Abstract
Background and aims: Dietary fat plays a role in the pathophysiology of symptoms in functional dyspepsia (FD). In healthy subjects, cognitive factors enhance postprandial fullness; in FD patients, attention increases gut perception. We hypothesised that the information given to patients about the fat content of a meal would affect dyspeptic symptoms.
Methods: Fifteen FD patients were each studied on four occasions in a randomised double blind fashion. Over two days they ingested a high fat yoghurt (HF) and over the other two days a low fat yoghurt (LF). For each yoghurt, the patients received the correct information about its fat content on one day (HF-C, LF-C) and the opposite (wrong) information on the other day (HF-W, LF-W). Dyspeptic symptoms, plasma cholecystokinin (CCK) concentrations, and gastric volumes were evaluated.
Results: Both the fat content and information about the fat content affected fullness and bloating scores—both were higher after HF-C compared with LF-C, and LF-W compared with LF-C, with no differences between HF-C and HF-W. Nausea scores were higher after HF compared with LF, with no effect of the information about fat content. No differences between discomfort and pain scores were found between study conditions. Plasma CCK and gastric volumes were greater following HF compared with LF, with no effect of the information given to the patients. All differences are p<0.05.
Conclusions: Cognitive factors contribute to symptom induction in FD. Low fat foods may also elicit symptoms if patients perceive foods as high in fat, while CCK and gastric volumes do not appear to be affected by cognitive factors.
Keywords: dietary fat, cholecystokinin, cognitive factors, dyspepsia, functional dyspepsia
There is growing evidence that nutrients, particularly fat, play an important role in the pathophysiology of functional dyspepsia (FD).1–4 Many patients with FD report that their symptoms are exacerbated after ingestion of foods containing fat. Ingestion of a high fat soup induces more severe symptoms than the same soup without fat.3 Moreover, duodenal fat infusion results in a dose related increase in the severity of dyspeptic symptoms in these patients.5 This effect appears to be specific for fat as only duodenal infusion of fat, but not glucose, induces the symptomatic response.6
A number of mechanisms may, at least in part, mediate the effects of fat on dyspeptic symptoms. Fat may act indirectly via changes in gastrointestinal motility, for example through an increase in proximal gastric relaxation.7 However, our data suggest that duodenal lipid induces symptoms independently of any changes in proximal gastric motor function;5,8 increasing the dose of duodenal fat is associated with an increase in the severity of symptoms while the increase in proximal gastric volume is not related to the fat dose. In contrast, fat digestion is required for the induction of “dyspeptic” symptoms in healthy subjects,9 and the beneficial effect of the cholecystokinin (CCK)-A receptor antagonist dexloxiglumide on symptoms induced by duodenal fat infusion during gastric distension in patients with FD suggests that CCK is also involved.5 As these factors do not entirely explain the symptoms, other factors need to be taken into consideration. These include other gastrointestinal peptides and psychological factors, among others; their contribution to symptom development after food ingestion, particularly in relation to fat, in FD is unknown.
Cognitive factors play a role in appetite regulation; in healthy subjects, a nutrient containing soup induces a greater perception of fullness when subjects are informed of the nature of the soup compared with the control condition in which the subjects are not explicitly given this information.10 In patients with FD, both attention (due to anticipatory knowledge) and distraction (by performance of a mental task) modulate perception of duodenal distension; attention increases and distraction attenuates gut perception.11 It is therefore conceivable that FD patients respond with symptoms to certain foods as a result of a previous negative learning experience or information they have received; the possibility that even foods with a low fat content may increase symptoms if patients perceive them as high in fat has not been evaluated.
The aim of our study was therefore to evaluate in patients with FD the role of cognitive factors on symptom induction, proximal gastric motor function, and plasma CCK concentrations in response to high fat and low fat yoghurts. We investigated the hypothesis that the information given to the subjects as to the fat content of the yoghurts would affect the severity of symptoms.
