Abstract
Background: Whether ileal absorption of bile acid is up or downregulated in chronic cholestasis is still debated, and most evidence has come from animal studies.
Aims: To compare ileal bile acid absorption in patients with primary biliary cirrhosis (PBC) and in healthy control subjects, and to assess the effect of ursodeoxycholic acid (UDCA).
Patients: We studied 14 PBC patients before and during (n=11) UDCA administration, 14 healthy control subjects, and 14 Crohn’s disease patients (as disease controls).
Methods: We used cholescintigraphy to measure retention in the enterohepatic circulation over five successive days of the bile acid analogue 75Se-homocholic acid-taurine (75SeHCAT) as an index of ileal bile acid absorption. Results were expressed as 75SeHCAT fractional turnover rate (FTR) and t½12.
Results: 75SeHCAT FTR was 0.19 (0.11)/day, 0.34 (0.11)/day (p<0.001), and 0.83 (0.32)/day in PBC patients, healthy controls (p<0.0001), and Crohn’s patients (p<0.001), respectively, which increased to 0.36 (0.16)/day in PBC patients during UDCA treatment (p<0.005). 75SeHCAT t½12 was 4.8 (2.1) days in PBC patients, 2.2 (0.5) days (p<0.001) in healthy controls, and 1.0 (0.5) days (p<0.001) in Crohn’s disease patients. 75SeHCAT t½12 decreased to 2.2 (0.93) days (p< 0.001) in PBC patients during UDCA treatment.
Conclusions: Our results support the concept that ileal bile acid absorption is upregulated in PBC patients, and that this effect may contribute towards damaging the cholestatic liver. This upregulation of bile acid absorption is abolished by UDCA.
Keywords: 75Se-homocholic acid-taurine, primary biliary cirrhosis, bile acid, ursodeoxycholic acid, intestinal absorption
Primary biliary cirrhosis (PBC) is a chronic autoimmune liver disease characterised by progressive cholestasis.1 As a consequence of chronic cholestasis, bile acid concentration may increase in serum and decrease in bile and in the small intestine, and these latter effects are likely to cause adaptive changes in expression and function of hepatic2 and intestinal transporters3 for bile acid. Intestinal adaptive changes are thought to be important in PBC4 because upregulation of intestinal bile acid transport due to reduced substrate availability may favour bile acid retention in the enterohepatic circulation to a point that the capacity of the canalicular bile acid export pump is exceeded. This effect may lead to accumulation of bile acids in the hepatocyte and to hepatocyte necrosis or apoptosis.5
The adaptive regulation of intestinal bile acid transport during cholestasis has been extensively studied but available information is based mainly on animal or ex vivo models of acute and severe cholestasis,6–12 experimental conditions unlikely to represent the pathophysiological situation of chronic cholestasis at steady state in humans. Furthermore, these animal studies have provided conflicting results as downregulation,6,8,9,11,12 upregulation,7 and lack of regulation10 of intestinal bile acid transporters or transport have been reported.
Very little information is available on ileal absorption of bile acid in humans with chronic cholestasis studied under physiological conditions. This objective can be achieved using a scintigraphic technique involving oral administration of 75Se-homocholic acid-taurine (75SeHCAT), a radiolabelled bile acid analogue of taurocholic acid.13 Previous studies have shown that 75SeHCAT behaves as the natural taurocholic acid in the overall turnover of the enterohepatic circulation14,15 with the only difference that 75SeHCAT undergoes negligible deconjugation by colonic bacteria14 and cannot be absorbed by passive non-ionic diffusion in the colon as for deconjugated bile acids. For this latter characteristic, 75SeHCAT fulfils the prerequisites for an ideal marker of ileal function16 in that it can only be absorbed by active bile acid uptake in the terminal ileum. Using 75SeHCAT scintigraphy it has been reported that intestinal absorption of bile acid in patients with chronic cholestatic liver diseases is normal17 but these results must be interpreted cautiously because the methodology used in these studies is inaccurate. By measuring 75SeHCAT retention over the whole intestinal area, this methodology cannot distinguish between 75SeHCAT activity within the enterohepatic circulation from that of 75SeHCAT retained within the colon. This colonic retention of the isotope has been reported by Ferraris and colleagues18 to cause overestimation of 75SeHCAT absorption to a variable extent in individual subjects and for different diseases by comparison with results obtained using the faecal isotope ratio, the gold standard for measurement of intestinal bile acid absorption.16,19
The aim of our study was to assess ileal absorption of bile acids in patients with chronic cholestasis due to PBC by using a validated cholescintigraphic technique.14,18 This technique involves sequential measurement of 75SeHCAT activity over the gall bladder area on five successive days following oral administration of the isotope. This technique is based on the observation in healthy subjects that a constant fraction of the bile acid pool is stored in the gall bladder in the fasting state,20 and has been shown to be independent of the phenomenon of colonic retention and to provide measurements similar to those obtained using the faecal isotope ratio.18 We have also tested the effect of ursodeoxycholic acid (UDCA), a bile acid currently used for the treatment of PBC,21 on intestinal retention of 75SeHCAT. We studied 14 patients with PBC and 14 healthy control subjects. We also studied, as a disease control group, 14 patients with ileal Crohn’s disease, a condition known to cause intestinal bile acid malabsorption. Eleven of the 14 PBC patients were studied twice, before and during UDCA administration.
