Abstract
Background: The role of proinflammatory cytokines in the pathogenesis of portal hypertension is unclear.
Aims and methods: This study tests the hypothesis that tumour necrosis factor α (TNF-α) is an important mediator of the circulatory disturbances in alcoholic hepatitis (AH) and evaluates the acute and short term effect of a single infusion of the monoclonal chimeric anti-TNF-α antibody (Infliximab) on portal and systemic haemodynamics in 10 patients with severe biopsy proven AH. Cardiovascular haemodynamics, hepatic venous pressure gradient (HVPG), and hepatic and renal blood flow were measured before, 24 hours after Infliximab, and prior to hospital discharge.
Results: Serum bilirubin (p<0.05), C reactive protein (p<0.001), and white cell count (p<0.01) were reduced significantly, as were plasma levels of interleukin (IL)-6 and IL-8 after treatment. Of the 10 patients, nine were alive at 28 days. Mean HVPG decreased significantly at 24 hours (23.4 (2.8) to 14.3 (1.9) mm Hg; p<0.001) with a sustained reduction prior to discharge (12.8 (1.9) mm Hg; p<0.001). Mean arterial pressure and systemic vascular resistance increased significantly (p<0.001and p<0.01, respectively), mirrored by a reduction in cardiac index (5.9 (0.5) to 4.7 (0.5) l/min/m2; p<0.05) prior to discharge. Hepatic and renal blood flow also increased significantly (506.2 (42.9) to 646.3 (49.2) ml/min (p=0.001) and 424.3 (65.12) to 506.3 (85.7) ml/min (p=0.001), respectively) prior to discharge.
Conclusion: The results of this study illustrate that anti-TNF-α treatment in AH patients produces a highly significant, early, and sustained reduction in HVPG, possibly through a combination of a reduction in cardiac output and intrahepatic resistance. In addition, there was a reduction in hepatic inflammation and improved organ blood flow, suggesting an important role for TNF-α in mediating the circulatory disturbances in AH.
Keywords: tumour necrosis factor, inflammation, alcoholic hepatitis, haemodynamics, portal pressure
Cirrhosis is characterised by a hyperdynamic circulation manifest as splanchnic vasodilatation and increased cardiac output (CO). These circulatory disturbances combined with increased intrahepatic resistance contribute to the development and maintenance of portal hypertension1 and often present as the major complications of cirrhosis in the form of variceal bleeding, fluid retention, and reduced renal blood flow (RBF).
Tumour necrosis factor α (TNF-α), a proinflammatory cytokine, is believed to exert vascular effects by increasing vascular permeability and causing vasodilatation, thought to be mediated through nitric oxide (NO) dependant pathways.2 TNF-α has been reported to be elevated in alcoholic liver disease and, in particular, high levels of TNF-α are found in alcoholic hepatitis (AH).3 In a recently published pilot study, we have shown that administration of a chimeric anti-TNF-α antibody, Infliximab, to patients with severe AH is safe and results in an improvement in liver function and a reduction in inflammatory mediators.4
The role of TNF-α in the pathogenesis of portal hypertension is suggested by studies on portal vein ligated rats treated with anti-TNF-α therapies, demonstrating a blunting of the hyperdynamic circulation and a reduction in portal pressure.5,6 We hypothesised that in view of the reported role of TNF-α in the pathogenesis of AH, administration of an anti-TNF- α antibody to AH patients would result in a reduction in portal pressure and an improvement in the systemic haemodynamic derangement. Our study tests this hypothesis, evaluating the acute and short term effect of a single infusion of Infliximab, on the portal, cardiovascular, and renal haemodynamics in patients with severe AH.
METHODS
Patients
Ten patients (eight males, mean age 53 (8) years) with severe AH were recruited into this study. (The clinical outcome of three of these patients have been described in our previous report.4) The local committee on ethics of human research granted ethical approval, and all patients/next of kin gave written informed consent/assent. Following admission, patients were observed for 72 hours prior to inclusion. Patients were included if they had severe AH defined by a compatible clinical history (alcohol consumption >80 g alcohol/day for men and >60 g/day for women up until the time of admission), a Maddrey discriminant function (DF)7 of >32, histological evidence of AH (balloon degeneration of hepatocytes; Mallory bodies; neutrophilic infiltrates and apoptotic acidophilic bodies), and deterioration in liver function tests over the observation period, despite supportive medical management. Patients were excluded if they were <18 or >75 years and had any of the following: renal dysfunction (plasma creatinine >130 μmol/l), severe cardiovascular or cerebrovascular disease, active or latent tuberculosis, hepatic or extrahepatic malignancy, and treatment with vasoactive agents or corticosteroids therapy.
