Abstract
The invasive stages of Toxoplasma gondii, an Apicomplexan parasite, actively invade their host cells in an actin-dependent way. However, despite containing biochemically significant amounts of actin, actin filaments have never been observed in these parasites. Jasplakinolide, a membrane-permeable actin-polymerizing and filament-stabilizing drug, induced the polymerization of actin filaments at the anterior end of each tachyzoite in association with the conoid, where they formed, in many cases, a prominent membrane-enclosed apical projection reminiscent of acrosomal processes of invertebrate sperm. These jasplakinolide-induced filaments decorated with myosin subfragment 1, demonstrating unequivocally that they were indeed actin. Jasplakinolide-treated tachyzoites were unable to invade host cells, but once the drug was removed the parasites were able to enter host cells. Actin polymerization at the apical end of the parasite is consistent with the role of the apical end in host-cell invasion powered by a jackhammer-like extension and retraction of the conoid complex coupled to the secretion and rearward capping of surface proteins.
Actin is the single most abundant protein in eukaryotic cells. As well as providing part of the framework for the structural organization of the cell, the dynamic assembly and disassembly of actin filaments drives cell motility (1). The invasive stages of many members of the phylum Apicomplexa, including Plasmodium sp., the causative agent of malaria, and Toxoplasma gondii, a major contributor to death in AIDS patients, are highly motile and actively invade target host cells. It is well established that reagents that interfere with actin dynamics in these parasites block motility and host-cell invasion (2–6). Although actin is present biochemically in significant amounts (7–9), little is known about the structural organization of the actin cytoskeleton in these organisms. Actin and myosin have been localized by immunoelectron microscopy to the apical end of the parasite and in the space between the plasma membrane and the outer face of the inner membrane complex (IMC) in both Toxoplasma and in Plasmodium falciparum merozoites (10–14). Nevertheless, examination of parasites fixed by using a variety of electron microscopy protocols fails to reveal any filamentous material within the parasites that might represent actin (15). In fact, recent evidence from Toxoplasma implies that most of the cellular actin is sequestered as monomeric G-actin (9).
To gain a better understanding of the organizational dynamics of the actin cytoskeleton in an apicomplexan parasite, we have used jasplakinolide, a membrane-permeable actin-polymerizing and filament-stabilizing drug (16), to visualize actin filaments in T. gondii tachyzoites. When we treated isolated tachyzoites with jasplakinolide, actin filaments were found primarily at the anterior end of each tachyzoite in association with the conoid, where they formed, in many cases, a prominent membrane-enclosed apical projection reminiscent of acrosomal processes of invertebrate sperm (17). Our results define the conoid and apical complex as a major site of actin polymerization in Toxoplasma, consistent with the role of the apical end in host-cell invasion and polarized secretion.
MATERIALS AND METHODS
Parasites and Host Cells.
The RH strain of T. gondii was maintained by serial passage in confluent monolayers of primary human foreskin fibroblasts (18). Parasites were harvested shortly after complete lysis of the host-cell monolayer and purified by filtration through a 3-μm pore-sized polycarbonate filter.
Reagents and Treatment of Isolated Tachyzoites.
Jasplakinolide (Molecular Probes) was prepared as a 1-mM stock in DMSO and aliquots stored at −20°C.
Samples of isolated tachyzoites (≈107 parasites/ml) in suspension were incubated with 1 μM jasplakinolide for 5 min or 60 min at 37°C before being processed for electron microscopy or myosin subfragment 1 (S1) decoration, as described below. To examine the energy-dependent nature of filament formation, isolated tachyzoites were treated with 1 mM potassium cyanide and 10 mM sodium azide for 5 min immediately before the addition of 1 μM jasplakinolide. The samples were then incubated for 60 min and fixed for electron microscopy.
Host-Cell Invasion Assay.
