Abstract
Background: IgA serum autoantibodies against tissue transglutaminase (tTG) have an established diagnostic value in coeliac disease, and high efficacy tests are widely available for their detection. However, serological evaluation of IgA deficient subjects is still difficult.
Aims: To evaluate the diagnostic potential of IgG class anti-tTG autoantibodies measured quantitatively using an enzyme linked immunosorbent assay (ELISA) compared with immunofluorescent detection of coeliac autoantibodies.
Patients: We tested serum samples from 325 IgA deficient subjects, including 78 patients with coeliac disease, 73 disease controls, and 174 blood donors.
Methods: IgG antibodies against human recombinant tTG were measured with an ELISA. IgG antiendomysium antibodies (EMA) were assayed by indirect immunofluorescence on human jejunum and appendix sections.
Results: The IgG anti-tTG ELISA had a sensitivity of 98.7% and a specificity of 98.6%, and the correlation with IgG EMA titres was high (rs=0.91). One coeliac patient, initially negative in all autoantibody tests, displayed both IgG anti-tTG antibodies and IgG EMA during later gluten exposure. IgG anti-tTG antibodies and EMA titres showed significant decreases (p<0.001) in treated patients. The frequency of IgG anti-tTG autoantibody positivity was 9.8% among IgA deficient blood donors and 11 of the 12 positive subjects with known HLA-DQ haplotypes carried DQ2 or DQ8 alleles.
Conclusions: IgG anti-tTG and IgG EMA autoantibody tests are highly efficient in detecting coeliac disease in IgA deficient patients. The high prevalence of coeliac antibodies among symptom free IgA deficient blood donors who also carry coeliac-type HLA-DQ genes indicates that all IgA deficient persons should be evaluated for coeliac disease.
Keywords: coeliac disease, selective IgA deficiency, IgG transglutaminase autoantibodies, IgG endomysial autoantibodies
Selective IgA deficiency is the most common primary immunodeficiency in humans, occurring with an incidence of 1:400–500.1,2 Genetic studies imply that one important susceptibility locus is associated with the ancestral haplotype, HLA-A1,Cw7,B8,DR3,DQ2.3 The underlying immunoregulatory defect in B lymphocyte maturation is not fully understood and may be of variable severity. Joining of the variable immunoglobulin regions to IgA-type constant segments is altered, resulting in a defective IgA switch, while the majority of patients are still able to mount an effective immune response with IgG class antibodies.1
Most IgA deficient subjects have no obvious clinical symptoms but a variety of infections, allergies, and autoimmune disorders can be associated with this condition,4 including a 10–20-fold increased risk of coeliac disease, one other disorder associated with HLA-DQ2 and DQ8.5–8 The clinical course, therapy outcome, and rate of complications do not differ substantially between IgA deficient and IgA competent coeliac patients.6,8,9 However, serological detection and therapy monitoring of coeliac patients with IgA deficiency is more difficult as they will be negative in conventional assays detecting serum IgA antibodies against endomysium (EMA), reticulin (ARA), and tissue transglutaminase (tTG).
In IgA deficient patients with enteral symptoms and suspected of having coeliac disease, the traditional diagnostic approach comprised either routine use of jejunal biopsies or preselection of patients with IgG antigliadin antibody (AGA) tests.5,7 However, IgG AGA positivity, even in IgA deficient subjects, did not predict coeliac disease in population and case finding studies and did not reduce effectively the unnecessary invasive procedures.10,11 Several reports on small numbers of patients show that the IgG class counterparts of EMA, ARA, and tTG autoantibodies are often detectable in IgA deficient coeliac patients.12–18 However, both immunofluorescent methods and enzyme linked immunosorbent assays (ELISA) detecting IgG antibodies are, in general, technically difficult,19 and none of these assays has been validated against a large number of biopsied IgA deficient controls. Recently, recombinant human tTG antigens of high purity became available for ELISA detection of coeliac IgA antibodies20,21 and the aim of our study was to evaluate the diagnostic potential of IgG anti-tTG antibodies measured with a human tTG-ELISA in a large group of IgA deficient clinical patients (coeliac and non-coeliac) and in a group of IgA deficient blood donors.
PATIENTS AND METHODS
Patients
To identify IgA deficient coeliac patients, the following policy was used at the Heim Pál Children’s Hospital, Budapest. Serum samples submitted for IgA EMA analysis and found to be negative were analysed for IgG EMA in such cases where serum total IgA was low. Cut off values for IgG EMA testing were established from age related serum IgA ranges of normal Hungarian children22: <0.2 g/l in children aged 0–10 years and <0.4 g/l in older patients.