SUBJECTS AND METHODS
Subjects
Fifteen patients (nine women, six men; aged 24–56 years (median 42)) with FD participated in the study. All patients were of normal body weight for height (body mass index 22.8 (0.9) kg/m2), non-smokers, and did not take any medication during the course of the study. The study was carried out with the approval of the ethics committee at the University Hospital Zürich. All patients gave their informed written consent prior to participation. Patients were recruited by advertisement in local newspapers describing the symptomatic criteria required for entry into the study. In a telephone interview, patients were selected for further investigation depending on the presence and severity of symptoms. Patients had at least three of the following symptoms for more than six months of at least a moderate severity: postprandial fullness/early satiety, bloating, epigastric pain, and nausea/vomiting. Severity was scored on a 0–3 scale with 0 representing “symptoms not experienced”, 1 “slight symptoms, but not bothering”, 2 “moderate symptoms, bothering, but not impairing daily activities”, and 3 “severe symptoms, impairing daily activities”. Patients who had previously undergone gastrointestinal surgery or were on medications that could not be discontinued for the duration of the study were not included. Each patient underwent laboratory tests, upper gastrointestinal endoscopy, abdominal ultrasound, a 13C-urea breath test for Helicobacter pylori status, and a H2 breath test to assess lactose intolerance. If all investigations were negative, patients were admitted into the study. Each subject was required to visit the laboratory once before the start of the study. During this visit, subjects were familiarised with the requirements of the study, the gastric (barostat) tube, the study procedures, and the symptom questionnaires.
Methods
Test meals
Two yoghurts (weight 300 g each) were used as test meals. The low fat yoghurt (LF) consisted of 150 g fat free yoghurt, 75 g low fat milk, and 75 g raspberries (143 kcal, energy from fat 8.1% (1.3 g), from carbohydrate 63.6% (21.6 g), and from protein 28.3% (9.8 g)). The high fat yoghurt (HF) consisted of 75 g whole fat yoghurt, 75 g cream, 75 g whole fat milk, and 75 g raspberries (330 kcal, energy from fat 66.6% (23.6 g), from carbohydrate 23.5% (18.5 g), and from protein 9.9% (8.0 g)). The ingredients were blended with an electric mixer to obtain a thick consistency for both yoghurts. Both yoghurts were appetising, and of similar taste, consistency, and colour. Prior to administration to patients, samples of both yoghurts were given to 16 healthy subjects in a randomised order and immediately following each other to test for palatability and whether subjects noticed any differences, including sweetness, fat content, taste, or acidity between the two yoghurts. All subjects rated both shakes as very palatable, and five subjects did not notice a difference between the yoghurts. The remaining 11 subjects indicated that the yoghurts differed with regard to their sweetness (seven subjects), fat content (two subjects), taste (eight subjects), and acidity (five subjects). None of the subjects was able to correctly assign fat content to the appropriate yoghurt.
Gastric tube and barostat
Changes in gastric volume in response to the test meals were evaluated in seven (four women, three men) of the 15 patients (the other eight patients did not tolerate the barostat tube, as revealed during the prestudy visit). For this purpose, each patient was intubated with a single lumen tube (OD 3.5 mm, ID 2.8 mm; Tygon Tubing, Upchurch Scientific, Oak Harbor, Washington, USA), which had a flaccid thin walled polyethylene bag (capacity 1100 ml) attached to its distal end. The proximal end of the tube was connected via a three way tap to the measurement and balloon ports of a gastric barostat (Distender Series II; G&J Electronics Inc., Willowdale, Ontario, Canada). Functioning of the barostat has been described in detail elsewhere.7 In brief, it is capable of measuring changes in gastric volume at a fixed pressure. Thus when the gastric wall relaxes, air is injected into the gastric bag to maintain the pressure while air is withdrawn when the stomach contracts. The barostat bag was positioned in the fundus of the stomach, as described previously.2 The bag was unfolded by inflating it with air, positioned in the fundus by gently pulling the tube back until its passage was restricted by the lower oesophageal sphincter, and then pushed back in by 3 cm. The tubes were then secured to the side of the face. Subjects tolerated the tubes well and did not sense the empty bag in the stomach.