Human foreskin fibroblasts were grown in 35 mm Falcon Petri dishes (Becton Dickinson) containing a 22 mm no. 1 glass coverslip (Corning). To examine the effects of jasplakinolide on parasite entry, free tachyzoites were incubated with 1 μM drug for 10 min at 37°C and then added to human foreskin fibroblasts. The parasites were allowed 30 min at 37°C to invade host cells, after which all free uninvaded parasites were aspirated and the cultures washed several times with medium to remove all attached parasites. The cultures were then incubated for ≈6 h (37°C) in a humidified atmosphere containing 5% CO2. In a second set of experiments, to assess the reversibility of jasplakinolide on parasite invasion at the end of the initial 30-min infection period, all free uninvaded parasites were carefully aspirated. The cultures were carefully washed several times with medium to remove any trace of drug but not sufficiently to dislodge attached parasites and then incubated for 24 h (37°C).
For both sets of experiments, at the end of the incubation period the coverslips were taken and the infected cells fixed in methanol at −20°C. To assess the effects of jasplakinolide on parasite entry, both the numbers of host cells infected and the numbers of parasites per infected host cell were counted by direct visualization by using a Zeiss microscope with phase objectives. By 6-h postentry, internalized parasites are surrounded by a clear halo within the parasitophorous vacuole; only those parasites clearly within a parasitophorous vacuole were scored as internalized. To assess the reversibility of jasplakinolide on parasite invasion, parasite replication was assessed by counting the numbers of parasites per vacuole after 24-h incubation. To ensure random counting, fields from all regions of the coverslip were selected without prior microscopic examination, and all vacuoles within each field were counted. In all experiments, the number of parasites per vacuole was determined for between 500–700 vacuoles from at least three separate experiments. Statistical comparisons of the infection rates and the numbers of parasites per infected host cell were performed by using Student’s t test with a P value of 0.05 as the cutoff for significance.
Electron Microscopy.
For electron microscopy, all samples of isolated extracellular tachyzoites were fixed in suspension with a freshly prepared mixture containing 1% glutaraldehyde (made up from an 8% stock purchased from Electron Microscopy Sciences, Fort Washington, PA) and 1% osmium tetroxide in 50 mM phosphate buffer (pH 6.2). The fixative was added at room temperature and the samples then placed on ice (4°C) for 45 min. After fixation, all samples were then pelleted, rinsed with distilled water to remove excess phosphate ions, and en bloc stained with 0.5% aqueous uranyl acetate for 6–16 h at 4°C. Samples were dehydrated with acetone and embedded in an Epon–Araldite mix.
Ultrathin sections (50–70 nm thick) were cut, double stained with uranyl acetate and lead citrate, and examined by using a Philips 200 electron microscope.
Decoration of Filaments with S1.
Isolated tachyzoites were incubated with 1 μM jasplakinolide (60 min at 37°C) and pelleted. As controls, free tachyzoites to which an equivalent amount of DMSO had been added were also incubated for 60 min at 37°C. Samples were detergent extracted for 5 min at 4°C by using 0.2% Triton X-100 in 50 mM phosphate buffer (pH 6.8) with 3 mM MgCl2. The detergent solution was decanted and 5 mg/ml S1 in 100 mM phosphate buffer (pH 6.8) was added. The samples were decorated for 10 min on ice and then for a further 20–30 min at room temperature, after which the specimens were gently washed in 100 mM phosphate buffer for 20 min to remove unbound S1. The samples were fixed in a mixture of 1% glutaraldehyde and 2% tannic acid in 50 mM phosphate buffer (pH 6.8) for 30 min at room temperature, washed several times with buffer, and postfixed in 1% OsO4. Samples were rinsed with distilled water to remove excess phosphate ions and en bloc stained with 0.5% aqueous uranyl acetate. Samples were dehydrated with acetone and embedded in an Epon–Araldite mix.
Immunoelectron Microscopy.