Study design
Haemodynamic assessment was performed prior to entry into the study, 24 hours after administration of Infliximab, and on the day prior to discharge. The study was terminated either on day 28 or the day of discharge, whichever occurred earlier.
Day –2
Baseline haemodynamic assessment was performed in nine patients at the time of undergoing a transjugular liver biopsy. In the 10th patient, a biopsy had been performed prior to hospital transfer and it was therefore difficult to justify a baseline haemodynamic assessment.
Day 0
Forty eight hours following the biopsy and histological confirmation of AH, Infliximab was administered intravenously (Schering-Plough Corporation, Kenilworth, New Jersey, USA) over a two hour period, at a dose of 5 mg/kg body weight.4
Day 1
Repeat haemodynamic assessment was performed the next morning, 24 hours after Infliximab infusion, in eight patients through an indwelling 9 French internal jugular sheath (William Cook Europe, Bjaeverskov, Denmark), negating further venepuncture. One patient declined a repeat haemodynamic study at this time.
Prior to the day of discharge or day 28
A further haemodynamic assessment was performed at a mean of 19 (4) days in five of the patients who consented to the third phase of the study.
Sampling
Blood was collected before the study began and then daily for one week after infusion; thereafter, twice per week until day 28 for routine haematology, biochemistry, and C reactive protein concentration. During each haemodynamic assessment, blood was collected from an artery, and the hepatic and renal veins in precooled heparinised tubes. Plasma was separated by centrifugation and stored at −70° C for subsequent analysis. A 24 hour urine collection enabled determination of sodium excretion.
Measurements
All haemodynamic studies were started after an overnight fast, at 0700 hours, after patients had been supine for at least one hour. Patients were sedated for the procedure using midazolam (mean dose 3 mg; Phoenix Pharma Ltd, Gloucester, UK).
Hepatic venous pressure gradient (HVPG)
The right hepatic vein was cannulated with a Cobra 2 catheter (Cordis, Roden, the Netherlands) under fluoroscopic screening (Toshiba Spot Film Device Model: SA-900U; Tochigi-ken, Japan) and wedged catheter measurements assessed in triplicate in at least two radicals after injection of 2 ml of contrast medium (iohexol (Omnipaque); Amersham Health, Little Chalfont, UK). Careful attention to fluoroscopic examination ensured wedged positions obtained were without drainage by local venous shunts. Wedged (WHVP) and free hepatic venous pressure (FHVP) measurements were recorded via pressure transducer sets (Medex Medical, Rossendale, Lancashire, UK) on a Hewlett Packard monitor (model 86S; HP-Palo Alto, California, USA). HVPG was calculated as the difference between WHVP and FHVP. The coefficient of variation of measurements of HVPG within a given patient was 3% (0.42) for the study group.
Cardiovascular haemodynamics
Heart rate, oxygen saturation, and ECG were recorded continuously, and mean arterial pressure (MAP) (1/3 (systolic−diastolic)+diastolic pressure) was measured prior to catheterisation and every five minutes thereafter (Hewlett-Packard, Model 86S). The pulmonary artery was catheterised via the internal jugular sheath using a Swan-Ganz catheter (Edward Lifesciences, Irvine, California, USA) and CO calculated by thermodilution and displayed electronically (Vigilance monitor; Critical Care Edwards Lifesciences, Irvine, California, USA). Each measurement was performed in triplicate and an electronic mean calculated. Swan-Ganz measurements were performed in seven patients on day 0 as two patients developed runs of ventricular ectopics on introduction of the catheter into the right ventricular outflow tract, albeit there were no sequelae on catheter withdrawal.
Blood flow
Hepatic blood flow (HBF) was determined using a primed (12 mg) continuous infusion (1 mg/min) of indocyanine green (ICG) (Akorn, Inc., Buffalo Grove, Illinois, USA) and RBF using a primed (0.56 ml/kg body weight) continuous infusion (50 ml/h) of para- aminohippuric acid (PAH) (Apotheek Academisch Ziekenhuis, Maastricht, the Netherlands). Both solutions were started one hour prior to sampling to ensure steady state concentrations. RBF was determined by measurement of PAH in arterial and renal venous blood. HBF was measured by simultaneous sampling of arterial and hepatic venous blood. PAH and ICG were determined spectrophotometrically by standard techniques. Plasma flow rate of the liver and kidneys were calculated using formulae based on the method of indicator dilution and Fick’s principle.8,9 PAH determined plasma flow was converted to blood flow using the haematocrit.
Cytokine analysis
TNF-α, interleukin (IL)-6, and IL-8 were assayed in all patients according to the manufacturer’s instructions using specific ELISA assays (BioSource International, Nivelles, Belgium) in EDTA anticoagulated peripheral blood samples,4 at baseline and 24 hours following Infliximab administration.