Blood sampling
Venous blood samples were taken repeatedly throughout the study (fig 1 ▶) to determine plasma levels of CCK at baseline and following meal ingestion. Samples were collected in chilled EDTA tubes, centrifuged at 2°C for 15 minutes, and stored at −70°C until extraction. Plasma CCK was determined by a sensitive and specific commercially available radioimmunoassay kit (Euro-Diagnostica BV, Arnhem, the Netherlands). Cross reactivity with sulphated gastrins was 0.5%, while cross reactivity with unsulphated gastrins was <0.01%. The sensitivity of the assay was 0.3 pmol/l with a confidence limit of 2 SD. The coefficients of variation were 5.5% at 4.4 pmol/l and 2.0% at 20.6 pmol/l for the intra-assay variation, and 13.7% at 4.2 pmol/l and 4.1% at 20.6 pmol/l for interassay variation.
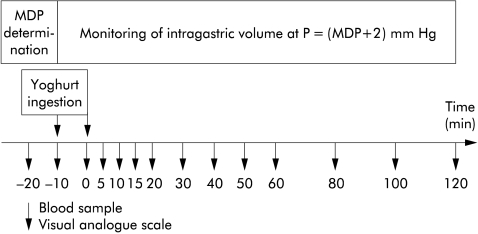
Figure 1.
Experimental protocol. Patients ingested a high fat or a low fat yoghurt (each on two occasions; on one occasion patients were correctly informed about the fat content of the yoghurt—that is, “this is a high fat yoghurt” or “this is a low fat yoghurt”, respectively—while on the other occasion subjects were given the wrong information—that is, “this is a low fat yoghurt” or “this is a high fat yoghurt”, respectively. Patients (n = 15) rated dyspeptic symptoms (including bloating, fullness, nausea, and discomfort, pain) at regular intervals and blood samples for determination of plasma cholecystokinin concentrations were taken at the same time points. In addition, changes in gastric volume in response to the four study conditions were recorded in seven of the 15 patients using an electronic barostat. MDP, minimal distending pressure (“baseline” pressure).
Assessment of symptoms
The severity of postprandial fullness, bloating, epigastric discomfort, pain, and nausea was assessed by means of previously validated visual analogue scales (VAS)12 at baseline and repeatedly after meal ingestion (fig 1 ▶). The VAS was a 10 cm line, with 0 representing “sensation/symptom not present” and 10 “sensation/symptom extremely strong/uncomfortable”.
Experimental protocol
Each patient was studied on four separate occasions. All studies were carried out in the morning after an overnight fast, 5–10 days apart, and each study took approximately three hours. Patients who underwent the barostat study were intubated, and the minimal distending pressure (MDP) was determined first by raising intrabag pressure with the barostat in steps of 1 mm Hg/min. MDP has previously been defined as the pressure that is necessary to overcome intra-abdominal pressure resulting in a bag volume of at least 30 ml. Intragastric pressure was set at 2 mm Hg above MDP (t = −20 minutes) and the corresponding volume was monitored until a stable recording was obtained for at least 10 minutes (baseline, t = −10 minutes). Then patients ingested the test meal within 10 minutes (end of yoghurt ingestion: t = 0 minutes), each yoghurt on two occasions. The two occasions differed with regard to the information the patients received about the composition of the yoghurt. On one occasion, patients received the correct information regarding the fat content of the yoghurt (that is “you will now be invited to eat a high fat yoghurt” in the case of the high fat yoghurt (condition HF-C) or “you will now be invited to eat a low fat yoghurt” in the case of the low fat yoghurt (condition LF-C)); on the other occasion they received the wrong information (that is, “you will now be invited to eat a low fat yoghurt” in the case of the high fat yoghurt (condition HF-W) or “you will now be invited to eat a high fat yoghurt” in the case of the low fat yoghurt (condition LF-W)), in a standardised fashion, and patients were presented with the empty containers of the ingredients. In the seven patients with the barostat tube in place, monitoring of gastric volume continued for two hours, and in all patients blood samples were drawn and symptoms assessed at regular intervals—that is, fasting (baseline) samples at t = −20, and −10 min, post-meal samples immediately after meal ingestion (t = 0 min), and then every five minutes for the first 20 minutes, every 10 minutes for the subsequent 40 minutes, and every 20 minutes for the final 60 minutes (until t = 120 minutes).