Jasplakinolide-treated parasites were fixed in suspension in a mixture of 4% formaldehyde and 0.5% glutaraldehyde in 100 mM phosphate buffer (pH 7.0). The parasites were pelleted, dehydrated through increasing concentrations of ethanol to 70%, and embedded in LR white (Electron Microscopy Sciences). Polymerization was carried out at 37°C for 5 days and 50- to 70-nm sections cut and mounted on nickel grids. Sections were incubated with an antiactin monoclonal (Amersham Pharmacia; diluted 1:100 (vol/vol) in PBS-glycine) followed by a rabbit anti-mouse IgG conjugated to 5-nm gold particles (British BioCell International, Cardiff, U.K.). Sections were stained with 1% uranyl acetate in 30% methanol and examined by using a Philips 200 electron microscope.
Scanning Electron Microscopy.
Jasplakinolide-treated tachyzoites were allowed to attach to poly-l-lysine coated coverslips, and then fixed in 2% glutaraldehyde in 100 mM phosphate buffer (pH 7.0). The samples were dehydrated in ethanol, critical point dried, sputter coated, and examined in an AMR 1000 (Amray, Bedford, MA) scanning electron microscope.
RESULTS
Background.
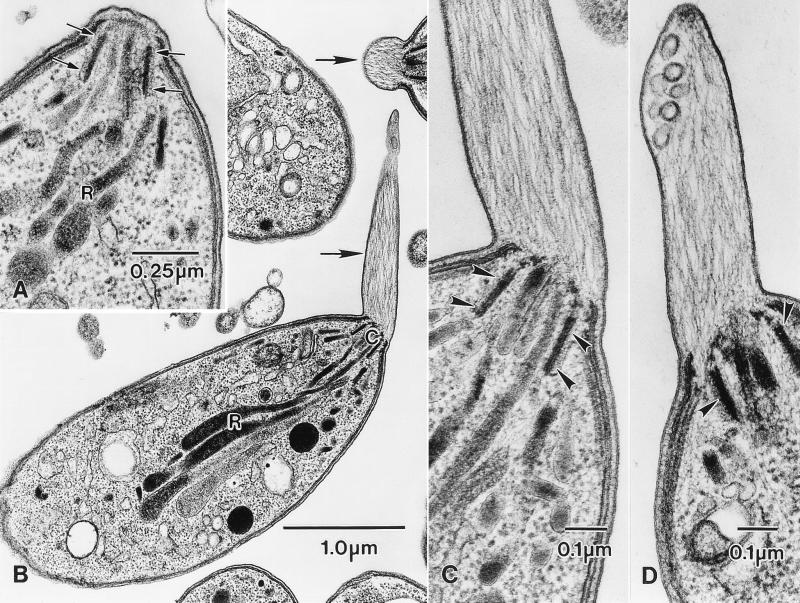
Toxoplasma tachyzoites are highly polarized cells with an elaborate cytoskeleton that has been described in detail previously (Fig. 1A; refs. 19, 20). Briefly, the cytoskeleton consists of a subpellicular cytoskeleton comprising the IMC (a pair of closely apposed membranes that form a discontinuous set of sheets immediately beneath the outer plasma membrane) and an associated array of 22 microtubules, and the apical conoid complex. The conoid complex consists of a set of polar rings and a hollow cone of spirally arranged tubular structures (20). It is through the center of the conoid complex that the parasite discharges the contents of its secretory organelles (the rhoptries and micronemes) during host-cell entry. Of importance to the present study is the fact that nothing resembling actin filaments has been found in any part of the parasite. The only filamentous material seen in the parasite is found associated with the anterior end of the nucleus around the Golgi complex.
Figure 1.