NOx (nitrate/nitrite) production
A modified Greiss reaction was used to determine NOx levels in peripheral venous and hepatic venous blood, pre and post Infliximab administration. Plasma was filtered according to the protocol of Giovannoni and colleagues,10 and nitrate converted to nitrite catalytically using the method of Miranda and colleagues.11
Statistical analysis
Data are shown as mean (SEM). One way analysis of variance was used to define differences between means of normally distributed data, using a commercially available package (Graph Pad Prism, version 3.0; Graph Pad Software, Inc., San Diego, California, USA). For statistical evaluation of hepatic and renal blood flow and urinary sodium excretion data, which were not normally distributed and had a limited number of observations, a Wilcoxon matched pairs test was used. Results were considered significant if p<0.05.
RESULTS
Patients
Ten patients were deeply jaundiced at the time of entry into the study, with a mean bilirubin of 335.9 (41.6) μmol/l and an alanine transaminase (ALT) level of 46 (3.5) U/l. All had ascites and six had mild hepatic encephalopathy. Mean Maddrey DF was 68.9 (8.9) (table 1 ▶). Liver biopsy showed evidence of established cirrhosis in all patients, in addition to features suggestive of a superimposed AH. Treatment with Infliximab resulted in a significant reduction in prothrombin time (p<0.05) by one week and also a significant improvement in serum bilirubin levels (p<0.05) by the time of discharge, with no significant change in ALT. There was a significant reduction in Maddrey score at one week (p<0.01) which was sustained at 28 days (62.9 (9) at day 0; 53.6 (9) at day 7; 42.3 (5) at day 28; p<0.05). Systemic inflammatory response syndrome (SIRS) score and its components showed a sustained improvement (2.5 (0.4) at day 0 to 0.5 (0.27) at day 28; p<0.001), as evidenced by a reduction in white blood cell count, heart rate, respiratory rate, and temperature (table 2 ▶). There was also a substantial reduction in C reactive protein level (mg/l) 24 hours after treatment with Infliximab (86.8 (14.4) to 53.1 (9.2); p<0.001). These improvements in inflammatory indices were mirrored by a significant reduction at 24 hours post Infliximab in peripheral venous IL-6 (pg/ml) (54 (12.3) to 37.7 (8.1); p<0.01) and IL-8 (pg/ml) (76 (29) to 55.2 (22.9); p<0.05). There was a trend towards a significant reduction in peripheral venous TNF-α levels (pg/ml) post Infliximab administration in seven patients in whom levels were detectable above the assay detection threshold of 5 pg/ml (57 (36) at baseline to 45.5 (28.6) post administration; p=0.06).
Table 1.
Patient characteristics on entry into the study
| Age (y) | 52.7 (2.6) |
| Sex (M/F) | 8/2 |
| Bilirubin (μmol/l) | 311 (46) [3–17]* |
| Alanine transaminase (U/l) | 46 (3.5) [8–63]* |
| Prothrombin time (s) | 21.2 (1.5) [10–13]* |
| Ascites (n) | |
| Mild | 4 |
| Moderate | 5 |
| Severe | 1 |
| Encephalopathy grade | 1.4 (0.16) |
| Child score | 11.8 (0.3) |
| Maddrey score | 68.9 (8.9) |
| SIRS score | 2.6 (0.3) |
SIRS, systemic inflammatory response syndrome.
Values are mean (SEM) [range] or number.
*Normal reference range.
Table 2.
Changes in liver function and SIRS components prior to and after treatment with Infliximab
| Baseline (day –2) | Day 1 | Day 7 | Day 14 | Day 28 | |
| Prothrombin time [10–13] (s) | 20.7 (1.7) | 20.1 (1.8) | 18.8 (1.6)* | 18.3 (1.2)* | 17.8 (0.9)** |
| Bilirubin [3–17] (μmol/l) | 335.9 (41.6) | 285.5 (48.2) | 267.6 (43.4) | 219.2 (40.4)* | 141.1 (17.7)* |
| C reactive protein [0–5] (mg/l) | 86.8 (13.7) | 53.1 (9.2)** | 48.7 (10.3)** | 39.2 (5.6)*** | 32.7 (4.6)*** |
| White blood cell count [3–10] (×109/l) | 17.5 (2.7) | 13.5 (1.6)** | 13.8 (1.6)** | 13.5 (1.2)** | 12.3 (1.8)*** |
| Temperature (°C) | 37.7 (0.3) | 37 (0.2)* | 37 (0.3)* | 36.8 (0.2)* | 36.8 (0.2)* |
| Heart rate (beats/min) | 93.8 (3.7) | 85.3 (3.1)* | 85.7 (2.9)* | 79 (3.2)** | 73.5 (1.6)*** |
| Respiratory rate (/min) | 22.3 (1.5) | 18.7 (1.1)* | 18.5 (2.1)* | 17.1 (0.8)* | 17.7 (1.1)* |
*p<0.05, **p<0.01, ***p<0.001 compared with baseline values.