Data analysis
Changes in gastric volume during the different study conditions were calculated by averaging the volume readings obtained during the baseline period (at MDP +2 mm Hg) and during consecutive 10 minute periods following meal ingestion. Data obtained at t = −20 and −10 minutes for the different parameters (VAS scores, plasma CCK concentrations, gastric volumes) were then averaged to obtain a baseline value. As these values varied slightly between study conditions, they were adjusted using study day LF-C as a reference, and then entered into the analysis.
Statistical analysis
Data were analysed using repeated measures analysis of variance (two way ANOVA) with time and treatments as factors. A p value <0.05 was considered statistically significant. Data are presented as means (SEM).
RESULTS
Symptomatic responses
Fullness (fig 2A ▶)
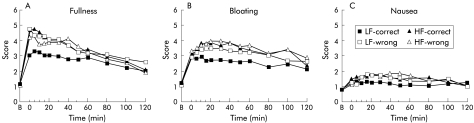
Figure 2.
Scores for fullness (A), bloating (B), and nausea (C) following ingestion of a high fat (HF) or a low fat (LF) yoghurt with patients receiving either the correct (that is, “this is a HF yoghurt” or “this is a LF yoghurt”, respectively) or the wrong (that is, “this is a LF yoghurt” or “this is a HF yoghurt”, respectively) information with regard to the fat content of the yoghurts. Data are means of n = 15 patients.
Scores for fullness increased following ingestion of all yoghurts (p = 0.0001). Both the fat content of the yoghurt and the information given to the subjects as to the fat content of the yoghurts tended to affect ratings for fullness (p = 0.067). Scores for fullness were higher following ingestion of the high fat yoghurt compared with the low fat yoghurt (HF-C v LF-C; p = 0.041). The information given to subjects as to the fat content of the yoghurt markedly affected scores for fullness following ingestion of the low fat yoghurt; subjects felt significantly more full when told that the yoghurt was high in fat (LF-C v LF-W; p = 0.013) while the information given to the subjects did not increase the severity of fullness following ingestion of the high fat yoghurt any further (HF-C v HF-W; p = 0.803). In contrast, there was no difference in scores for fullness between the high fat yoghurt when subjects were told it was low in fat and the low fat yoghurt when subjects were told it was high in fat (HF-W v LF-W; p = 0.474).
Bloating (fig 2B ▶)
Bloating scores increased following ingestion of all yoghurts (p = 0.0001). Both the fat content of the yoghurt and the information given to the subjects as to the fat content of the yoghurts affected ratings for bloating (p = 0.038). Scores for bloating were higher following ingestion of the high fat yoghurt compared with the low fat yoghurt (HF-C v LF-C; p = 0.042). The information given to subjects as to the fat content of the yoghurt affected scores for bloating following ingestion of the low fat yoghurt; subjects tended to feel more bloated when told that the yoghurt was high in fat (LF-C v LF-W; p = 0.056). In contrast, the information given to the subjects did not increase the severity of bloating following ingestion of the high fat yoghurt any further (HF-C v HF-W; p = 0.412).
Nausea (fig 2C ▶)
Ratings for nausea were slightly higher following ingestion of the high fat yoghurt when subjects were informed that the yoghurt was high in fat compared with the low fat yoghurt when subjects were informed that the yoghurt was low in fat (HF-C v LF-C; p = 0.012). In contrast, the information given to subjects did not affect nausea ratings within the type of yoghurt (HF-C v HF-W (p = 0.716); LF-C v LF-W (p = 0.176)).
Discomfort
Scores for discomfort increased following ingestion of all yoghurts (p = 0.001). However, there was no difference in scores between the four study conditions (p = 0.165).
Pressure/pain
Pain scores increased only slightly following ingestion of all yoghurts (p = 0.001) but there was no difference in scores between the four study conditions (p = 0.424).