During the years 1989–2001, serum samples from 1807 new patients with low serum IgA levels were prospectively tested for IgG EMA, and a small intestinal biopsy was offered to patients with positive EMA results. IgA deficient patients with a clinical suspicion of coeliac disease underwent small intestinal biopsy also, independent of the EMA results. In total, 78 IgA deficient patients (median age 7.9 years (range 0.9–73), median serum IgA concentration 0.01 g/l (range 0.00–0.11)) were diagnosed with coeliac disease according to the revised ESPGHAN criteria,23 and follow up samples were collected from 36 of these patients after a prolonged period on a gluten free diet (range 0.4–4.7 years).
Furthermore, serum samples from 73 IgA deficient non-coeliac patients (median age 2 years (range 1–40)) who were negative for both IgA and IgG EMA and had total serum IgA levels <0.2 g/l were included in the study. Forty six of these control patients (controls I) had a jejunal biopsy performed and all had normal villous architecture; the other 27 patients (controls II) had no small intestinal biopsy performed. Table 1 ▶ shows the clinical symptoms of the IgA deficient coeliac and non-coeliac patients in whom diarrhoea, weight loss, hypoproteinaemia, and vitamin K deficiency were equally distributed. IgA deficient coeliac patients had however a significantly higher frequency of iron deficiency, short stature, and hypocalcaemia (table 1 ▶).
Table 1.
Presence of clinical symptoms (signs) and associated diseases in IgA deficient coeliac patients and non-coeliac control patients (%)
| Coeliac patients (n=78) | Controls I† (n=46) | Controls II† (n=27) | All controls* (n=73) | p Value‡ | |
| Clinical symptoms and signs | |||||
| Chronic or recurrent diarrhoea | 53 (67.9%) | 32 (69.6%) | 11 (40.7%) | 43 (58.9%) | 0.248 |
| Distended abdomen, bloating | 34 (43.6%) | 14 (30.4%) | 6 (22.2%) | 20 (27.4%) | 0.038 |
| Slow weight gain or weight loss | 57 (73.1%) | 32 (69.6%) | 11 (40.7%) | 43 (58.9%) | 0.067 |
| Short stature | 34 (43.6%) | 11 (23.9%) | 3 (11.1%) | 14 (19.2%) | 0.001 |
| Iron deficiency | 65 (83.3%) | 21 (45.7%) | 9 (33.3%) | 30 (41.1%) | <0.0001 |
| Hypocalcaemia, tetany | 10 (12.8%) | 1 ( 2.2%) | 0 | 1 (1.4%) | 0.007 |
| Hypoproteinaemia | 9 (11.5%) | 6 (13.0%) | 2 (7.4%) | 8 (11.0%) | 0.910 |
| Vitamin K deficiency | 5 (6.4%) | 1 (2.2%) | 1 (3.7%) | 2 (2.7%) | 0.284 |
| Associated diseases | (0.18) | ||||
| Insulin dependent diabetes mellitus | 4 (5.1%) | 1 (2.2%) | 0 | 1 (1.4%) | |
| Other autoimmune diseases | 2 (2.6%) | 1 (2.2%) | 0 | 1 (1.4%) | |
| Gastro-oesophageal reflux disease (GORD) | 0 | 4 (8.7%) | 0 | 4 (5.5%) | |
| Recurrent abdominal pain | 2 (2.6%) | 6 (13.0%) | 1 (3.7%) | 7 (9.6%) | |
*Final diagnoses: cow’s milk and/or soy protein enteropathy (n=24), jejunal bacterial overgrowth (n=6), Crohn’s disease (n=5), toddler’s diarrhoea (n=5), GORD (n=4), Giardia lamblia infection (n=2), cystic fibrosis (n=2), lymphangiectasia, Helicobacter pylori infection, cytomegalovirus infection (n=1 each), no gastrointestinal disease (n=14), asymptomatic IgA deficient family members of known coeliac patients (n=8).
†Controls I had a jejunal biopsy performed and all had normal villous architecture; controls II had no small intestinal biopsy performed.
‡The p values refer to the difference between coeliac disease patients and all control patients using the χ2 test.