Jasplakinolide induces the formation of actin-containing apical extensions in isolated T. gondii tachyzoites. (A) High magnification of the apical end of a tachyzoite from an untreated control culture showing the walls of the conoid (arrows) with the necks of the rhoptries (R) closely associated with it. Apart from some fuzzy material associated with the conoid, there is no filamentous material in any part of the apical region. (B) Low-power electron micrograph of isolated tachyzoites treated with 1 μM jasplakinolide for 60 min at 37°C. Filament-containing apical extensions are present in two of the parasites (arrows). C, conoid complex; R, rhoptries. (C and D) Higher-magnification micrographs of the apical end of jasplakinolide-treated tachyzoites showing the membrane-bounded apical extensions, each of which contains many filaments extending from the conoid. Note also the presence of membrane-bounded vesicles and membrane-associated dense material at the anterior end of the protrusions (D). In C and D, arrowheads denote the walls of the conoid.
Jasplakinolide Induces the Assembly of Actin Filaments Around the Conoid that Results in the Formation of an Acrosomal-Like Apical Protrusion.
When we incubated isolated tachyzoites with jasplakinolide (1–5 μM at 37°C), the parasites became more active; they twisted and flexed and moved over a substratum more rapidly than control tachyzoites. When we examined the isolated tachyzoites treated with jasplakinolide (1 μM for 60 min at 37°C) by thin-section electron microscopy, we found extensive accumulations of filamentous material in most but not all of the parasites examined. The bulk of this filamentous material originated from the apical end of each parasite and in many cases produced a prominent membrane-bounded protrusion from the conoid complex (Fig. 1 B–D), reminiscent of acrosomal processes of invertebrate sperm (17). These apical protrusions can be up to 2 μM long, with the filaments aligned approximately parallel to the longitudinal axis of the protrusion (Fig. 1B). In all parasites examined, jasplakinolide-induced polymerization of actin filaments at the apical end of the parasite did not result in the extrusion of the conoid or in the loss of any of the apical secretory organelles. In untreated parasites, nothing resembling the apical protrusion or the presence of filaments around the conoid was ever observed.
Although the jasplakinolide-induced apical protrusion and the filaments around the conoid complex were the most dramatic effects observed, in some of these parasites filamentous material was also found directly beneath the subpellicular IMC-microtubule cytoskeleton, and in the posterior regions of some parasites associated with the basal ring complex. Again the filaments in these bundles were not randomly orientated within the cytoplasm but were aligned with the main axis of each bundle running parallel to the apical-basal axis of the cell. It should be emphasized that in untreated parasites processed by using the same fixation protocols as well as others, actin filaments or anything resembling these accumulations of filamentous material have never been observed.
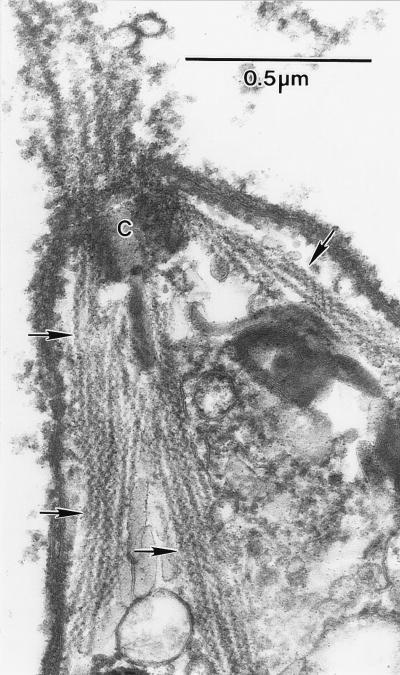
To confirm that these jasplakinolide-induced filaments were indeed actin, permeabilized parasites were treated with the S1. All the jasplakinolide-induced filaments labeled with the S1 fragment (Fig. 2) demonstrating unequivocally that these filaments were indeed actin. Again, the parallel arrays of S1 labeled filaments demonstrates that the jasplakinolide-induced polymerization/stabilization of actin filaments was not a random process. In contrast, no filaments or structures were labeled with S1 fragments in untreated parasites consistent with actin filaments have never been observed in these parasites. Unfortunately, the S1-decorated filaments were too close together to enable us to accurately determine the polarity of individual filaments. In addition to S1 decoration, we labeled sections of LR white-embedded jasplakinolide-treated parasites with an antibody against actin and a gold-conjugated secondary antibody. Both the filaments in the apical protrusions and the bundles of filaments in the body of the parasites were labeled (data not shown). We also attempted to stain jasplakinolide-treated parasites with rhodamine-conjugated phalloidin but were not successful, consistent with previous reports showing that phalloidin does not bind to Toxoplasma actin (9).