Plasma CCK concentrations
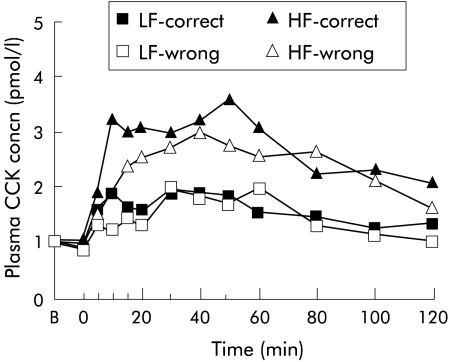
Both yoghurts increased plasma CCK concentrations (p = 0.0001) but the high fat yoghurt increased plasma CCK more than the low fat yoghurt (HF-C v LF-C; p = 0.036). Plasma CCK concentrations did not differ whether subjects were given the correct or wrong information as to the fat content of the yoghurt, although levels tended to be higher during condition HF-correct (HF-C) compared with condition HF-wrong (HF-W) (fig 3 ▶).
Figure 3.
Plasma cholecystokinin (CCK) concentrations following ingestion of a high fat (HF) or low fat (LF) yoghurt with patients receiving either the correct (that is “this is a HF yoghurt” or “this is a LF yoghurt”, respectively) or the wrong (that is, “this is a LF yoghurt” or “this is a HF yoghurt”, respectively) information with regard to the fat content of the yoghurts. Data are means of n = 15 patients.
Gastric volume changes
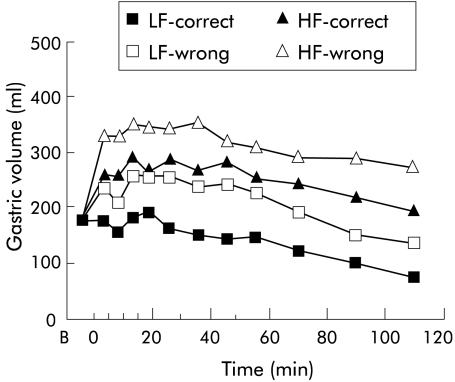
Gastric volumes increased following ingestion of both yoghurts (p = 0.001), except for condition LF-C (fig 4 ▶). In addition, gastric volumes were greater following ingestion of the high fat yoghurt compared with the low fat yoghurt (HF-C v LF-C; p = 0.012). The information about the fat content of the yoghurt did not significantly affect gastric volumes.
Figure 4.
Gastric volumes following ingestion of a high fat (HF) or low fat (LF) yoghurt with patients receiving either the correct (that is, “this is a HF yoghurt” or “this is a LF yoghurt”, respectively) or the wrong (that is, “this is a LF yoghurt” or “this is a HF yoghurt”, respectively) information with regard to the fat content of the yoghurts. Data are means of n = 7 patients.
DISCUSSION
Our data demonstrate that: (1) cognitive factors contribute to exacerbation of symptoms, particularly fullness, in FD, (2) symptoms after ingestion of a low fat meal are particularly affected by cognitive factors, while (3) plasma CCK concentrations and gastric volumes appear to be unaffected by cognitive factors. In addition, our data confirm previous reports that a high fat meal induces greater dyspeptic symptoms and results in greater plasma CCK concentrations and gastric volumes than a low fat meal.
The most interesting and novel finding of our study is that some of the most frequent dyspeptic symptoms, fullness and bloating, are exacerbated not only by ingestion of a meal high in fat but also by a meal that is perceived by the patients as high in fat. This has not been demonstrated previously and indicates that the situation with regard to the role of fat in dyspeptic symptom induction is more complex than assumed previously.6 Our data therefore suggest that at least two factors are involved in the induction of dyspeptic symptom related to fat: (1) the actual fat content of the meal—symptoms were more severe following the high fat yoghurt compared with the low fat yoghurt; and (2) the perceived fat content of the meal—patients associate fat ingestion with the occurrence of symptoms and, as a result, experience them. The mechanism(s) underlying this phenomenon is unknown. It has been described previously that anticipatory knowledge of gut distension enhances perception of this stimulus in patients with FD,11 and awareness of the nutrient content of a soup increases the feeling of fullness in healthy subjects.10 These previous findings indicate that the occurrence of symptoms in patients with FD may perhaps be the result of some form of conditioning due to the experience of exacerbation of symptoms after ingestion of fatty foods in the past. These previous findings also indicate that cognitive factors influencing perception related to food ingestion occur in both healthy subjects and in patients with FD. The substantial effect of placebo treatment on symptom improvement of up to 50%,13,14 which in some studies was similar to the effect of the drug under study,13 is well documented in patients with FD. This effect appears to occur independently of any changes in gastrointestinal motility or gastric perception,15 and a reduction in anxiety, as a result of the patient’s belief that the treatment will relieve symptoms, has been discussed as a possible underlying mechanism.15 It is therefore conceivable that this effect is reversed in a situation in which patients are confronted with a food, which they perceive as having detrimental effects on their symptoms, as in our study. Certainly, further studies are required to investigate these findings in more detail as an understanding of the role of cognitive factors may potentially provide opportunities for novel therapeutic approaches. In contrast, symptoms following the high fat yoghurt were not affected by the information about the fat content. This may indicate that the fat contained in the yoghurt per se had a dominant independent effect on symptoms that could not be further modulated by cognitive factors—that is, the cognitive influence was weaker than the effect of fat.