PATIENTS AND METHODS
Patients
Fourteen female PBC patients entered the study. Patients characteristics are shown in table 1 ▶. Diagnosis of PBC was based on histological, serological, and immunological criteria, and all patients tested positive for antimithochondrial antibodies. None of the patient was overtly icteric on admission to the study, and serum bilirubin was slightly above the normal limit in only one patient.
Table 1.
Characteristics of the primary biliary cirrhosis (PBC) patients studied
| Pretreatment | During UDCA | ||||||||||
| Patient No | Age (y) | Body weight (kg) | Bilirubin (mg/dl) (0.3–1.2) | AP (mU/ml) (30–85) | γGT (mU/ml) (5–50) | ALT (mU/ml) (5–50) | Histology (stage) | Bilirubin (mg/dl) | AP (mU/ml) | γGT (mU/ml) | ALT (mU/ml) |
| 1† | 64 | 75 | 0.7 | 133 | 73 | 60 | II | 0.6 | 92 | 25 | 30 |
| 2† | 55 | 47 | 0.6 | 103 | 92 | 32 | II | 0.6 | 83 | 46 | 30 |
| 3† | 63 | 47 | 0.6 | 236 | 419 | 60 | II–III | 0.6 | 110 | 152 | 29 |
| 4† | 23 | 82 | 0.4 | 591 | 400 | 360 | I–II | 0.4 | 414 | 303 | 250 |
| 5† | 52 | 58 | 0.4 | 320 | 120 | 104 | I–II | 0.8 | 240 | 80 | 80 |
| 6† | 50 | 55 | 0.4 | 500 | 202 | 80 | I | 1.0 | 340 | 82 | 55 |
| 7† | 46 | 61 | 1.0 | 448 | 180 | 92 | I–II | 0.8 | 208 | 102 | 48 |
| 8† | 70 | 56 | 0.5 | 182 | 311 | 50 | II–III | 0.6 | 95 | 105 | 36 |
| 9† | 49 | 77 | 1.4 | 750 | 836 | 182 | III–IV | 1.4 | 180 | 190 | 42 |
| 10† | 40 | 60 | 0.6 | 512 | 260 | 48 | I | 0.8 | 138 | 88 | 40 |
| 11† | 50 | 50 | 0.6 | 694 | 193 | 123 | III | 0.5 | 272 | 82 | 86 |
| 12 | 57 | 58 | 1.0 | 652 | 297 | 113 | I–II | 1.0 | 657 | 317 | 50 |
| 13 | 48 | 43 | 0.5 | 748 | 226 | 81 | II | 0.6 | 236 | 309 | 57 |
| 14 | 65 | 50 | 1.0 | 282 | 310 | 125 | IV | 0.8 | 133 | 124 | 50 |
| Mean | 52 | 59 | 0.7 | 439 | 280 | 108 | 0.8 | 228*** | 143*** | 63*** | |
| SD | 12 | 12 | 0.3 | 230 | 191 | 83 | 0.3 | 158 | 98 | 56 | |
†Patients were studied twice with the 75SeHCAT test: pretreatment and during ursodeoxycholic acid (UDCA) administration.