Values are mean (SEM) [normal range].
SIRS, systemic inflammatory response syndrome.
Clinical evidence of improvement was sustained during the study period in all but one patient who died at 28 days from aspiration pneumonia and subsequent septicaemia. Apart from this patient, no other individual required any vasoactive agents, admission to the intensive care unit, or antibiotic therapy, or developed any signs of progressive hepatic encephalopathy. Ascites in three patients required paracentesis.
Portal haemodynamics
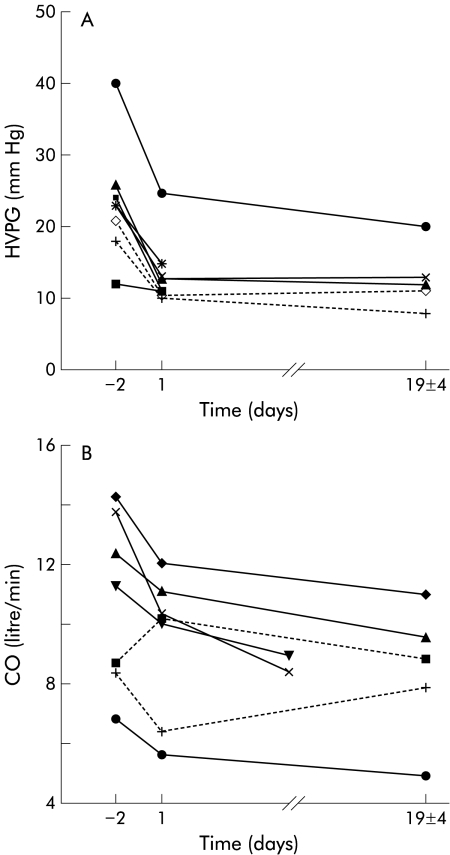
HVPG was reduced by more than 30% of baseline values in seven of eight patients who had a repeat assessment within 24 hours of treatment with Infliximab. Overall, mean HVPG was reduced by 38.9% from a mean of 23.4 (2.8) mm Hg at baseline to 14.3 (1.9) mm Hg on day 1 (p<0.001), and mean HVPG on the day prior to discharge (day 19 (4)) was 12.8 (1.9) mm Hg (p<0.001) (fig 1A ▶).
Figure 1.
(A) Individual patient HVPG data following treatment with Infliximab are plotted against time. HVPG was measured 48 hours before (day –2) and 24 hours after (day 1) treatment with Infliximab, and then after a mean discharge interval period of 19 (4) days. A significant reduction (p<0.001) was noted by day 1 in all but one patient (who entered the study with a low baseline HVPG), with a sustained reduction by the day of discharge (p<0.001). (B) Individual patient CO (l/min) data with Infliximab treatment plotted against time. The broken lines represent the two patients in whom CO increased at day 1 or by the end of the study, respectively, despite reduction in their HVPG, as shown in (A).
Cardiovascular haemodynamics
In all 10 patients, MAP increased significantly from baseline values (71.6 (1.7) mm Hg to 77 (1.9) mm Hg on day 1; p<0.001) and remained elevated on the day prior to discharge (mean 81.1 (3.2) mm Hg; p<0.001). This was associated with a significant increase in systemic vascular resistance (SVR) from 477.7 (72) dyn.s/cm5 (baseline) to 786.2 (124) dyn.s/cm5 prior to discharge (p<0.01). There was a corresponding 22% reduction in CO (p<0.05) by the end of the study (table 3 ▶, fig 1B ▶), in addition to a significant reduction in heart rate (day 1: p<0.05; day prior to discharge: p<0.001), as shown in table 2 ▶. No significant changes in central venous pressure were observed following Infliximab therapy.
Table 3.