The observation that patients with FD may also experience symptoms after ingestion of low fat foods as a result of cognitive influences, and not only after ingestion of foods containing fat, raises the question as to the value of dietary modification—for example, the adoption of a low fat diet—in the management of dyspeptic symptoms apparently related to fat ingestion. This may be particularly relevant in patients in whom cognitive effects are suspected to have a major influence on symptoms, and suggests that cognitive-behavioural training may be a therapeutic strategy in patients that complain of symptoms associated with eating, or ingestion of specific foods, but are refractory to dietary modification.
In considering underlying pathophysiological mechanisms, studies in both humans and experimental animals have demonstrated marked effects of somatic and mental stress and anxiety on gut motility and visceral perception.11,16–20 In addition, it has been suggested that plasma concentrations of some gastrointestinal peptides, including CCK and somatostatin, are significantly more elevated in response to a stressful interview in patients with FD compared with healthy subjects.21 We were unable to find differences in postprandial plasma CCK concentrations or gastric volumes (as a measure of gastric relaxation) in relation to the information about fat content. Our data indicate that CCK is not involved in exacerbation of symptoms by cognitive influences. While plasma CCK concentrations tended to be higher, although not statistically significant, when patients were informed that the yoghurt was high in fat, the severity of symptoms did not differ between the two high fat study conditions. Therefore, our data do not provide evidence that CCK is involved in symptom induction related to the perceived fat content of a meal. The role of other gastrointestinal peptides or stress related hormones, including corticotropin releasing factor,22 may warrant further investigation. In addition, while our data suggest that proximal gastric relaxation does not play a role in the exacerbation of symptoms by cognitive influences, the lack of a statistically significant difference may be the result of a type 2 error and warrants further investigation.
In conclusion, our data provide evidence for a role of cognitive factors in symptom induction in functional dyspepsia, particularly after ingestion of low fat meals if patients perceive them as containing high fat quantities, while CCK and gastric volumes do not appear to be affected significantly by cognitive factors. Our findings may have important clinical implications and strongly suggest that cognitive influences need to be taken into consideration in the treatment of functional dyspepsia.
Acknowledgments
The study was supported by a grant (No SNF 32-56068.98) from the Swiss National Science Foundation. C Feinle-Bisset is currently supported by a Florey Research Fellowship from the Royal Adelaide Hospital, Adelaide, South Australia.