Figure 2.
Anterior end of an isolated tachyzoite that had been detergent extracted and decorated with S1 before fixation. Although the majority of filaments in the anterior extension have been lost, those adjacent to the conoid (C) and extending into the body of the parasite are clearly label with S1 (arrows).
The Formation of the Apical Protrusion and the Bundles of Actin Filaments Within the Parasite Is an Energy-Dependent Process.
When we pretreated isolated tachyzoites with a mixture of 10 mM sodium azide and 1 mM KCN for 5 min before adding 1 μM jasplakinolide and incubating the parasites for 60 min at 37°C, few apical protrusions or accumulations of filamentous material were seen. This result implies that the jasplakinolide-induced formation of the apical protrusion and bundles of actin filaments was an energy-dependent process. The energy-dependent nature of jasplakinolide-induced actin polymerization helps explain why not all treated tachyzoites possessed an apical protrusion because populations of isolated tachyzoites invariably contain a proportion of dead or dying cells.
Jasplakinolide Causes a Reversible Inhibition of Tachyzoite Entry.
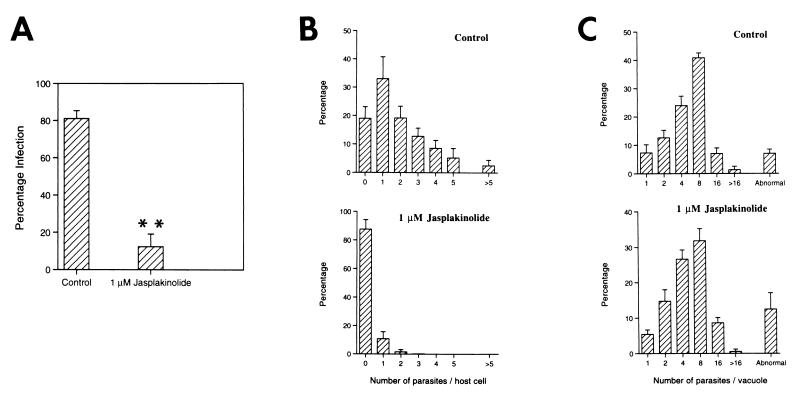
Because tachyzoite motility and host-cell entry are actin-dependent processes that are blocked by cytochalasins, we examined the effects of jasplakinolide on parasite entry. Although jasplakinolide caused isolated tachyzoites to move more rapidly, pretreating them with 1 μM jasplakinolide (10 min at 37°C) before adding them to host cells in the continued presence of jasplakinolide significantly blocked parasite invasion (Fig. 3 A and B). When the drug and all unattached parasites were carefully aspirated and replaced by clean medium, those parasites attached to host cells were able to invade and initiate intracellular growth and replication (Fig. 3C), showing that the inhibition was reversible. In addition, the percentage of host cells infected after jasplakinolide treatment and washout was not significantly lower than untreated control cultures. The reversibility of jasplakinolide-induced inhibition of cell invasion is consistent with the recent report of an actin depolymerizing factor in Toxoplasma (21).
Figure 3.