Normal ranges for alkaline phosphatase (AP), γ-glutamyl transpeptidase (γGT), and alanine aminotransferase (ALT) are shown in parentheses.
***p<0.001 compared with pretreatment.
All 14 PBC patients underwent a 75SeHCAT study while off bile acid treatment and 11 agreed to have a repeat study during UDCA treatment. In order to avoid a sequence effect, four patients were first studied during UDCA and underwent a repeat study three months after stopping UDCA. Seven patients were studied during the opposite sequence: off treatment first and then three months after starting UDCA treatment. UDCA was administered in divided doses at mealtime, and the dose ranged from 13 to 15 mg/kg/day in individual patients.
Fourteen healthy control subjects were also studied (13 postmenopausal females and one male), of comparable age and body weight to patients with PBC (54 (4) v 52 (3) years and 61 (3) v 59 (3) kg, respectively). None of the control subjects had diarrhoea at the time of investigation. Fourteen patients with chronic or recurrent diarrhoea due to Crohn’s disease were also studied (seven males, seven females, aged 42–70 years). Crohn’s disease was diagnosed on the basis of characteristic clinical, radiological, and endoscopic findings. All 14 patients had involvement of the terminal ileum, and seven had colonic involvement. Eight of these patients had undergone previous surgical treatment with resection of the terminal ileum that was longer than 100 cm in only one patient. Written informed consent was obtained from each subject, and the study was approved by the local ethics committee.
75SeHCAT test
The 75SeHCAT test was performed as follows. On the day of the study, patients and healthy control subjects were admitted to a day case unit after fasting, and blood samples were taken for measurement of serum enzymes and serum bile acids. A capsule containing 370 kBq (10 μCi) 75SeHCAT (Amersham International, Saluggia (VC), Italy)22 was administered orally and anterior and posterior abdominal γ-camera (model SP6; Elscint, GE Medical System, Milan, Italy) counting was carried out for 300 seconds on successive 4–5 early mornings and in a fasting state. 75Se activity was calculated by selecting an area of interest over the gall bladder and following correction for background.
Laboratory methods
Serum concentrations of bilirubin, alkaline phosphatase, γ-glutamyltranspeptidase, and alanine aminotransferase were measured by standard automated laboratory techniques. Serum bile acid composition was measured by gas chromatography-mass spectrometry, as described in details elsewhere.23
Calculation and statistical methods
In order to correct for different depths of 75Se activity within the abdominal cavity, total counts over the gall bladder area of interest were obtained by relating anterior (ant) to posterior (post) counts according to the formula:
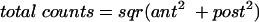 |
Assuming first order kinetics, the slope K of the decrease in 75Se activity over the gall bladder area was obtained by relating the ln of 75Se activity measured on successive days (y) versus time in days (x). The t½12 of 75SeHCAT was calculated according to the formula:
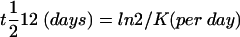 |
where K represents the fractional turnover rate (FTR) of 75SeHCAT.18
Results are expressed as means (SD). Differences between groups were tested for statistical significance using the Student’s t test for paired and unpaired observations, as appropriate. A p value <0.05 was used to indicate statistical significance of differences. Parameters of linear regression were calculated using the least squares method.
RESULTS
PBC patients versus healthy control subjects and Crohn’s disease patients
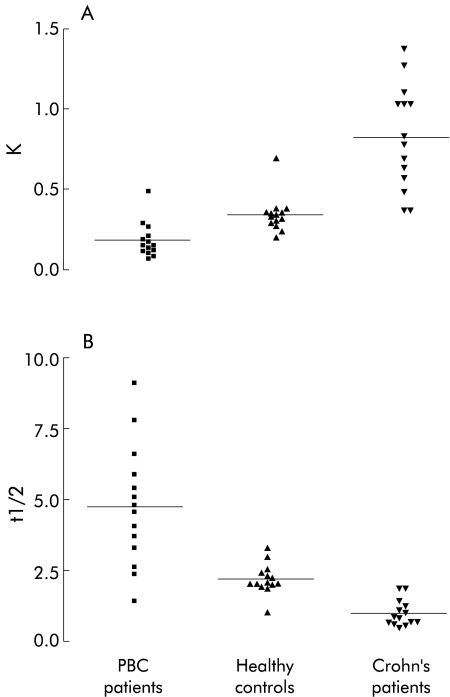
75SeHCAT FTR ranged from 0.041 to 0.487/day in PBC patients, from 0.207 to 0.690/day in healthy controls, and from 0.368 to 1.380/day in Crohn’s disease patients (fig 1A ▶). The mean value for 75SeHCAT FTR was significantly lower in PBC patients (0.182 (0.107)/day) compared with healthy controls (0.341 (0.112)/day) (p<0.0001) and Crohn’s disease patients 0.829 (0.325)/day) (p<0.0001).