Renal function and haemodynamic changes following treatment with Infliximab
| Baseline (day –2) | Day 1 | Prior to discharge | |
| MAP (mm Hg) | 71.6 (1.7) | 77 (1.9)*** | 81.1 (3.2)*** |
| SVR (dyn.s/cm5) | 466.1 (81.4) | 552.7 (66.2)* | 786.2 (124)** |
| CO (l/min) | 10.9 (1) | 10 (0.8)* | 8.4 (0.8)* |
| Cardiac index (l/min/m2) | 5.9 (0.5) | 5.3 (0.4)* | 4.7 (0.5)* |
| WHVP (mm Hg) | 38.6 (2.4) | 29.1 (1.4)*** | 27.4 (2.8)*** |
| FHVP (mm Hg) | 15.3 (1.8) | 14.9 (2) | 14.6 (1.7) |
| HVPG (mm Hg) | 23.4 (2.8) | 14.3 (1.9)*** | 12.8 (1.9)*** |
| HBF (ml/min) | 506.2 (42.9) | 591.8 (55.2)* | 646.3 (49.2)*** |
| Central venous pressure (mm Hg) | 12.9 (1.1) | 11.8 (0.7) | 11.7 (0.9) |
| RBF (ml/min) | 424.3 (65.1) | 451.8 (76)* | 506.3 (85.7)** |
| Urinary sodium [>10] (mmol/l) | 10.3 (2.5) | 19 (5.1)* | 23.3 (4.3)** |
| Urine volume [>700] (ml/24 h) | 895.8 (140) | 975.8 (113) | 1122 (109) |
| Creatinine [80–133] (μmol/l) | 90.9 (21.5) | 89 (14.7) | 83.5 (10.6) |
*p<0.05, **p<0.01, ***p<0.001 compared with baseline values.
Numbers of patients included in each part of the haemodynamic assessments (baseline; day 1; prior to discharge): portal haemodynamics (n=9; 8; 5); cardiac haemodynamics (n=7; 7; 5); hepatic/renal blood flow (n=6; 6; 5); and MAP (n=10; 10; 9).
CO, cardiac output; FHVP, free hepatic venous pressure; HBF, hepatic blood flow; HVPG, hepatic venous pressure gradient; MAP, mean arterial pressure; RBF, renal blood flow; SVR, systemic vascular resistance; WHVP, wedged hepatic venous pressure.
Hepatic blood flow
Although HBF was measured in nine patients, data were analysed in six patients who had a hepatic extraction of greater than 10% (mean %ICG extraction: baseline 11.9 (0.9)%; day 1 12.5 (0.7)%). A significant increase in HBF was observed in six patients in whom it was measurable, from 506.2 (42.9) ml/min at baseline to 591.8 (55.2) ml/min on day 1.
Renal blood flow and renal function
None of the patients developed renal impairment following Infliximab treatment. There was a significant increase in RBF from 424.3 (65.1) ml/min at baseline to 506.3 (85.7) ml/min at the time of discharge (p<0.01). This increase in RBF was mirrored by an increase in urinary sodium excretion from 10.3 (2.5) mmol/l on day 0 to 19 (5.1) mmol/l on day 1 (p<0.05). This improvement in sodium excretion was sustained at the time of discharge (23.3 (4.3) mmol/l) and was statistically significant (p<0.01). Similarly, urine output increased from 895.8 (140) ml pretreatment to 1122 (109) ml at the time of discharge (p=0.21) (table 3 ▶).
NOx
Measurements of NOx in peripheral and hepatic venous blood were available in four patients. Mean baseline peripheral venous NOx was 62.9 (22.3) μmol/l and this was found to be reduced to 45.8 (22.3) μmol/l after Infliximab administration. The hepatic venous concentration of NOx increased after Infliximab treatment from 66 (32.3) μmol/l (baseline) to 89.8 (43.2) μmol/l. The small numbers of observations (n=4) make statistical testing difficult to interpret.
DISCUSSION
This study is the first to measure changes in portal and systemic haemodynamics in AH patients following treatment with Infliximab, and demonstrates an early significant reduction in HVPG (mean 38%; p<0.001) which was sustained over several weeks and was coupled with a significant increase in HBF. This observation further highlights the critical role played by TNF-α in the development of portal hypertension in AH. In addition, the results show a significant improvement in systemic haemodynamics; witnessed as a reduction in CO and heart rate with a concomitant increase in SVR and MAP, resulting in an increase in renal blood flow and sodium excretion.
The results of the present study confirm those of our previous observation4 that treatment of AH patients with Infliximab is well tolerated, with nine patients surviving and demonstrating significant improvements in their prothrombin time and serum bilirubin levels and, as a consequence, decreased Maddrey DF (p<0.01). Improvement in C reactive protein and white cell count 24 hours after Infliximab therapy was associated with a reduction in SIRS components, and circulating IL-6 and IL-8, highlighting the central role of TNF-α in sustaining the inflammatory response in these patients. This is further supported by our previous data showing a decrease in intrahepatic IL-8 mRNA synthesis 28 days following Infliximab administration to AH patients.4 The lack of significant reduction in TNF-α levels in patients in whom it was measured following Infliximab administration has been observed previously by us4 and others,12 and may reflect the relatively small numbers of patients included in this study and/or the complex interaction between TNF-α and its bound and free receptors. Soluble TNF receptors have been shown to be upregulated in severe AH13 while TNF levels in patients with AH are widely variable in the literature, ranging from values as low as 5 pg/ml to as high as 400 pg/ml,14–17 suggesting that other factors such as concomitant infection, degree of endotoxaemia, genetic factors, and soluble receptor expression may be important in determining measured levels of TNF.
