Abstract
Background: The mucus layer protects the gastrointestinal mucosa from mechanical, chemical, and microbial challenge. Mucin 2 (MUC-2) is the most prominent mucin secreted by intestinal epithelial cells. There is accumulating evidence that subepithelial myofibroblasts regulate intestinal epithelial cell function and are an important source of prostaglandins (PG). PG enhance mucin secretion and are key players in mucoprotection. The role of bacterial fermentation products in these processes deserves further attention.
Aims: We therefore determined whether the effect of short chain fatty acids (SCFA) on MUC-2 expression involves intermediate PG production.
Methods: Both mono- and cocultures of epithelial cells and myofibroblasts were used to study the effects of SCFA on MUC-2 expression and PG synthesis. Cell culture supernatants were used to determine the role of myofibroblast derived prostaglandins in increasing MUC-2 expression in epithelial cells.
Results: Prostaglandin E1 (PGE1) was found to be far more potent than PGE2 in stimulating MUC-2 expression. SCFA supported a mucoprotective PG profile, reflected by an increased PGE1/PGE2 ratio in myofibroblast supernatants and increased MUC-2 expression in mono- and cocultures. Incubation with indomethacin revealed the latter to be mediated by PG.
Conclusions: SCFA can differentially regulate PG production, thus stimulating MUC-2 expression in intestinal epithelial cells. This mechanism involving functional interaction between myofibroblasts and epithelial cells may play an important role in the mucoprotective effect of bacterial fermentation products.
Keywords: coculture, mucin, myofibroblast, prostaglandin, short chain fatty acid
Chronic relapsing mucosal inflammation is a hallmark of inflammatory bowel disease (IBD). Concentrations of proinflammatory cytokines are dramatically increased in the intestinal mucosa of IBD patients.1–3 Local production of proinflammatory cytokines may compromise intestinal barrier integrity (for example, increase epithelial permeability and change mucus production and quality).4–8 The mucus layer forms a physical-chemical barrier on the epithelial layer separating the gut lumen from the lamina propria, thus having an important function in scavenging dietary and microbial antigens. Mucus secretion by epithelial cells can be influenced by a variety of physiological and immune mediators, such as prostaglandins (PG).9,10 It is now well established that epithelial cell functions (for example, proliferation, differentiation, secretion, and motility) are regulated by myofibroblasts which form a thin layer of cells underlying the epithelium.11–15 These myofibroblasts are an important source of PG and therefore may play a crucial role in mucoprotection. Myofibroblasts constitutively express cyclooxygenase (COX)-1 whereas during inflammation COX-2 is induced, and these enzymes are responsible for prostaglandin E1 (PGE1) and PGE2 production.12,13,16,17 COX inhibition with non-steroidal anti-inflammatory drugs (NSAIDs) can result in detrimental side effects such as induction of gastrointestinal lesions.18,19 Administration of PGE1 analogues in this respect is believed to support mucoprotection.20,21 Certain bacteria of the intestinal flora are beneficial for gut health. Apart from immunomodulating capacities, these bacteria can also improve mucosal barrier integrity.22 The mucoprotective effects of metabolic products from the intestinal flora deserve further study. Short chain fatty acids (SCFA) are the end products of microbial fermentation of non-digestible carbohydrates and have been reported to increase mucus secretion.23,24 SCFA are absorbed by the distal ileum and colon, and butyrate in particular is an important source of nutrition for epithelial cells.23,25 Butyrate has gained much attention as it promotes mucosal restitution, induces differentiation, and inhibits inflammation and tumour growth.25,26 Hence in this study we determined the effect of SCFA on PGE1 and PGE2 production and assessed the implications for epithelial mucin 2 (MUC-2) expression. To do this, we used a coculture model representing the spatial interaction between epithelial and mesenchymal cells.8,14,27
MATERIAL AND METHODS
Cell culture
Monolayers (MC) of intestinal epithelial T84 (passages 57–64) and LS174T (passages 110–120) (ATCC, Manassas, USA) cells or intestinal myofibroblasts CCD-18Co (passages 10–14) were cultured in 24 or 96 well tissue culture plates (Corning BV, Acton, USA). Parallel with MC, cocultures (CC) were set up by culturing epithelial cells directly on a confluent layer of CCD-18Co cells, as previously described.8 In brief, CCD-18Co cells were seeded in a twofold dilution in the culture plates and grew confluent within one week. T84 and LS174T cells were added in a fivefold dilution on top of the CCD-18Co layer (CC) or in a separate plate (MC). Cells were cultured in DMEM/F12 glutamax I (Invitrogen Life Technologies, Carlsbad, USA) with penicillin (100 IU/ml), streptomycin (100 μg/ml) (Invitrogen Life Technologies), and 5% heat inactivated fetal bovine serum (Invitrogen Life Technologies). Medium was refreshed every two days. CCD-18Co monolayers were used when grown confluent while CC and MC were used when the epithelial cell layer had grown subconfluent.