Figure 1.
Daily fractional turnover rate (K) (A) and t½12 (B) of 75Se-homocholic acid-taurine (75SeHCAT) in healthy controls, Crohn’s disease patients, and in patients with primary biliary cirrhosis (PBC). Bars represent the mean value for each group.
75SeHCAT retention in the enterohepatic circulation expressed as t½12 ranged from 1.4 to 9.1 days in PBC patients, from 1.0 to 3.3 days in normal controls, and from 0.5 to 1.9 days in Crohn’s disease patients (fig 1B ▶). The mean value for 75SeHCAT t½12 was significantly higher in PBC patients (4.8 (2.1) days) compared with healthy control subjects (2.2 (0.5) days) (p<0.001) and Crohn’s disease patients (1.0 (0.5) days) (p<0.0001).
Values of 75SeHCAT FTR and t½12 in PBC patients did not correlate with the histological stage of the disease or with biochemical parameters of cholestasis or cytolysis.
Effect of UDCA treatment
During treatment, the bile acid pool enriched with UDCA and the proportion of this bile acid in serum bile acids increased from 6 (5)% before treatment to 26 (5)% during UDCA (p<0.01). Corresponding values for the primary bile acids cholic acid and chenodeoxycholic acid decreased from 30 (15)% to 21 (12)% and from 33 (11)% to 25 (11)%, respectively, but these differences were not statistically significant. The proportion of lithocholic acid increased from 8 (10)% to 13 (12)% (NS) and deoxycholic acid remained virtually unchanged (17 (9)% v 16 (11)% for pretreatment and during UDCA, respectively).
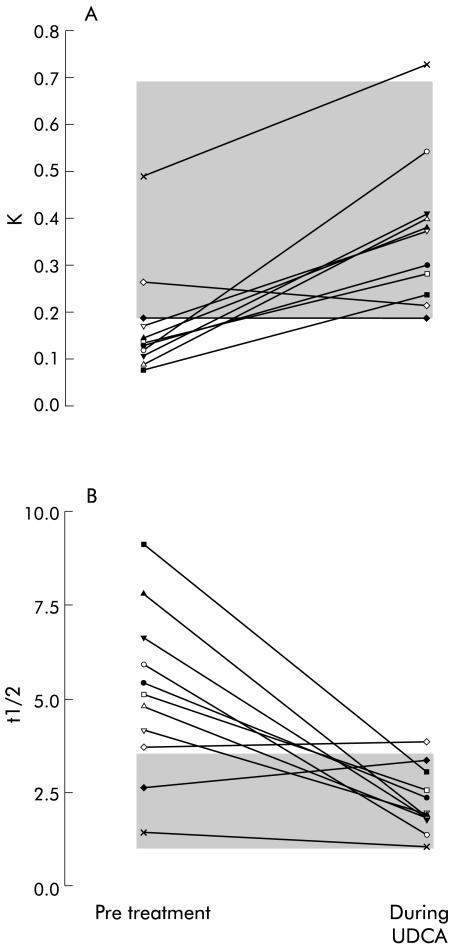
During UDCA, 75SeHCAT FTR increased in nine individual PBC patients and slightly decreased in two of 11 patients studied before and during UDCA (fig 2A ▶). These two latter patients were among the four patients with an FTR within the normal range before treatment. The mean value for 75SeHCAT FTR increased from 0.173 (0.116)/day pretreatment to 0.363 (0.157)/day (p<0.005) during UDCA.
Figure 2.
Effect of ursodeoxycholic acid (UDCA) treatment on daily fractional turnover rate (K) (A) and on t½12 (B) of 75Se-homocholic acid-taurine (75SeHCAT) in patients with primary biliary cirrhosis. The shaded area represents the range of values for healthy controls.
