrointest Endosc 2002;55:863–9. [DOI] [PubMed] [Google Scholar]
- 84.Fritscher-Ravens A, Sriram PV, Bobrowski C, et al. Mediastinal lymphadenopathy in patients with or without previous malignancy: EUS-FNA-based differential cytodiagnosis in 153 patients. Am J Gastroenterol 2000;95:2278–84. [DOI] [PubMed] [Google Scholar]
- 85.Romagnuolo J, Scott J, Hawes RH, et al. Helical CT versus EUS with fine needle aspiration for celiac nodal assessment in patients with esophageal cancer. Gastrointest Endosc 2002;55:648–54. [DOI] [PubMed] [Google Scholar]
- 86.Fritscher-Ravens A, Brand L, Knofel WT, et al. Comparison of endoscopic ultrasound- guided fine needle aspiration for focal pancreatic lesions in patients with normal parenchyma and chronic pancreatitis. Am J Gastroenterol 2002;97:2768–75. [DOI] [PubMed] [Google Scholar]
- 87.Bounds BC, Brugge WR. EUS diagnosis of cystic lesions of the pancreas. Int J Gastrointest Cancer 2001;30:27–31. [DOI] [PubMed] [Google Scholar]
- 88.Hernandez LV, Mishra G, Forsmark C, et al. Role of endoscopic ultrasound (EUS) and EUS-guided fine needle aspiration in the diagnosis and treatment of cystic lesions of the pancreas. Pancreas 2002;25:222–8. [DOI] [PubMed] [Google Scholar]
- 89.Ando N, Goto H, Niwa Y, et al. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc 2002;55:37–43. [DOI] [PubMed] [Google Scholar]
- 90.tenBerge J, Hoffman BJ, Hawes RH, et al. EUS-guided fine needle aspiration of the liver: indications, yield, and safety based on an international survey of 167 cases. Gastrointest Endosc 2002;55:859–62. [DOI] [PubMed] [Google Scholar]
- 91.Nguyen PT, Chang KJ. EUS in the detection of ascites and EUS-guided paracentesis. Gastrointest Endosc 2001;54:336–9. [DOI] [PubMed] [Google Scholar]
- 92.Chang KJ, Erickson RA, Nguyen P. Endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration of the left adrenal gland. Gastrointest Endosc 1996;44:568–72. [DOI] [PubMed] [Google Scholar]
- 93.Van Dam J, Brady PG, Freeman M, et al. Guidelines for training in electronic ultrasound: guidelines for clinical application. From the ASGE. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc 1999;49:829–33. [DOI] [PubMed] [Google Scholar]
- 94.Caletti G, Deviere J, Fockens P, et al. Guidelines of the European Society of Gastrointestinal Endoscopy (ESGE) Part II: Retroperitoneum and large bowel, training. The European Endosonography Club Working Party. Endoscopy 1996;28:626–8. [PubMed] [Google Scholar]
- 95.Schlick T, Heintz A, Junginger T. The examiner’s learning effect and its influence on the quality of endoscopic ultrasonography in carcinoma of the esophagus and gastric cardia. Surg Endosc 1999;13:894–8. [DOI] [PubMed] [Google Scholar]
- 96.Fockens P, Van den Brande JH, van Dullemen HM, et al. Endosonographic T-staging of esophageal carcinoma: a learning curve. Gastrointest Endosc 1996;44:58–62. [DOI] [PubMed] [Google Scholar]
- 97.Bhutani MS, Hoffman BJ, Hawes RH. A swine model for teaching endoscopic ultrasound (EUS) imaging and intervention under EUS guidance. Endoscopy 1998;30:605–9. [DOI] [PubMed] [Google Scholar]
- 98.Giovannini M, Pesenti C, Rolland AL, et al. Endoscopic ultrasound-guided drainage of pancreatic pseudocysts or pancreatic abscesses using a therapeutic echo endoscope. Endoscopy 2001;33:473–7. [DOI] [PubMed] [Google Scholar]
- 99.Seifert H, Wehrmann T, Schmitt T, et al. Retroperitoneal endoscopic debridement for infected peripancreatic necrosis. Lancet 2000;356:653–5. [DOI] [PubMed] [Google Scholar]
- 100.Gunaratnam NT, Sarma AV, Norton ID, et al. A prospective study of EUS-guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc 2001;54:316–24. [DOI] [PubMed] [Google Scholar]
- 101.Gress F, Schmitt C, Sherman S, et al. Endoscopic ultrasound-guided celiac plexus block for managing abdominal pain associated with chronic pancreatitis: a prospective single center experience. Am J Gastroenterol 2001;96:409–16. [DOI] [PubMed] [Google Scholar]
- 102.Gress F, Schmitt C, Sherman S, et al. A prospective randomized comparison of endoscopic ultrasound- and computed tomography-guided celiac plexus block for managing chronic pancreatitis pain. Am J Gastroenterol 1999;94:900–5. [DOI] [PubMed] [Google Scholar]
- 103.Bhutani MS. Endoscopic ultrasonography (DDW report 2002). Endoscopy 2002;34:888–9. [DOI] [PubMed] [Google Scholar]