IgG EMA and anti-tTG antibodies were also analysed in serum samples from 174 blood donors who had selective IgA deficiency with a total serum IgA level <0.02 g/l. These samples were collected during 1991–1992 at the Finnish Red Cross Blood Transfusion Service, Helsinki, Finland.24
Measurement of serum autoantibodies
Anti-tTG antibodies were measured from number coded serum aliquots stored at −40°C. IgA anti-tTG specific antibodies were measured with the Celikey assay (Pharmacia Diagnostics Freiburg, Germany) according to the manufacturer’s instructions. IgG anti-tTG antibodies were measured with a research ELISA (Pharmacia Diagnostics), as previously described.25 Antibody content of the sera was calculated in U/ml using a standard curve and values above 5 U/ml and 10 U/ml, respectively, were considered positive for IgA and IgG anti-tTG antibodies.
IgG EMA were assessed using an indirect immunofluorescent method on a composite block of monkey oesophagus, human jejunum, and appendix, or on human umbilical cord from premature newborns (blood donor samples), as described elsewhere.19,26 Positive results (1:⩾2.5 dilution) were quantified by further titration using appendix sections.
Genetic typing
Whole blood was available from 19 IgA deficient coeliac patients and from 12 IgG EMA positive blood donors for determination of HLA-DQB1* alleles, which was performed with the Dynal AllSet SSP DQ low resolution (Dynal AS, Oslo, Norway) and Olerup SSP DQ low resolution (GenoVision, Saltsjöbaden, Sweden) kits.
Immunoglobulin levels
Total serum IgA was determined in clinical patients using the Boehringer Mannheim/Hitachi 912 Analyzer (Boehringer Mannheim Corporation, Indianapolis, Indiana, USA) with Tina-quant IgA (Roche Diagnostics GmbH, Mannheim, Germany) reagents. In blood donors, serum IgA levels were measured by an enzyme immunoassay.27
Statistics
Antibody levels are expressed as median (5th and 95th centiles) values. The Mann-Whitney U test (two tailed) was used to estimate differences in antibody levels between groups, the Wilcoxon signed rank test to compare antibody levels before and after the diet, and the Spearman rank method (two tailed) to calculate the correlation between IgG EMA and anti-tTG antibody levels. The cut off level of the anti-tTG assay was calculated from receiver operating characteristics (ROC) analysis21 carried out with coeliac disease patients and all of the control subjects (controls I+controls II).
RESULTS
Serum antibodies on a normal diet
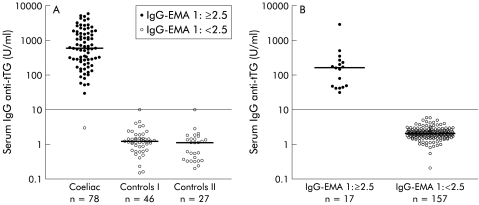
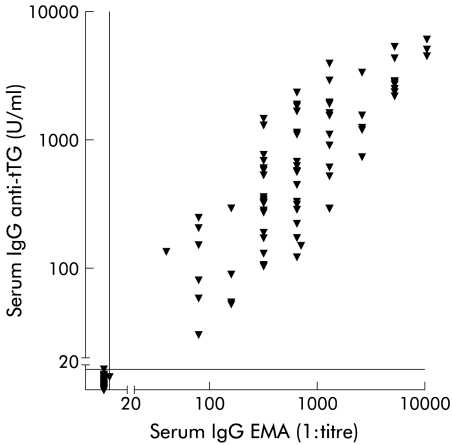
Serum levels of IgG anti-tTG antibodies of IgA deficient patients with coeliac disease, control patients, and blood donors are shown in fig 1A ▶ and 1B ▶. The area under the ROC curve was 0.996, and with a cut off value of 10 U/ml, the sensitivity and specificity of the IgG anti-tTG assay in clinical patients were 98.7% and 98.6%, respectively. The frequency of IgG anti-tTG autoantibody positivity was 9.8% among IgA deficient blood donors. There was a positive correlation (rs=0.91, p<0.0001) between IgG anti-tTG antibody and IgG EMA levels (fig 2 ▶).
Figure 1.
Serum levels of IgG antibodies against recombinant human tissue transglutaminase (tTG) in (A) coeliac disease patients, control subjects who had a small intestinal biopsy performed (controls I), and non-biopsied controls (controls II) and in (B) 174 IgA deficient blood donors. Positivity (titre 1:⩾2.5) and negativity (titre 1:<2.5) for serum IgG antibodies against endomysium (EMA) are represented. Horizontal lines represent median values. Cut off=10 U/ml.
Figure 2.
Serum levels of IgG antibodies against recombinant human tissue transglutaminase (tTG) plotted against the reciprocal serum titres of IgG antibodies against endomysium (EMA). Spearman correlation coefficient was 0.91 (95% confidence interval 0.877–935; p<0.0001). Vertical and horizontal lines represent cut off levels.