Jasplakinolide reversibly blocks tachyzoite entry into host cells. Tachyzoites were treated with 1 μM jasplakinolide for 10 min (37°C) before being added to human foreskin fibroblasts and allowed 30 min to invade still in the presence of drug. Control cultures had an equivalent amount of DMSO added. All extracellular parasites (both free and those attached to the host-cell surface) were removed by washing the monolayers several times with clean medium, and the cultures were incubated for ≈6 h (A and B) or 24 h (C) at 37°C before parasite numbers were counted. Jasplakinolide caused significant inhibition of parasite entry, as determined by both the percentage of infected host cells (A) and the numbers of parasites per host cell (B). Note in A, ∗∗, percentage of infected host cells significantly different from controls, P > 0.001. (C) When jasplakinolide and all free unattached parasites were removed from the cultures, those parasites bound to the host cells were able to invade and replicate in the host cells in an identical manner to untreated control cultures. Counts of the numbers of parasites per vacuole after 24 h. In all cases, the results represent the means ± SD from three replicate experiments.
DISCUSSION
In this study, we used jasplakinolide to polymerize and stabilize actin filaments in isolated tachyzoites and found filaments primarily at the anterior end of each tachyzoite in association with the conoid, where they formed a prominent membrane-enclosed apical projection reminiscent of acrosomal processes of invertebrate sperm. It is possible that the formation of these actin-containing apical protrusions in Toxoplasma tachyzoites was either the consequence of there being a large pool of actin monomer in the parasite (9) and/or of the existence of a localized region of actin nucleation or of a drug-induced redistribution of preexisting actin filaments. Although we cannot totally discount either possibility, the highly organized nature of the jasplakinolide-induced filaments in the apical protrusions implies that the drug is not polymerizing/stabilizing filaments in a random manner. In addition, by immunoelectron microscopy we can still localize actin to the space between the plasma membrane and the outer face of the IMC, demonstrating that the formation of the apical protrusions was unlikely the result of a drug-induced redistribution of preexisting actin filaments. Thus, what is interesting in Toxoplasma is that jasplakinolide-induced polymerization and/or stabilization of actin filaments occurred at specific sites and not randomly throughout the parasite. This localized polymerization of actin filaments is in contrast to the effects of jasplakinolide in other cell types, including the host cells in which we grow tachyzoites. In these cases, the drug causes the formation of large accumulations of randomly orientated actin filaments throughout the cytoplasm (ref. 22; M.K.S. and L.G.T., unpublished observations). By contrast, when we treated 24-h-old replicating intracellular parasites with jasplakinolide (1–5 μM), although large accumulations of randomly orientated actin formed in the host cells, only small numbers of actin filaments developed in the body of the parasites, and apical protrusions were only commonly seen parasites in older cultures (30–48 h after entry) (M.K.S. and L.G.T., unpublished observations). The lack of apical protrusions in 24-h-old intracellular tachyzoites is consistent with the fact that at this age the parasites do not move within the host cell. Nevertheless, 24-h-old intracellular tachyzoites clearly have everything they need to be motile, because treating them with ionophore or DTT induces them to move and rapidly egress from the host cell. Collectively, our data imply that the effect of jasplakinolide on extracellular tachyzoites was to enhance a normal cellular process and points to the apical region being a major site of actin filament polymerization in Toxoplasma.
Because jasplakinolide seems to enhance a normal cellular process, it raises the question of the biological significance of localized actin polymerization in Toxoplasma tachyzoites. To put it another way, what is special about the conoid and apical ends of the parasite? R. Mondragon and E. Frixione (23) showed that the tachyzoite reversibly extends the apical conoid complex in a Ca2+- and actin-dependent manner during gliding and host-cell entry. During host-cell entry, the conoid seems almost to act as a jackhammer moving back and forth as the parasite pushes its way into the host cell. Localized cycles of polymerization and depolymerization of actin at the apical end of the parasite could provide this force. This interpretation is consistent with the observation that jasplakinolide-treated parasites (in which actin has been assembled into filaments) fail to enter the host cells, but if the drug is washed out, parasites can enter. Interestingly, however, jasplakinolide-induced polymerization at the apical end of the parasite did not result in the extrusion of the conoid or in the loss of any of the apical secretory organelles.