The significant reduction in HVPG following treatment with Infliximab found in the present study is likely to be due to a combination of an alteration in both the “forward” and “backward” components of portal hypertension. Changes in the forward component, which regulates splanchnic inflow, largely through a reduction in CO and increase in SVR, have been documented in studies in animal models.5,18,19 However, as shown in fig 1B ▶, in this study there was an increase in CO in two patients (one after 24 hours and one prior to discharge), despite a sustained reduction in their HVPG. This would appear to suggest that in addition to regulating splanchnic inflow, there is an equally important role for TNF-α in the modulation of intrahepatic resistance—that is, the “backward component” of portal hypertension. This hypothesis is further supported by our observation of a reduction in HVPG despite an increase in HBF, as a consequence of a mean reduction in intrahepatic resistance of 43%. Additionally, no significant change in HVPG was found in studies in portal hypertensive patients with cirrhosis demonstrating a reduction in CO and a significant increase in SVR and MAP after systemic inhibition of nitric oxide synthase (NOS) with the inhibitor NG-monomethyl-l-arginine.20,21 This suggests that modulation of systemic regulators of the forward component alone may not be sufficient to reduce HVPG.
“Intrahepatic resistance” is thought to have a “fixed” component relating to fibrosis and a change in cellular architecture and size, and a “variable” component that can be modified by many factors, including the activity of hepatic stellate cells (HSC). Following liver injury, HSCs undergo transdifferentiation to a myofibroblastic phenotype, significantly impairing segmental blood flow, while also proliferating and increasing production of the extracellular matrix. TNF-α has been shown to be instrumental in the activation of rat HSCs and also increases the synthesis of extracellular matrix proteins,22 albeit there are no human studies. Moreover, spontaneous apoptosis in activated HSCs is significantly downregulated by TNF-α, thus prolonging the survival of activated HSC in chronic liver disease and maintaining increased intrahepatic resistance.23 It is possible that in alcoholic hepatitis, where there is overproduction of TNF-α,24 prolonged activation of HSC may give rise to significant elevation of HVPG through increased intrahepatic resistance. It is interesting therefore to hypothesise that the mechanism of reduction in intrahepatic resistance after administration of anti-TNF results either from apoptosis of HSCs, their deactivation, or from remodelling of the extracellular matrix. However, there is no evidence to suggest massive HSC apoptosis can occur within 24 hours of Infliximab administration to account for our observed reduction in HVPG, or data to show that HSC activation can be reversed. Similarly, there are no human data to support rapid remodelling of the extracellular matrix, albeit this has been described within 24 hours using cultured HSC.23
An alternative explanation for the decrease in intrahepatic resistance following Infliximab administration stems from research demonstrating decreased intrahepatic endothelial nitric oxide synthase (eNOS) activity in cirrhosis.25,26 Although the mechanisms for the local intrahepatic deficiency of NO and the differential tissue regulation of NO production remain ill defined, there is evidence from cardiovascular research suggesting that methylarginine derivatives are important in regulating NOS activity following endothelial injury.27,28 Of interest, asymmetrical dimethylarginine is in turn regulated by dimethylarginine hydrolase, an enzyme whose activity can be modified by TNF-α.29 One may thus speculate that administration of Infliximab may reduce the intrahepatic concentration of eNOS inhibitors, resulting in a compartmentalised increase in intrahepatic NO. There is no current paradigm in the literature to support this, but the increased hepatic venous NOx we measured in a subset of patients post Infliximab would be compatible with this hypothesis.
Another important finding in our study was the early improvement in HBF and RBF following Infliximab treatment. These findings coupled with the fact that there was no significant difference in central venous pressure or FHVP before and after treatment, discounts a reduction in central volume as a possible explanation for the observed reduction in HVPG. Furthermore, most treatments aimed at lowering portal pressure and splanchnic vasodilatation have a deleterious effect on renal perfusion, largely through a reduction in MAP. The significant acute and sustained increase in MAP reported in this study is likely to have played an important role in the increase in RBF and sodium excretion observed. This improvement in RBF may explain why none of our patients developed hepatorenal syndrome despite their severe presentations. A similar observation was also reported by Akriviadis et al in their placebo controlled trial of AH patients treated with pentoxifylline, a TNF-α inhibitory agent.30 This study found a significant reduction in hepatorenal syndrome related deaths (12% v 43%) in the treatment arm compared with placebo, reflecting the importance of maintaining renal perfusion. A further possible explanation for the improvement in renal perfusion and function we have observed is that the reduction in HVPG may result in a favourable change in the “hepatorenal axis”,31 independent of the improvement in MAP. This hypothesis is supported by our data showing an improvement in natriuresis and urine output, in addition to a significant increase in renal blood flow, concurrent with improvement in liver function and systemic haemodynamics.