Stimulation of CC and MC with PG or SCFA
CCD-18Co monolayers or MC/CC T84 or LS174T were incubated for 24 hours with a concentration range (0.025-4.0 mM) of acetic acid, propionic acid, or butyric acid (VWR International, West Chester, USA). To determine the effects of PG on MUC-2 expression, MC/CC T84 were incubated for 24 hours with 0.01–100 ng/ml PGE1 or PGE2 (dissolved in 100% ethanol, diluted to 1 mg/ml stocks in phosphate buffered saline, and stored at −80°C, Sigma-Aldrich BV, St Louis, Missouri, USA). In addition, supernatants of CCD-18Co which had been stimulated for 24 hours with butyrate in the absence or presence of 10−6 M indomethacin (to block prostaglandin production; Sigma-Aldrich BV) were transferred to MC T84. Supernatants and/or cells were collected and PG concentration or MUC-2 expression was determined.
Dot blotting MUC-2
We used a dot blot technique to determine MUC-2 expression in cell cultures as mucins are extremely large glycoproteins (over 500 kDa) which makes them difficult to handle in western blotting techniques.28,29 We used the anti-HCM (human colon mucin) antibody raised against purified mucins from mucosal scrapings which recognises peptide epitopes of MUC-2 in colonic goblet cells.28 Our method was validated using preimmune serum (T84 stained negative), negative control cells (CCD-18Co), and bovine serum albumin. Cell samples were collected in laemmli (protein isolation buffer) and protein determination was performed using the DC protein assay (Biorad, Hercules, California, USA) according to the manufacturer’s protocol with minor modifications. Samples (0.3–0.7–1.0 μg/2 μl) were dotted onto nitrocellulose membranes (Schleicher and Schuell, Riviera Beach, USA). Membranes were blocked in TBST/5% Protivar (Nutricia Roetermeer, the Netherlands) followed by one hour of incubation with anti-MUC-2 antibody (kindly donated by Dr Einerhand, Erasmus University, Rotterdam, the Netherlands). After washing, blots were incubated with goat antirabbit-horseradish peroxidase (Santa Cruz Biotechnology Santa Cruz, USA) and for substrate detection ECL (Roche Diagnostics Indianapolis, Indiana, USA) was used. Densitometry was performed using the Lumi-Imager (Roche Diagnostics) and the signal was expressed in light units (BLU). BLUs were also expressed relative to control incubations (%BLU). SCFA incubations were performed in parallel with MC and CC, and MUC-2 expression was analysed within the same dot blot. To compare the stimulatory effect of SCFA on MUC-2 expression in MC and CC, we deducted basal MUC-2 expression levels.
Measurement of PGE1 and PGE2
Supernatants from CCD-18Co were analysed for PG production using ELISAs for PGE1 (R&D Minneapolis, USA; 21% cross reactivity with PGE2) and PGE2 (Biotrak Amersham Biosciences corp., Piscataway, USA; 4% cross reactivity with PGE1).30 Concentrations measured during SCFA incubations were expressed relative to basal secretion. The PGE1/PGE2 ratio was calculated to determine a shift towards a more mucoprotective PG profile.