The youngest coeliac disease patient (11 months), who initially was negative for all autoantibodies, displayed elevated levels of IgG EMA and IgG anti-tTG antibodies during later gluten exposure. Two of the control patients had IgG anti-tTG antibody levels close to the cut off level and one of the coeliac disease patients (aged three years) had positive IgA anti-tTG antibody levels (12 U/ml) despite low total IgA levels (0.04 g/l). This patient and three additional coeliac patients negative for IgA anti-tTG antibodies and total serum IgA <0.05 g/l resumed normal serum IgA levels on a gluten free diet. None of the other subjects had elevated levels of IgA anti-tTG antibodies.
The 17 IgA deficient blood donors positive for IgG EMA had lower anti-tTG antibody levels (median 162 (40–990) U/ml) than the 78 coeliac disease patients with clinically manifest disease (median 602 (58–4330) U/ml). Also, IgG EMA titres of the blood donors (median 1:160 (1:20–1:2560)) were lower than for coeliac disease patients (median 1:640 (1:<2.5–1:10 240)).
Serum antibodies during a gluten free diet
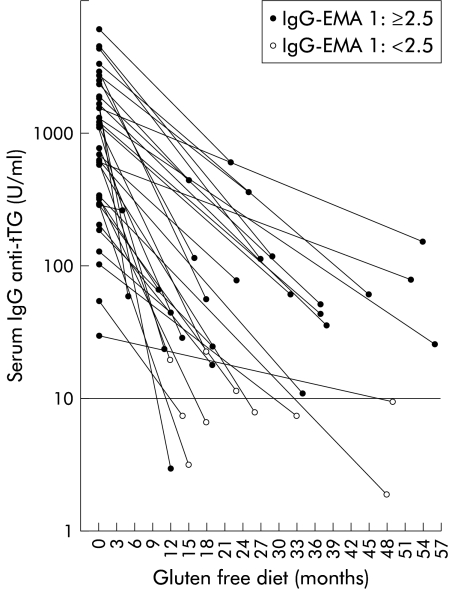
Initial IgG anti-tTG antibody levels of 36 coeliac patients and levels after various times on a gluten free diet are shown in fig 3 ▶. Median level of IgG EMA antibodies decreased from 1:640 (range 1:80–1:10 240) to 1:10 (range 1:<2.5–1:640) in the follow up sample (p<0.001). Initial IgG anti-tTG antibody values (median 928 (92–4346) U/ml) also showed a significant decrease in the follow up sample (median 40 (3–384) U/ml; p<0.0001).
Figure 3.
Serum levels of IgG antibodies against tissue transglutaminase (tTG) in 36 coeliac disease patients before and during a gluten free diet. IgG antiendomysium (EMA) titres 1:⩾2.5 (positive) and IgG anti-endomysium titres 1:<2.5 (negative) are represented. Cut off=10 U/ml.
Only 10 of 36 patients regained normal IgG EMA titres and eight of 36 normal IgG anti-tTG antibody levels after a long term gluten free diet. However, control jejunal biopsies performed after a gluten free diet in 17 coeliac patients with negative (n=9) or low (1:<20) IgG EMA titres (n=8) revealed villous recovery in all. One of the coeliac patients showed no reduction in IgG EMA or anti-tTG antibody levels after four months on a gluten free diet whereas another coeliac patient with unchanged IgG EMA titres displayed a fivefold reduction in IgG anti-tTG antibody levels (from 2326 to 448 U/ml) after 15 months on a gluten free diet. Concordance in positive and negative results between IgG EMA and anti-tTG antibodies was observed for 32 of 36 patients after a gluten free diet.
Genetic typing
Eighteen of 19 coeliac patients and 10 of 12 IgG anti-tTG positive blood donors were positive for HLA-DQ2, and one in each group was positive for HLA-DQ8. The single DQ2 and DQ8 negative antibody positive blood donor had only the DQB1*02 allele (that is, only the DQB1 part, but not the DQA1 part of the DQ2 heterodimer).
DISCUSSION
Circulating IgA class anti-tTG autoantibodies and EMA are highly prevalent and characteristic in active coeliac disease.15,20,21 Our results show that in as many as 98.7% of IgA deficient coeliac patients, disease specific IgG anti-tTG antibodies and EMA can be detected. Therefore, the humoral response targeting tTG as an autoantigen should be considered as a regular and diagnostically useful feature in all coeliac patients, both IgA competent and IgA deficient.