We know from the work of several groups (2–6, 24, 25) that parasite motility and host-cell entry are actin dependent and involve the secretion of proteins onto the parasite surface and the subsequent rearward translocation of these proteins. When we forced the parasite to nucleate its actin by treating tachyzoites with jasplakinolide, although they moved more rapidly, they did not enter host cells consistent with invasion being dependent on a dynamic actin–myosin motor system. In the present study, we did not determine whether parasite attachment or internalization was the point of inhibition of host-cell entry. Because the percentage of host cells infected after jasplakinolide treatment and washout was not significantly lower than untreated control cultures, we suspect that the internalization stage is the point of inhibition. In addition, whereas the treatment and washout experiments show that this jasplakinolide-induced blocking of invasion is reversible, we do not know whether tachyzoites with apical protrusions are able to attach to host cells and/or whether they can subsequently invade.
Current models for the organization of the actin–myosin motor system in apicomplexan parasites envisage actin filaments lying parallel to the plasma membrane in the space between the IMC and plasma membrane (14). In one model, the actin filaments are associated with the cytoplasmic tails of transmembrane surface molecules and are moved by myosin molecules attached to the outer face of the IMC. Movement of the actin filaments in a posterior direction would result in the rearward capping of associated surface molecules, thereby driving gliding and host-cell entry. At present, the limited spatial resolution of current immunoelectron microscopy methods precludes the precise localization of the actin and myosin molecules in relation to the IMC and plasma membrane. However, the actin-driven rearward capping of surface proteins would require the continual polymerization of actin at the apical end of the parasite. The ability of jasplakinolide to induce actin filament polymerization at the apical end of motile Toxoplasma tachyzoites would be consistent with a motor system based on the dynamic nucleation and translocation of actin filaments.
Thus, in Toxoplasma, the presence of highly localized actin polymerization specifically at the apical end of the parasites is consistent with both gliding motility and host-cell invasion powered by the secretion and rearward capping of surface proteins and with conoid extension and retraction. The invasive stages of other apicomplexan parasites (e.g., Plasmodium sp., Cryptosporidium parvum) are also motile and invade host cells in an actin-dependent manner. Our results, therefore, raise the possibility that highly localized polymerization of actin filaments at the apical end of the parasite may provide the driving force for actin-dependent motility and invasion in these related parasites.
Acknowledgments
We are grateful to David Roos for a source of Toxoplasma tachyzoites and to Kelly Vranich for technical help. This work was supported by National Institutes of Health Grant GM-52857 to L.G.T.
ABBREVIATIONS
- IMC
inner membrane complex
- S1
myosin subfragment 1
References
- 1.Mitchison T J, Cramer L P. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- 2.Miller L H, Aikawa M, Johnson J G, Shiroishi T. J Exp Med. 1979;149:172–184. doi: 10.1084/jem.149.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell D G, Sinden R E. J Cell Sci. 1981;50:345–359. doi: 10.1242/jcs.50.1.345. [DOI] [PubMed] [Google Scholar]