Viability measurements
As SCFA have been reported to be cytotoxic at high doses, we measured the viability of CCD-18Co, MC, and CC using a WST-1 assay (Roche Diagnostics). After 24 hours of incubation, the medium was refreshed and cells were incubated with WST-1. After one hour for MC and CC, and three hours for CCD-18Co, 100 μl sample of the supernatant was measured at A450-A655 in a spectrophotometer (Biorad).
Data analysis
All data are presented as mean (SEM). Data were analysed with the univariate ANOVA using SPSS software version 10.
RESULTS
MUC-2 expression of MC/CC T84 after incubation with PG
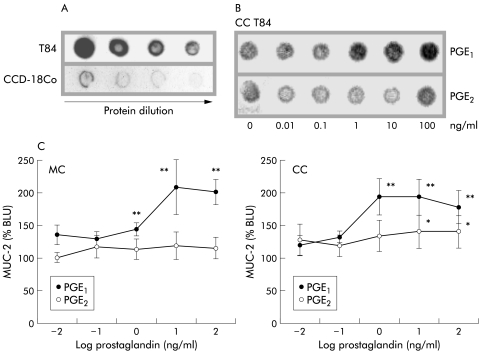
Protein titration of T84 or CCD-18Co homogenates revealed T84 to be positive for MUC-2, and as expected CCD-18Co were negative in the dot blot analyses (fig 1A ▶). The differential effects of PGE1 and PGE2 on MUC-2 expression by CC T84 are shown in a representative dot blot (fig 1B ▶). Densitometry revealed that MUC-2 expression in both MC and CC T84 was stimulated by PGE1 rather than PGE2 (fig 1C ▶). MUC-2 expression in both MC and CC T84 was dose dependently increased after 24 hours of incubation with PGE1 (1–100 ng/ml; p<0.002) whereas PGE2 only marginally increased MUC-2 expression in CC T84 cultures at the highest doses (10–100 ng/ml; p<0.01).
Figure 1.
Prostaglandin stimulation of mucin 2 (MUC-2) expression in monocultures (MC)/cocultures (CC) of T84. (A) Dot blot analyses showed MC T84 cells to be positive for MUC-2 whereas CCD-18Co were negative. (B) Representative dot blot showing the effects of prostaglandins PGE1 and PGE2 on MUC-2 expression in CC T84. (C) Densitometric analysis revealed PGE1 to enhance MUC-2 expression in both MC and CC T84 (1–100 ng/ml; **p<0.002) whereas PGE2 only marginally affected MUC-2 expression in CC T84 (10–100 ng/ml; *p<0.01). MUC-2 expression is presented relative to controls (%BLU).
Effect of SCFA on PGE1 versus PGE2
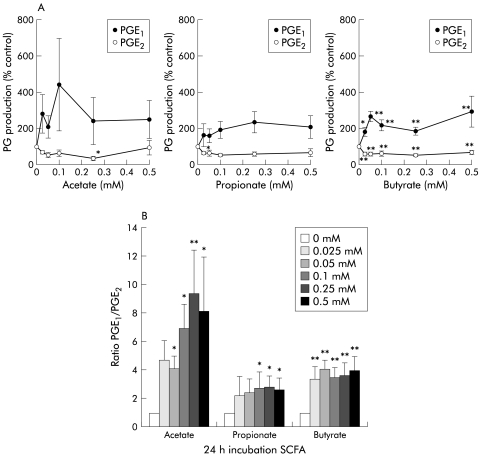
Supernatants of unstimulated CCD-18Co contained more PGE2 than PGE1 (633 (371) v 891 (437) pg/ml; p<0.05, n = 6). Butyrate in particular enhanced PGE1 and reduced PGE2 concentrations in all dosage groups (fig 2A ▶; p<0.01, p<002). Acetate and propionate incubations followed the same trend. As a result, the PGE1/PGE2 ratio increased after SCFA incubation (fig 2B ▶; p<0.01, p<0.002). Culture medium levels for PGE1 and PGE2 were low or undetectable. PG levels, as measured in butyrate containing culture medium spiked with PG, did not differ from spiked medium controls. Moreover, none of the incubations compromised cell viability, as determined by WST analysis (data not shown).