To date, there have been no published placebo controlled trials of Infliximab therapy in AH from which one can draw comparisons. Our study did not have a control arm since its inherent design was to establish whether TNF-α had a role in the development of portal hypertension, and it did not assess Infliximab treatment efficacy. Clearly, it would be difficult to justify intensive repeat haemodynamic assessments and a transjugular liver biopsy in patients not receiving any active therapy. It is also important to note that the significant reduction in HVPG and MAP occurred within 24 hours of administration of Infliximab and was sustained thereafter until discharge. This would suggest that it would be highly unlikely for the improvements in HVPG and systemic haemodynamics to have occurred as part of the natural course of AH. Indeed, early work by Maddrey and colleagues7 reported HVPG data in AH patients treated with corticosteroids or placebo, and showed no significant difference in HVPG between the treatment groups over the 28–32 day study period. Moreover, in our study, improvement in haemodynamics coincided with the significant improvement in inflammatory indices 24 hours after Infliximab therapy, supporting the hypothesis that reducing a TNF-α driven inflammatory cytokine cascade restores the imbalance in vascular mediators that promotes a hyperdynamic state.
In conclusion, we report a marked acute and sustained improvement in portal and systemic haemodynamics in patients with severe AH following treatment with the anti-TNF-α therapy Infliximab. These beneficial haemodynamic changes are complemented by a reduction in SIRS components and inflammatory indices and suggest that TNF-α driven inflammatory processes are important in the development of portal hypertension and the hyperdynamic state in AH.
Abbreviations
AH, alcoholic hepatitis
ALT, alanine transaminase
CO, cardiac output
DF, discriminant function
FHVP, free hepatic venous pressure
HBF, hepatic blood flow
HSC, hepatic stellate cell
HVPG, hepatic venous pressure gradient
ICG, indocyanine green
IL, interleukin
MAP, mean arterial pressure
NO, nitric oxide
NOS, nitric oxide synthase
NOx, nitrate/nitrite
PAH, para-aminohippuric acid
RBF, renal blood flow
SIRS, systemic inflammatory response syndrome
SVR, systemic vascular resistance
TNF-α, tumour necrosis factor α
WHVP, wedged hepatic venous pressure
REFERENCES
- 1.Bosch J, Garcia-Pagan JC. Complications of cirrhosis. I. Portal hypertension. J Hepatol 2000;32:141–56. [DOI] [PubMed] [Google Scholar]
- 2.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med 1993;329:2002–12. [DOI] [PubMed] [Google Scholar]
- 3.McClain CJ, Barve S, Deaciuc I, et al. Cytokines in alcoholic liver disease. Semin Liver Dis 1999;19:205–19. [DOI] [PubMed] [Google Scholar]
- 4.Tilg H, Jalan R, Kaser A, et al. Anti-tumor necrosis factor-alpha monoclonal antibody therapy in severe alcoholic hepatitis. J Hepatol 2003;38:419–25. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Talavera JC, Merrill WW, Groszmann RJ. Tumor necrosis factor alpha: a major contributor to the hyperdynamic circulation in prehepatic portal-hypertensive rats. Gastroenterology 1995;108:761–7. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Talavera JC, Cadelina G, Olchowski J, et al. Thalidomide inhibits tumor necrosis factor alpha, decreases nitric oxide synthesis, and ameliorates the hyperdynamic circulatory syndrome in portal-hypertensive rats. Hepatology 1996;23:1616–21. [DOI] [PubMed] [Google Scholar]
- 7.Maddrey WC, Boitnott JK, Bedine MS, et al. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978;75:193–9. [PubMed] [Google Scholar]
- 8.Uusaro A, Ruokonen E, Takala J. Estimation of splanchnic blood flow by the Fick principle in man and problems in the use of indocyanine green. Cardiovasc Res 1995;30:106–12. [DOI] [PubMed] [Google Scholar]
- 9.Ten Have GA, Bost MC, Suyk-Wierts JC, et al. Simultaneous measurement of metabolic flux in portally-drained viscera, liver, spleen, kidney and hindquarter in the conscious pig. Lab Anim 1996;30:347–58. [DOI] [PubMed] [Google Scholar]
- 10.Giovannoni G, Land JM, Keir G, et al. Adaptation of the nitrate reductase and Griess reaction methods for the measurement of serum nitrate plus nitrite levels. Ann Clin Biochem 1997;34:193–8. [DOI] [PubMed] [Google Scholar]