- 4.Morisaki J H, Heuser J E, Sibley L D. J Cell Sci. 1995;108:2457–2464. doi: 10.1242/jcs.108.6.2457. [DOI] [PubMed] [Google Scholar]
- 5.Dobrowolski J M, Sibley L D. Cell. 1996;84:933–939. doi: 10.1016/s0092-8674(00)81071-5. [DOI] [PubMed] [Google Scholar]
- 6.Forney J R, Vaughan D K, Yang S, Healey M C. J Parasitol. 1998;84:908–913. [PubMed] [Google Scholar]
- 7.Field S J, Pinder J C, Clough B, Dluzewski A R, Wilson R J M, Gratzer W B. Cell Motil Cytoskeleton. 1993;25:43–48. doi: 10.1002/cm.970250106. [DOI] [PubMed] [Google Scholar]
- 8.Webb S E, Fowler R E, O’Shaughnessy C, Pinder J C, Dluzewski A R, Gratzer W B, Bannister L H, Mitchell G H. Parasitology. 1996;112:451–457. doi: 10.1017/s0031182000076915. [DOI] [PubMed] [Google Scholar]
- 9.Dobrowolski J M, Niesman I R, Sibley L D. Cell Motil Cytoskeleton. 1997;37:253–262. doi: 10.1002/(SICI)1097-0169(1997)37:3<253::AID-CM7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Schwartzman J D, Pfefferkorn E R. J Protozool. 1983;30:657–661. doi: 10.1111/j.1550-7408.1983.tb05339.x. [DOI] [PubMed] [Google Scholar]
- 11.Cintra W M, DeSouza W. J Submicrosc Cytol. 1985;17:503–508. [PubMed] [Google Scholar]
- 12.Yasuda T, Yagita K, Nakamura T, Endo T. Parasitol Res. 1988;75:107–113. doi: 10.1007/BF00932709. [DOI] [PubMed] [Google Scholar]
- 13.Dobrowolski J M, Carruthers V B, Sibley L D. Mol Microbiol. 1997;26:163–173. doi: 10.1046/j.1365-2958.1997.5671913.x. [DOI] [PubMed] [Google Scholar]
- 14.Pinder J C, Fowler R E, Dluzewski A R, Bannister L H, Lavin F M, Mitchell R J M, Gratzer W B. J Cell Sci. 1998;111:1831–1839. doi: 10.1242/jcs.111.13.1831. [DOI] [PubMed] [Google Scholar]
- 15.Bannister L H, Mitchell G H. Ann Trop Med Parasitol. 1995;89:105–111. doi: 10.1080/00034983.1995.11812940. [DOI] [PubMed] [Google Scholar]
- 16.Bubb M R, Senderowicz A M J, Sausville E A, Duncan K L M, Korn E D. J Biol Chem. 1994;269:14869–14871. [PubMed] [Google Scholar]
- 17.Tilney L G. In: Biology of Fertilization. Metz C, Monroy A, editors. Vol. 2. New York : Academic; 1985. pp. 157–213. [Google Scholar]
- 18.Roos D S, Donald R G D, Morrissette N S, Moulton A L C. Methods Cell Biol. 1994;45:28–61. doi: 10.1016/s0091-679x(08)61845-2. [DOI] [PubMed] [Google Scholar]
- 19. Chobotar B, Scholtyseck E. In: The Biology of the Coccidia. Long P L, editor. Baltimore: Univ. Park; 1982. pp. 101–165. [Google Scholar]
- 20.Nichols B, Chiappino M. J Protozool. 1987;34:217–226. doi: 10.1111/j.1550-7408.1987.tb03162.x. [DOI] [PubMed] [Google Scholar]
- 21.Allen M L, Dobrowolski J M, Muller H, Sibley L D, Mansour T E. Mol Biochem Parasitol. 1997;88:43–52. doi: 10.1016/s0166-6851(97)00069-8. [DOI] [PubMed] [Google Scholar]
- 22.Holzinger A, Meindl U. Cell Motil Cytoskeleton. 1997;38:365–372. doi: 10.1002/(SICI)1097-0169(1997)38:4<365::AID-CM6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Mondragon R, Frixione E. J Eukaryotic Microbiol. 1996;43:120–127. doi: 10.1111/j.1550-7408.1996.tb04491.x. [DOI] [PubMed] [Google Scholar]
- 24.Sultan A A, Thathy V, Frevert U, Robson K L H, Crisanti A, Nussenweig V, Nussenweig R S, Menard R. Cell. 1997;90:511–522. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- 25.Carruthers, V. B. & Sibley, L. D. Eur. J. Cell Biol.73, 114–123. [PubMed]