During UDCA, 75SeHCAT t½12 decreased in nine patients and slightly increased in two patients (fig 2B ▶). The mean value for t½12 decreased significantly from 5.1 (2.2) days pretreatment to 2.2 (0.9) days (p<0.001) during UDCA, and visual inspection of fig 1 ▶ clearly indicates that the size of this effect of UDCA was greater for patients with higher pretreatment t½12 values. The t½12 values measured during treatment fell within the range observed in healthy controls in all patients except one. The mean value for 75SeHCAT t½12 during UDCA was the same as that observed in control subjects (2.2. (0.9) days v 2.2 (0.5) days, respectively).
Serum concentrations of alkaline phosphatase, γ-glutamyltranspeptidase, and alanine aminotransferase decreased in each individual patient during UDCA (table 1 ▶). Treatment was well tolerated by all patients. No side effects were reported.
DISCUSSION
Our study indicates that retention in the enterohepatic circulation of the bile acid analogue 75SeHCAT is increased in patients with PBC compared with healthy controls, a finding in keeping with the low FTR of primary bile acid reported by other authors24,25 in these patients. Differences between PBC patients and control subjects were striking, as indicated by visual inspection of fig 1 ▶. Values for PBC patients varied widely for both 75SeHCAT FTR and t½12, probably as a result of the variable degrees of perturbation of the enterohepatic circulation in individual patients. This phenomenon may also explain the slight overlap between the two populations. As expected, 75SeHCAT FTR was increased and t½12 was reduced in patients with ileal disease or resection compared with healthy controls and PBC patients (fig 1 ▶), a finding confirming the intrinsic validity of 75SeHCAT as a test of ileal absorption.
In contrast with our results, Chazouilleres and colleagues17 reported no difference in 75SeHCAT retention in 12 PBC patients compared with control subjects but the validity of their results is questionable for two reasons. Firstly, they measured whole abdominal retention of 75SeHCAT, a measurement influenced by colonic retention of isotope, thus introducing a large error in their measurements,18 as admitted by Chazouilleres and colleagues,17 in relation to their own studies in healthy volunteers.26 Secondly, Chazouilleres and colleagues17 included five cholecystectomised patients among a total of 12 PBC patients studied. Abolition of gall bladder storage function in cholecystectomised patients has been reported to result in a decreased bile acid pool size27,28 and in an increased FTR of primary bile acids,29,30 and this latter phenomenon may have contributed to the increase in FTR of 75SeHCAT in the subgroup of cholecystectomised PBC patients studied by Chazouilleres and colleagues.17 In contrast with these authors, our study was carried out using a validated cholescintigraphic technique for measurement of ileal bile acid absorption,18 and all 14 PBC patients studied had functioning gall bladders, as ascertained by 75SeHCAT accumulation in the gall bladder area during cholescintigraphy.
We are well aware that increased retention of 75SeHCAT in the enterohepatic circulation does not prove that all natural bile acid species are retained in the same way as 75SeHCAT. 75SeHCAT handling within the enterohepatic circulation has been shown to be similar to that of taurocholic acid,14 but no information is available on comparisons between 75SeHCAT and chenodeoxycholic or deoxycholic acid. The observation that biliary bile acid composition shows little difference between healthy subjects and patients with early cholestatic disease31,32 indirectly suggests that in our study there was retention of all endogenous bile acids.
Our study strongly supports the view that chronic cholestasis in humans is accompanied by upregulation of ileal bile acid absorption. Cholestasis and/or sluggish gall bladder contraction may have theoretically contributed to the reduced 75SeHCAT FTR in our study. We believe that the effect of cholestasis was marginal because serum bile acid composition was normal in our patients, suggesting very mild cholestasis. Furthermore, gall bladder motility has been reported by others33 as normal in PBC patients.