Figure 2.
Short chain fatty acids (SCFA) increased the prostaglandin PGE1/PGE2 ratio produced by CCD-18Co. (A) Butyrate increased PGE1 and decreased PGE2 concentrations (*p<0.01, **p<0.002); acetate and propionate followed the same tendency. Prostaglandin production is presented relative to controls (% control). (B) SCFA increased the PGE1/PGE2 ratio (n = 5; *p<0.01, **p<0.002), resulting in a preferred mucoprotective profile.
MUC-2 expression of MC and CC T84 or LS174T after SCFA incubation
T84 and LS174T cells were cultured directly on CCD-18Co monolayers and MUC-2 expression was determined. Basal MUC-2 expression was significantly higher in CC T84 compared with MC T84 while MUC-2 expression in CC LS174T was not increased compared with MC (fig 3 ▶; p<0.05).
Figure 3.
Increased mucin 2 (MUC-2) expression in cocultures (CC) of T84 compared with monocultures (MC) of T84. Basal MUC-2 expression increased when T84 cells were cocultured with CCD-18Co, the latter however was not apparent using LS174T cells (n = 4, *p<0.05). BLU, light units.
SCFA enhanced MUC-2 expression more in CC compared with MC in both cell lines, except for propionate in MC/CC T84 (p<0.05). Propionate and acetate effectively induced MUC-2 expression in MC and CC of both cell lines (fig 4A, B ▶; p<0.01, p<0.002). Butyrate stimulated MUC-2 expression in MC/CC T84 and CC LS174T but not in MC LS174T (p<0.01, p<0.002). As it is likely that intestinal epithelial cells are exposed to higher levels of SCFA than subepithelial myofibroblasts, we used a broader concentration range in our epithelial mucin expression studies. WST data revealed no toxicity of these SCFA concentrations in both MC and CC (data not shown).
Figure 4.
Short chain fatty acids (SCFA) increased mucin 2 (MUC-2) expression in monocultures (MC) and cocultures (CC) of T84 and LS174T. (A, B) SCFA stimulation of MUC-2 expression in the presence of CCD-18Co was found to be more pronounced compared with epithelial monolayers alone (p<0.05). Acetate and propionate dose dependently increased MUC-2 expression in MC/CC T84 (n = 4) and MC/CC LS174T (n = 3) (*p<0.01, **p<0.002). Butyrate was effective in MC/CC T84 and CC LS174T (p<0.01, p<0.002) but did not enhance MUC-2 expression in MC LS174T cells. MUC-2 expression was presented after deduction of basal expression levels of either MC or CC. BLU, light units.
MUC-2 expression of MC T84 after incubation with CCD-18Co supernatant
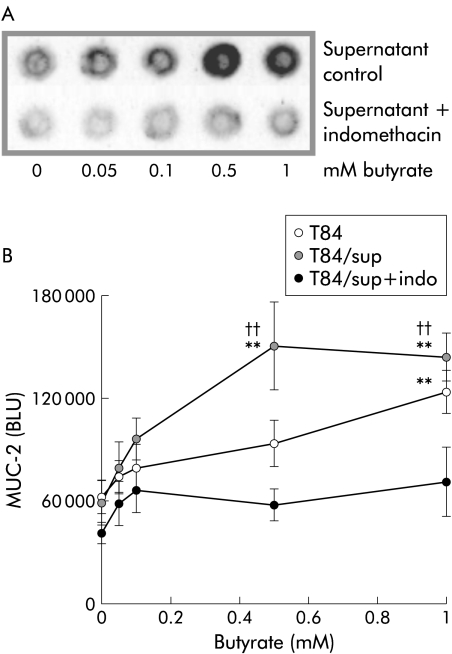
SCFA stimulated MUC-2 expression to a higher extent in CC compared with MC. Therefore, we tested whether mucin expression in cocultures was regulated by CCD-18Co derived PG by blocking PG production with indomethacin. MUC-2 expression in MC T84 incubated with supernatants of CCD-18Co stimulated with butyrate in the presence or absence of indomethacin are shown in fig 5A ▶. Densitometric analysis showed butyrate to enhance MUC-2 expression in MC T84 (fig 5B ▶; 1 mM, p<0.002). T84 incubation with CCD-18Co supernatants increased MUC-2 expression at lower butyrate doses, representing the additional effect of CCD-18Co derived soluble mediators (0.5–1 mM; p<0.002). The butyrate effect was found to be mediated by PG derived from both CCD-18Co and T84 as indomethacin completely blocked stimulation of MUC-2 expression (0.5–1 mM; p<0.01). In additional analyses, we confirmed that indomethacin reduced PG concentrations in butyrate stimulated CCD-18Co culture supernatants (PGE1, p<0.02; PGE2, p<0.02; n = 5). Enhanced MUC-2 expression in MC T84 incubated with butyrate, acetate, or propionate was also abrogated by indomethacin (data not shown).