- 11.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001;5:62–71. [DOI] [PubMed] [Google Scholar]
- 12.Spahr L, Rubbia-Brandt L, Frossard J, et al. Combination of steroids with infliximab or placebo in severe alcoholic hepatitis: a randomized controlled pilot study. J Hepatol 2002;37:448. [DOI] [PubMed] [Google Scholar]
- 13.Naveau S, Emilie D, Balian A, et al. Plasma levels of soluble tumor necrosis factor receptors p55 and p75 in patients with alcoholic liver disease of increasing severity. J Hepatol 1998;28:778–84. [DOI] [PubMed] [Google Scholar]
- 14.Spahr L, Rubbia-Brandt L, Pugin J, et al. Rapid changes in alcoholic hepatitis histology under steroids: correlation with soluble intercellular adhesion molecule-1 in hepatic venous blood. J Hepatol 2001;35:582–9. [DOI] [PubMed] [Google Scholar]
- 15.Taieb J, Mathurin P, Elbim C, et al. Blood neutrophil functions and cytokine release in severe alcoholic hepatitis: effect of corticosteroids. J Hepatol 2000;32:579–86. [DOI] [PubMed] [Google Scholar]
- 16.Hanck C, Rossol S, Bocker U, et al. Presence of plasma endotoxin is correlated with tumour necrosis factor receptor levels and disease activity in alcoholic cirrhosis. Alcohol Alcohol 1998;33:606–8. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto M, Uemura M, Nakatani Y, et al. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcohol Clin Exp Res 2000;24:48–54S. [PubMed] [Google Scholar]
- 18.Munoz J, Albillos A, Perez-Paramo M, et al. Factors mediating the hemodynamic effects of tumor necrosis factor-alpha in portal hypertensive rats. Am J Physiol 1999;276:G687–93. [DOI] [PubMed] [Google Scholar]
- 19.Van De Casteele M, Omasta A, Janssens S, et al. In vivo gene transfer of endothelial nitric oxide synthase decreases portal pressure in anaesthetised carbon tetrachloride cirrhotic rats. Gut 2002;51:440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forrest EH, Jones AL, Dillon JF, et al. The effect of nitric oxide synthase inhibition on portal pressure and azygos blood flow in patients with cirrhosis. J Hepatol 1995;23:254–8. [DOI] [PubMed] [Google Scholar]
- 21.Spahr L, Martin PY, Giostra E, et al. Acute effects of nitric oxide synthase inhibition on systemic, hepatic, and renal hemodynamics in patients with cirrhosis and ascites. J Invest Med 2002;50:116–24. [DOI] [PubMed] [Google Scholar]
- 22.Knittel T, Muller L, Saile B, et al. Effect of tumour necrosis factor-alpha on proliferation, activation and protein synthesis of rat hepatic stellate cells. J Hepatol 1997;27:1067–80. [DOI] [PubMed] [Google Scholar]
- 23.Saile B, Matthes N, Knittel T, et al. Transforming growth factor beta and tumor necrosis factor alpha inhibit both apoptosis and proliferation of activated rat hepatic stellate cells. Hepatology 1999;30:196–202. [DOI] [PubMed] [Google Scholar]
- 24.Enomoto N, Takei Y, Hirose M, et al. Thalidomide prevents alcoholic liver injury in rats through suppression of Kupffer cell sensitization and TNF-alpha production. Gastroenterology 2002;123:291–300. [DOI] [PubMed] [Google Scholar]
- 25.Sarela AI, Mihaimeed FM, Batten JJ, et al. Hepatic and splanchnic nitric oxide activity in patients with cirrhosis. Gut 1999;44:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah V, Toruner M, Haddad F, et al. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology 1999;117:1222–8. [DOI] [PubMed] [Google Scholar]
- 27.Leiper J, Vallance P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res 1999;43:542–8. [DOI] [PubMed] [Google Scholar]
- 28.Lin KY, Ito A, Asagami T, et al. Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation 2002;106:987–92. [DOI] [PubMed] [Google Scholar]
- 29.Ito A, Tsao PS, Adimoolam S, et al. Novel mechanism for endothelial dysfunction: dysregulation of dimethylarginine dimethylaminohydrolase. Circulation 1999;99:3092–5. [DOI] [PubMed] [Google Scholar]
- 30.Akriviadis E, Botla R, Briggs W, et al. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000;119:1637–48. [DOI] [PubMed] [Google Scholar]
- 31.Jalan R, Forrest EH, Redhead DN, et al. Reduction in renal blood flow following acute increase in the portal pressure: evidence for the existence of a hepatorenal reflex in man? Gut 1997;40:664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]