Abbreviations
FD, functional dyspepsia
CCK, cholecystokinin
VAS, visual analogue scale
MDP, minimal distending pressure
REFERENCES
- 1.Barbera R, Feinle C, Read NW. Abnormal sensitivity to duodenal lipid infusion in patients with functional dyspepsia. Eur J Gastroenterol Hepatol 1995;7:1051–7. [DOI] [PubMed] [Google Scholar]
- 2.Feinle C, Grundy D, Read NW. Effects of duodenal nutrients on sensory and motor responses of the human stomach to distension. Am J Physiol 1997;273:G721–6. [DOI] [PubMed] [Google Scholar]
- 3.Houghton LA, Mangnall YF, Dwivedi A, et al. Sensitivity to nutrients in patients with non ulcer dyspepsia. Eur J Gastroenterol Hepatol 1993;5:109–13. [Google Scholar]
- 4.Taggart D, Billington BP. Fatty foods and dyspepsia. Lancet 1966;27:465–6. [PubMed] [Google Scholar]
- 5.Feinle C, Meier O, Otto B, et al. Role of duodenal lipid and cholecystokinin A receptors in the pathophysiology of functional dyspepsia. Gut 2001;48:347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbera R, Feinle C, Read NW. Nutrient-specific modulation of gastric mechanosensitivity in patients with functional dyspepsia. Dig Dis Sci 1995;40:1636–41. [DOI] [PubMed] [Google Scholar]
- 7.Azpiroz F, Malagelada JR. Intestinal control of gastric tone. Am J Physiol 1985;249:G501–9. [DOI] [PubMed] [Google Scholar]
- 8.Feinle C, Grundy D, Otto B, et al. Relationship between increasing duodenal lipid doses, gastric perception, and plasma hormone levels in humans. Am J Physiol 2000;278:R1217–23. [DOI] [PubMed] [Google Scholar]
- 9.Feinle C, Rades T, Otto B, et al. Fat digestion modulates gastrointestinal sensations induced by gastric distention and duodenal lipid in humans. Gastroenterology 2001;120:1100–7. [DOI] [PubMed] [Google Scholar]
- 10.Cecil JE, Francis J, Read NW. Relative contributions of intestinal, gastric, oro-sensory influences and information to changes in appetite induced by the same liquid meal. Appetite 1998;31:377–90. [DOI] [PubMed] [Google Scholar]
- 11.Accarino AM, Azpiroz F, Malagelada JR. Attention and distraction: effects on gut perception. Gastroenterology 1997;113:415–22. [DOI] [PubMed] [Google Scholar]
- 12.Sepple CP, Read NW. Gastrointestinal correlates of the development of hunger in man. Appetite 1989;13:183–91. [DOI] [PubMed] [Google Scholar]
- 13.de Groot GH, de Both PS. Cisapride in functional dyspepsia in general practice. A placebo-controlled, randomized, double-blind study. Aliment Pharmacol Ther 1997;11:193–9. [DOI] [PubMed] [Google Scholar]
- 14.Talley NJ, Phillips SF. Non-ulcer dyspepsia: potential causes and pathophysiology. Ann Intern Med 1988;108:865–79. [DOI] [PubMed] [Google Scholar]
- 15.Mearin F, Balboa A, Zarate N, et al. Placebo in functional dyspepsia: symptomatic, gastrointestinal motor, and gastric sensorial responses. Am J Gastroenterol 1999;94:116–25. [DOI] [PubMed] [Google Scholar]
- 16.Camilleri M, Malagelada JR, Kao PC, et al. Gastric and autonomic responses to stress in functional dyspepsia. Dig Dis Sci 1986;31:1169–77. [DOI] [PubMed] [Google Scholar]
- 17.Coffin B, Azpiroz F, Malagelada JR. Somatic stimulation reduces perception of gut distention in humans. Gastroenterology 1994;107:1636–42. [DOI] [PubMed] [Google Scholar]
- 18.Gue M, Peeters T, Depoortere I, et al. Stress-induced changes in gastric emptying, postprandial motility, and plasma gut hormone levels in dogs. Gastroenterology 1989;97:1101–7. [DOI] [PubMed] [Google Scholar]
- 19.Monnikes H, Tebbe JJ, Hildebrandt M, et al. Role of stress in functional gastrointestinal disorders. Evidence for stress-induced alterations in gastrointestinal motility and sensitivity. Dig Dis 2001;19:201–11. [DOI] [PubMed] [Google Scholar]
- 20.Thompson DG, Richelson E, Malagelada JR. Perturbation of upper gastrointestinal function by cold stress. Gut 1983;24:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonsson BH, Uvnas-Moberg K, Theorell T, et al. Gastrin, cholecystokinin, and somatostatin in a laboratory experiment of patients with functional dyspepsia. Psychosom Med 1998;60:331–7. [DOI] [PubMed] [Google Scholar]
- 22.Monnikes H, Schmidt BG, Raybould HE, et al. CRF in the paraventricular nucleus mediates gastric and colonic motor response to restraint stress. Am J Physiol 1992;262:G137–43. [DOI] [PubMed] [Google Scholar]