Figure 5.
Mucin 2 (MUC-2) stimulation by butyrate is mediated by prostaglandins. (A) Representative dot blot of MUC-2 expression in monocultures of T84 incubated with supernatants of CCD-18Co that had been stimulated with butyrate in the presence or absence of indomethacin. (B) Densitometric analysis of four different experiments. Stimulation of MUC-2 expression by butyrate was found to be mediated by CCD-18Co and T84 derived prostaglandins as indomethacin (indo) blocked this effect (0.5-1 mM; ††p<0.01). CCD-18Co supernatants (sup) increased MUC-2 expression at lower butyrate concentrations (0.5–1 mM; **p<0.002) compared with MC T84 incubated with butyrate (1 mM; **p<0.002).
DISCUSSION
Bacterial fermentation products may play an important role in mucoprotection, being an energy source for intestinal epithelial cells and stimulating mucin secretion.23–25 Butyrate enemas have been found to reduce clinical symptoms in patients with ulcerative colitis, and high butyrate concentrations in the colon are protective against colon cancer.31,32 Also, PG are implicated in sustaining mucosal integrity, as revealed by the observation that PGE1 analogues were found to prevent formation of gastroduodenal lesions induced by NSAIDs.20,21
Our data revealed differential effects of PGE1 and PGE2 on MUC-2 expression in epithelial cells. PGE1 and PGE2 are known stimulators of mucin secretion by T84 and LS174T cells and the rat colon.29,33,34 In our experiments, PGE1 was more effective than PGE2 in enhancing MUC-2 expression. Mucin expression within the cells is the net effect of synthesis and secretion. This implies that PGE1 but not PGE2 incubation resulted in net MUC-2 synthesis. To our knowledge, the effect of PGE1 on mucin synthesis has not been studied previously; however, a PGE1 analogue has been reported to increase the intracellular mucus content of stomach epithelium.35 Our PGE2 data are in agreement with a study in which dmPGE2 enhanced mucin secretion by HT29-18N2 cells but reduced the amount of newly synthesised mucins, resulting in decreased intracellular mucus stores even at 24 hours.36 The observed difference between PGE1 and PGE2 cannot be attributed to differential bioactivity as in our laboratory they were found to be equally active in inhibiting cytokine secretion.30
There is consistent evidence that butyrate induces cell differentiation and downregulates inflammatory responses.25,37 Our data showed that SCFA enhanced the PGE1/PGE2 ratio secreted by subepithelial myofibroblasts, a profile that may support mucoprotection by enhancing epithelial mucin expression. Our concept of exposure of subepithelial myofibroblasts to bacterial fermentation products is biologically relevant as following epithelial absorption SCFA are found in the bloodstream.38
To study MUC-2 expression, we used colonic carcinoma cell lines T84 (columnar crypt epithelium) and LS174T (goblet cell type).29,39–41 When cocultured with subepithelial myofibroblasts, T84 expressed more MUC-2 compared with MC T84. Cell to cell contact, extracellular matrix, and soluble factors such as transforming growth factor β are known to induce epithelial differentiation which might cause T84 cells to increase basal MUC-2 expression.39,42 Basal MUC-2 expression of CC LS174T was not enhanced compared with MC LS174T, possibly because LS174T cells are not capable of further differentiation.