In vitro and in vivo animal studies on the adaptive changes in the expression and function of intestinal transporters for bile acids have provided conflicting results. Downregulation of ileal bile acid uptake has been reported in rats with decreased intestinal bile acid concentrations, resulting from acute extrahepatic cholestasis, and in studies involving measurement of bile acid uptake by ileal brush border membranes.3,6,8,11 In contrast with these findings, upregulation of ileal bile acid uptake was reported by other authors in anaesthetised bile fistula guinea pigs7 during administration of cholestyramine, a bile acid sequestrant resin. The effect on ileal absorption of increasing the bile acid load to the intestine by means of bile acid feeding is also controversial,10,12 adding further uncertainty to the adaptive changes in intestinal bile acid uptake to different bile acid loads. This controversy has been explained by several factors, including species specific differences3,7 or differences between measuring transport function of the whole intestine or transporter activity in a specific intestinal segment that may not detect changes in zonal distribution of transporters.4 Furthermore, all of these studies were carried out under conditions of acute or short term changes in intestinal bile acid load, a condition extremely different from the chronic and slowly progressive cholestatic condition that characterises PBC.1 To overcome these limitations, the importance of measuring intestinal transport function in humans has been authoritatively emphasised,4 and the method used in the present study is consistent with this recommendation.
The second important observation in our study was that 75SeHCAT FTR increased and retention within the enterohepatic circulation was reduced to normal in PBC patients during UDCA treatment. These findings are consistent with the current view that UDCA administration may cause endogenous bile acid malabsorption.34–36 This effect of UDCA on endogenous bile acid absorption is not the only mechanism advocated to explain the beneficial effect of UDCA in PBC and other cholestatic liver diseases. A cytoprotective effect37,38 and an improvement in hepatobiliary excretory function have also been reported for UDCA.39,40 The scintigraphic finding of Jazrawi and colleagues40 that the reduced hepatic excretion of intravenous 75SeHCAT observed in patients with PBC improved but was not corrected by UDCA in the majority of their patients indirectly suggests that normalisation of 75SeHCAT retention during UDCA observed in our study is partly independent of the choleretic effect of this bile acid and emphasises the effect of UDCA on intestinal absorption of the isotope. The observation of Invernizzi and colleagues41 that faecal bile acid excretion is increased in PBC patients during UDCA treatment, and that this increased excretion is mainly due to secondary bile acid (lithocholic and deoxycholic acid) excretion is consistent with the hypothesis of primary bile acid ileal malabsorption during UDCA treatment with consequent colonic biotransformation to secondary bile acid.
In conclusion, our findings indicate that intestinal bile acid absorption is upregulated in PBC patients, and that this upregulation is reversed by UDCA treatment. This ability of UDCA to compete with other bile acids for ileal absorption may prevent accumulation in the hepatocyte of toxic bile acids, thus representing a mechanism to explain the beneficial effect of UDCA in PBC.
Abbreviations
PBC, primary biliary cirrhosis
75SeHCAT
75Se-homocholic acid-taurine
UDCA, ursodeoxycholic acid
FTR, fractional turnover rate
REFERENCES
- 1.Metcalf JV, Mitchison HC, Palmer JM, et al. Natural history of early primary biliary cirrhosis. Lancet 1996;348:1399–402. [DOI] [PubMed] [Google Scholar]
- 2.Zollner G, Fickert P, Zenz R, et al. Hepatobiliary transporter expression in percutaneous liver biopsies of patients with cholestatic liver diseases. Hepatology 2001;33:633–46. [DOI] [PubMed] [Google Scholar]