SCFA dose dependently induced MUC-2 expression in MC/CC T84 and LS174T. SCFA induce mucin exocytosis in the rat colon through activation of cholinergic nerves.24,43 SCFA infusion in these studies was one hour or less. We observed that, independent of cholinergic activation, prolonged incubation with SCFA induced MUC-2 expression in epithelial cells, thus skewing the balance towards mucoprotection. Our finding is also supported by a study in which butyrate was found to increase mucin synthesis in colonic biopsies.44
In general, SCFA enhanced MUC-2 expression more potently in CC compared with MC. Monolayer LS174T cells were even unresponsive to butyrate incubations while cocultures were effectively stimulated. These data strongly support a role for subepithelial myofibroblasts in the regulation of MUC-2 production by epithelial cells.
Butyrate most effectively stimulated PGE1 production by CCD-18Co cells and when transferring these CCD-18Co supernatants to MC T84, MUC-2 expression was further increased compared with incubation of epithelial cells with butyrate alone. The butyrate effects mediated through CCD-18Co supernatant or directly by T84 (data not shown) were abrogated by indomethacin. This implies that enhanced mucin synthesis by SCFA is mediated by PG derived from both subepithelial myofibroblasts and intestinal epithelial cells. The latter is supported by the fact that mucosal epithelial cells constitutively express COX-1 and indeed epithelial cells have been reported to produce PG.26,45
In conclusion, SCFA increased the PGE1/PGE2 ratio produced by subepithelial myofibroblasts and PGE1 was found to be superior to PGE2 in enhancing MUC-2 expression in epithelial cells. SCFA stimulated epithelial MUC-2 expression was mediated by PG derived from subepithelial myofibroblasts and epithelial cells, as proved by our indomethacin inhibition experiments. The present study therefore suggests that bacterial fermentation products may have beneficial effects on gut health by supporting mucosal barrier integrity.
Acknowledgments
We appreciate the support of Rob Verdooren in the statistical evaluation.
Abbreviations
IBD, inflammatory bowel disease
COX, cyclo-oxygenase
NSAIDs, non-steroidal anti-inflammatory drugs
CC, coculture
MC, monoculture
PG, prostaglandins
SCFA, short chain fatty acids
MUC-2, mucin 2
BLU, light units
REFERENCES
- 1.Mahida YR, Wu K, Jewell DP. Enhanced production of interleukin 1-beta by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn’s disease. Gut 1989;30:835–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reimund JM, Wittersheim C, Dumont S, et al. Increased production of tumour necrosis factor-alpha, interleukin-1 beta, and interleukin-6 by morphologically normal intestinal biopsies from patients with Crohn’s disease. Gut 1996;39:684–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casellas F, Papo M, Guarner F, et al. Intracolonic release in vivo of interleukin-1 beta in chronic ulcerative colitis. Clin Sci Colch 1995;89:521–6. [DOI] [PubMed] [Google Scholar]
- 4.Madara JL, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest 1989;83:724–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKay DM, Croitoru K, Perdue MH. T cell-monocyte interactions regulate epithelial physiology in a coculture model of inflammation. Am J Physiol 1996;270:C418–28. [DOI] [PubMed] [Google Scholar]
- 6.Rhodes JM. Mucins and inflammatory bowel disease. QJM 1997;90:79–82. [DOI] [PubMed] [Google Scholar]
- 7.Tytgat KM, van der Wal JW, Einerhand AW, et al. Quantitative analysis of MUC2 synthesis in ulcerative colitis. Biochem Biophys Res Commun 1996;224:397–405. [DOI] [PubMed] [Google Scholar]
- 8.Willemsen LE, Schreurs CC, Kroes H, et al. A coculture model mimicking the intestinal mucosa reveals a regulatory role for myofibroblasts in immune-mediated barrier disruption. Dig Dis Sci 2002;47:2316–24. [DOI] [PubMed] [Google Scholar]
- 9.Cohan VL, Scott AL, Dinarello CA, et al. Interleukin-1 is a mucus secretagogue. Cell Immunol 1991;136:425–34. [DOI] [PubMed] [Google Scholar]
- 10.Plaisancie P, Barcelo A, Moro F, et al. Effects of neurotransmitters, gut hormones, and inflammatory mediators on mucus discharge in rat colon. Am J Physiol 1998;275:G1073–84. [DOI] [PubMed] [Google Scholar]
- 11.Powell DW, Mifflin RC, Valentich JD, et al. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol 1999;277:C1–9. [DOI] [PubMed] [Google Scholar]
- 12.Powell DW, Mifflin RC, Valentich JD, et al. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol 1999;277:C183–201. [DOI] [PubMed] [Google Scholar]
- 13.Mahida YR, Beltinger J, Makh S, et al. Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am J Physiol 1997;273:G1341–8. [DOI] [PubMed] [Google Scholar]
- 14.Berschneider HM, Powell DW. Fibroblasts modulate intestinal secretory responses to inflammatory mediators. J Clin Invest 1992;89:484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beltinger J, McKaig BC, Makh S, et al. Human colonic subepithelial myofibroblasts modulate transepithelial resistance and secretory response. Am J Physiol 1999;277:C271–9. [DOI] [PubMed] [Google Scholar]
- 16.Hinterleitner TA, Saada JI, Berschneider HM, et al. IL-1 stimulates intestinal myofibroblast COX gene expression and augments activation of Cl- secretion in T84 cells. Am J Physiol 1996;271:C1262–8. [DOI] [PubMed] [Google Scholar]
- 17.Kim EC, Zhu Y, Andersen V, et al. Cytokine-mediated PGE2 expression in human colonic fibroblasts. Am J Physiol 1998;275:C988–94. [DOI] [PubMed] [Google Scholar]
- 18.Bjarnason I, Hayllar J, MacPherson AJ, et al. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology 1993;104:1832–47. [DOI] [PubMed] [Google Scholar]
- 19.Davies NM, Saleh JY, Skjodt NM. Detection and prevention of NSAID-induced enteropathy. J Pharm Pharm Sci 2000;3:137–55. [PubMed] [Google Scholar]
- 20.Bardhan KD, Bjarnason I, Scott DL, et al. The prevention and healing of acute non-steroidal anti-inflammatory drug-associated gastroduodenal mucosal damage by misoprostol. Br J Rheumatol 1993;32:990–5. [DOI] [PubMed] [Google Scholar]
- 21.Grazioli I, Avossa M, Bogliolo A, et al. Multicenter study of the safety/efficacy of misoprostol in the prevention and treatment of NSAID-induced gastroduodenal lesions. Clin Exp Rheumatol 1993;11:289–94. [PubMed] [Google Scholar]
- 22.Madsen K, Cornish A, Soper P, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001;121:580–91. [DOI] [PubMed] [Google Scholar]
- 23.Cummings JH, Macfarlane GT. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol 1991;70:443–59. [DOI] [PubMed] [Google Scholar]
- 24.Shimotoyodome A, Meguro S, Hase T, et al. Short chain fatty acids but not lactate or succinate stimulate mucus release in the rat colon. Comp Biochem Physiol A Mol Integr Physiol 2000;125:525–31. [DOI] [PubMed] [Google Scholar]
- 25.D’Argenio G, Mazzacca G. Short-chain fatty acid in the human colon. Relation to inflammatory bowel diseases and colon cancer. Adv Exp Med Biol 1999;472:149–58. [DOI] [PubMed] [Google Scholar]
- 26.Awad AB, Kamei A, Horvath PJ, et al. Prostaglandin synthesis in human cancer cells: influence of fatty acids and butyrate. Prostaglandins Leukot Essent Fatty Acids 1995;53:87–93. [DOI] [PubMed] [Google Scholar]
- 27.Valentich JD, Popov V, Saada JI, et al. Phenotypic characterization of an intestinal subepithelial myofibroblast cell line. Am J Physiol 1997;272:C1513–24. [DOI] [PubMed] [Google Scholar]