- 3.Grober J, Zaghini I, Fujii H, et al. Identification of a bile acid-responsive element in the human ileal bile acid-binding protein gene. Involvement of the farnesoid X receptor/9-cis-retinoic acid receptor heterodimer. J Biol Chem 1999;274:29749–54. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann AF. Regulation of ileal bile acid transport: desirability of measuring transport function as well as transporter activity. Hepatology 1999;29:1335–7. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues CM, Steer CJ. Mitochondrial membrane perturbations in cholestasis. J Hepatol 2000;32:135–41. [DOI] [PubMed] [Google Scholar]
- 6.Higgins JV, Paul JM, Dumaswala R, et al. Downregulation of taurocholate transport by ileal BBM and liver BLM in biliary-diverted rats. Am J Physiol 1994;267(4 Pt 1):G501–7. [DOI] [PubMed] [Google Scholar]
- 7.Lillienau J, Crombie DL, Munoz J, et al. Negative feedback regulation of the ileal bile acid transport system in rodents. Gastroenterology 1993;104:38–46. [DOI] [PubMed] [Google Scholar]
- 8.Dumaswala R, Berkowitz D, Heubi JE. Adaptive response of the enterohepatic circulation of bile acids to extrahepatic cholestasis. Hepatology 1996;23:623–9. [DOI] [PubMed] [Google Scholar]
- 9.Coppola CP, Gosche JR, Arrese M, et al. Molecular analysis of the adaptive response of intestinal bile acid transport after ileal resection in the rat. Gastroenterology 1998;115:1172–8. [DOI] [PubMed] [Google Scholar]
- 10.Arrese M, Trauner M, Sacchiero RJ, et al. Neither intestinal sequestration of bile acids nor common bile duct ligation modulate the expression and function of the rat ileal bile acid transporter. Hepatology 1998;28:1081–7. [DOI] [PubMed] [Google Scholar]
- 11.Sauer P, Stiehl A, Fitscher BA, et al. Downregulation of ileal bile acid absorption in bile-duct-ligated rats. J Hepatol 2000;33:2–8. [DOI] [PubMed] [Google Scholar]
- 12.Stravitz RT, Sanyal AJ, Pandak WM, et al. Induction of sodium-dependent bile acid transporter messenger RNA, protein, and activity in rat ileum by cholic acid. Gastroenterology 1997;113:1599–608. [DOI] [PubMed] [Google Scholar]
- 13.Merrick MV, Eastwood MA, Anderson JR, et al. Enterohepatic circulation in man of a gamma-emitting bile-acid conjugate, 23-selena-25-homotaurocholic acid (SeHCAT). J Nucl Med 1982;23:126–30. [PubMed] [Google Scholar]
- 14.Jazrawi RP, Ferraris R, Bridges C, et al. Kinetics for the synthetic bile acid 75selenohomocholic acid-taurine in humans: comparison with [14C]taurocholate. Gastroenterology 1988;95:164–9. [DOI] [PubMed] [Google Scholar]
- 15.Galatola G, Jazrawi RP, Bridges C, et al. Hepatic handling of a synthetic gamma-labeled bile acid (75SeHCAT). Gastroenterology 1988;94:771–8. [DOI] [PubMed] [Google Scholar]
- 16.Fromm H, Thomas PJ, Hofmann AF. Sensitivity and specificity in tests of distal ileal function: prospective comparison of bile acid and vitamin B 12 absorption in ileal resection patients. Gastroenterology 1973;64:1077–90. [PubMed] [Google Scholar]
- 17.Chazouilleres O, Marteau P, Haniche M, et al. Ileal absorption of bile acids in patients with chronic cholestasis: SeHCAT test results and effect of ursodeoxycholic acid (UDCA). Dig Dis Sci 1996;41:2417–22. [DOI] [PubMed] [Google Scholar]
- 18.Ferraris R, Jazrawi R, Bridges C, et al. Use of a gamma-labeled bile acid (75SeHCAT) as a test of ileal function. Methods of improving accuracy. Gastroenterology 1986;90(5 Pt 1):1129–36. [DOI] [PubMed] [Google Scholar]
- 19.Woodbury JF, Kern F Jr. Fecal excretion of bile acids: a new technique for studying bile acid kinetics in patients with ileal resection. J Clin Invest 1971;50:2531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jazrawi RP, Bridges C, Joseph AE, et al. Effects of artificial depletion of the bile acid pool in man. Gut 1986;27:771–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poupon RE, Lindor KD, Cauch-Dudek K, et al. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology 1997;113:884–90. [DOI] [PubMed] [Google Scholar]
- 22.Soundy RG, Simpson JD, Ross HM, et al. Absorbed dose to man from the Se-75 labeled conjugated bile salt SeHCAT: concise communication. J Nucl Med 1982;23:157–61. [PubMed] [Google Scholar]
- 23.Scalia S, Scagliarini R, Pazzi P. Evaluation of ursodeoxycholic acid bioavailability from immediate- and sustained-release preparations using gas chromatography-mass spectrometry and high-performance liquid chromatography. Arzneimittelforschung 2000;50:129–34. [PubMed] [Google Scholar]
- 24.Vlahcevic ZR, Juttijudata P, Bell CC Jr, et al. Bile acid metabolism in patients with cirrhosis. II. Cholic and chenodeoxycholic acid metabolism. Gastroenterology 1972;62:1174–81. [PubMed] [Google Scholar]
- 25.Raedsch R, Lauterburg BH, Hofmann AF. Altered bile acid metabolism in primary biliary cirrhosis. Dig Dis Sci 1981;26:394–401. [DOI] [PubMed] [Google Scholar]
- 26.Fellous K, Jian R, Haniche M, et al. Measurement of ileal absorption of bile salts with the selenium 75 labelled homotaurocholic acid test. Validation and clinical significance. Gastroenterol Clin Biol 1994;18:865–72. [PubMed] [Google Scholar]
- 27.Pomare EW, Heaton KW. The effect of cholecystectomy on bile salt metabolism. Gut 1973;14:753–62. [PMC free article] [PubMed] [Google Scholar]
- 28.Shaffer EA, Small DM. Biliary lipid secretion in cholesterol gallstone disease. The effect of cholecystectomy and obesity. J Clin Invest 1977;59:828–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almond HR, Vlahcevic ZR, Bell CC Jr, et al. Bile acid pools, kinetics and biliary lipid composition before and after cholecystectomy. N Engl J Med 1973;289:1213–16. [DOI] [PubMed] [Google Scholar]
- 30.Roda E, Aldini R, Mazzella G, et al. Enterohepatic circulation of bile acids after cholecystectomy. Gut 1978;19:640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crosignani A, Podda M, Battezzati PM, et al. Changes in bile acid composition in patients with primary biliary cirrhosis induced by ursodeoxycholic acid administration. Hepatology 1991;14:1000–7. [PubMed] [Google Scholar]
- 32.Greim H, Trulzsch D, Czygan P, et al. Mechanism of cholestasis. 6. Bile acids in human livers with or without biliary obstruction. Gastroenterology 1972;63:846–50. [PubMed] [Google Scholar]
- 33.van de Meeberg PC, Portincasa P, Wolfhagen FH, et al. Increased gall bladder volume in primary sclerosing cholangitis. Gut 1996;39:594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stiehl A, Raedsch R, Rudolph G. Ileal excretion of bile acids: comparison with biliary bile composition and effect of ursodeoxycholic acid treatment. Gastroenterology 1988;94(5 Pt 1):1201–6. [DOI] [PubMed] [Google Scholar]
- 35.Stiehl A, Raedsch R, Rudolph G. Acute effects of ursodeoxycholic and chenodeoxycholic acid on the small intestinal absorption of bile acids. Gastroenterology 1990;98:424–8. [DOI] [PubMed] [Google Scholar]
- 36.Stiehl A. Intestinal absorption of bile acids: effect of ursodeoxycholic acid treatment. Ital J Gastroenterol 1995;27:193–5. [PubMed] [Google Scholar]
- 37.Galle PR, Theilmann L, Raedsch R, et al. Ursodeoxycholate reduces hepatotoxicity of bile salts in primary human hepatocytes. Hepatology 1990;12(3 Pt 1):486–91. [DOI] [PubMed] [Google Scholar]
- 38.Heuman DM, Pandak WM, Hylemon PB, et al. Conjugates of ursodeoxycholate protect against cytotoxicity of more hydrophobic bile salts: in vitro studies in rat hepatocytes and human erythrocytes. Hepatology 1991;14:920–6. [DOI] [PubMed] [Google Scholar]
- 39.Colombo C, Castellani MR, Balistreri WF, et al. Scintigraphic documentation of an improvement in hepatobiliary excretory function after treatment with ursodeoxycholic acid in patients with cystic fibrosis and associated liver disease. Hepatology 1992;15:677–84. [DOI] [PubMed] [Google Scholar]
- 40.Jazrawi RP, de Caestecker JS, Goggin PM, et al. Kinetics of hepatic bile acid handling in cholestatic liver disease: effect of ursodeoxycholic acid. Gastroenterology 1994;106:134–42. [DOI] [PubMed] [Google Scholar]
- 41.Invernizzi P, Setchell KD, Crosignani A, et al. Differences in the metabolism and disposition of ursodeoxycholic acid and of its taurine-conjugated species in patients with primary biliary cirrhosis. Hepatology 1999;29:320–7. [DOI] [PubMed] [Google Scholar]