- 28.Tytgat KM, Buller HA, Opdam FJ, et al. Biosynthesis of human colonic mucin: Muc2 is the prominent secretory mucin. Gastroenterology 1994;107:1352–63. [DOI] [PubMed] [Google Scholar]
- 29.McCool DJ, Marcon MA, Forstner JF, et al. The T84 human colonic adenocarcinoma cell line produces mucin in culture and releases it in response to various secretagogues. Biochem J 1990;267:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dooper MM, Wassink L, M’Rabet L, et al. The modulatory effects of prostaglandin-E on cytokine production by human peripheral blood mononuclear cells are independent of the prostaglandin subtype. Immunology 2002;107:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheppach W, Sommer H, Kirchner T, et al. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology 1992;103:51–6. [DOI] [PubMed] [Google Scholar]
- 32.D’Argenio G, Cosenza V, Delle Cave M, et al. Butyrate enemas in experimental colitis and protection against large bowel cancer in a rat model. Gastroenterology 1996;110:1727–34. [DOI] [PubMed] [Google Scholar]
- 33.Wright DH, Ford-Hutchinson AW, Chadee K, et al. The human prostanoid DP receptor stimulates mucin secretion in LS174T cells. Br J Pharmacol 2000;131:1537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belley A, Chadee K. Prostaglandin E(2) stimulates rat and human colonic mucin exocytosis via the EP(4) receptor. Gastroenterology 1999;117:1352–62. [DOI] [PubMed] [Google Scholar]
- 35.Svendsen LB, Stener Jorgensen F, Hart Hansen O, et al. Influence of the prostaglandin E1 analogue rioprostil on the human gastric mucosa. Digestion 1987;37:29–34. [DOI] [PubMed] [Google Scholar]
- 36.Phillips TE, Stanley CM, Wilson J. The effect of 16,16-dimethyl prostaglandin E2 on proliferation of an intestinal goblet cell line and its synthesis and secretion of mucin glycoproteins. Prostaglandins Leukot Essent Fatty Acids 1993;48:423–8. [DOI] [PubMed] [Google Scholar]
- 37.Segain JP, Raingeard de la Bletiere D, Bourreille A, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut 2000;47:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cummings JH, Pomare EW, Branch WJ, et al. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987;28:1221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halttunen T, Marttinen A, Rantala I, et al. Fibroblasts and transforming growth factor beta induce organization and differentiation of T84 human epithelial cells. Gastroenterology 1996;111:1252–62. [DOI] [PubMed] [Google Scholar]
- 40.van Klinken BJ, Oussoren E, Weenink JJ, et al. The human intestinal cell lines Caco-2 and LS174T as models to study cell-type specific mucin expression. Glycoconj J 1996;13:757–68. [DOI] [PubMed] [Google Scholar]
- 41.Halm DR, Halm ST. Secretagogue response of goblet cells and columnar cells in human colonic crypts. Am J Physiol 1999;277:C501–22. [DOI] [PubMed] [Google Scholar]
- 42.Kedinger M, Simon-Assmann P, Bouziges F, et al. Epithelial-mesenchymal interactions in intestinal epithelial differentiation. Scand J Gastroenterol Suppl 1988;151:62–9. [DOI] [PubMed] [Google Scholar]
- 43.Sakata T, Setoyama H. Local stimulatory effect of short-chain fatty acids on the mucus release from the hindgut mucosa of rats (Rattus norvegicus). Comp Biochem Physiol A Physiol 1995;111:429–32. [DOI] [PubMed] [Google Scholar]
- 44.Finnie IA, Dwarakanath AD, Taylor BA, et al. Colonic mucin synthesis is increased by sodium butyrate. Gut 1995;36:93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singer II, Kawka DW, Schloemann S, et al. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology 1998;115:297–306. [DOI] [PubMed] [Google Scholar]