The human recombinant tTG based ELISA used in this study clearly out performs the guinea pig substrate based IgG class anti-tTG antibody test, earlier shown to be highly unspecific.15 Immunofluorescent investigation of serum IgG EMA has severe technical drawbacks because secondary antibodies against human IgG often bind to the tissue connective fibres in a similar non-specific manner.28 While the positive EMA reaction is easy to recognise, up to 50% of negative samples may show endomysial staining when monkey oesophagus substrate is used.26 For more specific results, human IgG1 specific or monkey immunoglobulin preadsorbed secondary antibodies,18,29,30 or substrates with low background (human fetal tissues, jejunum, or appendix) are needed.14,19 Given these technical requirements, only few laboratories offer IgG class coeliac antibody testing on a routine basis. In contrast, although the performance of IgG AGA tests is not optimal, they are widely accessible because they can be performed with simple ELISA methods. Introduction of an efficient ELISA for IgG anti-tTG antibody testing could clearly enhance the feasibility of detection of IgG coeliac autoantibodies for laboratories that have not mastered the IgG EMA method.
An advantage of the measurement of IgG anti-tTG antibodies with ELISA is the more precise quantification of serum antibody concentrations which can make monitoring a gluten free diet effective and more convenient. In the present study, a clear decrease was detected, even in patients where IgG-EMA was still considerably positive.
Due to impaired responses to mucosal immunostimuli, IgA deficient subjects frequently present with enteral complaints1 but coeliac disease combined with IgA deficiency accounts in general for only a few of cases in each gastroenterology centre.9 The prospective use of IgG EMA enabled us to identify a high number of IgA deficient coeliac patients, and also those with moderate and mild symptoms, and therefore we included all IgA deficient patients evaluated by biopsy during the same period to avoid significant bias. The ELISA used here identified coeliac patients effectively whereas none of the non-coeliac subjects had IgG anti-tTG antibody levels in the range of untreated coeliac patients (fig 1 ▶).
Furthermore, the decrease in IgG autoantibodies seems to be very slow in IgA deficient coeliac patients and most of our patients were still positive after more than two or three years on a gluten free diet. In contrast, IgA competent coeliac patients became negative for both IgA and IgG anti-tTG antibodies by one year on a gluten free diet.31 Dietary interviews in our centre did not reveal more lapses in coeliacs with IgA deficiency than in those without (data not shown), and mucosal healing was observed at low levels of IgG autoantibody positivity. In fact, all of these patients had several fold reductions in IgG anti-tTG antibody concentrations on a gluten free diet, clearly showing that IgG coeliac autoantibodies are indeed gluten dependent. Specific antibody production is dependent on T helper lymphocyte function, and T cell priming, cytokine profiles, and B cell responsiveness show alterations in IgA deficiency.32,33 Therefore, the slow disappearance kinetics of IgG coeliac antibodies may be part of the immunoregulatory defect seen in IgA deficiency. However, there are a number of reports showing that individuals with HLA B8 DR3 haplotypes have alterations in their immune response, regardless of their IgA status, and hence it is not clear whether these features are directly due to IgA deficiency as such or associated HLA genes.34
We also found that 9.8% of apparently healthy IgA deficient adult blood donors were positive for both IgG EMA and anti-tTG antibodies and may have undetected coeliac disease. Biopsies were not performed in these patients but they were genetically similar to the clinically diagnosed IgA deficient coeliac patients. Given the high specificity of the EMA and anti-tTG antibody tests in our clinical cases, the results further support the fact that known IgA deficient subjects have at least a 10-fold increased risk of coeliac disease6 and should be an important target group for case finding.
However, IgA deficiency is often not known at the time of coeliac antibody testing and the firstline use of IgG EMA instead of serum IgA measurements was successful in population screening.14 The IgG anti-tTG antibody ELISA seems to be an easy tool to evaluate samples with negative IgA EMA or anti-tTG antibodies and unknown serum IgA levels. However, the cost effectiveness of this approach remains to be established.
Humoral IgA deficiency is usually defined as a total serum IgA of <0.05 g/l but levels may fluctuate as a response to mucosal antigens and cytokines.35 Four of our IgA deficient patients resumed normal serum IgA levels on a gluten free diet although IgA in IgA competent coeliac patients usually decreases on a gluten free diet.36 One prominent regulator of the IgA class switch is transforming growth factor β (TGF-β), and mice lacking the TGF-β receptor type II develop serum IgA deficiency.37 Although TGF-β1 is increased in the coeliac mucosa,38 its action to promote epithelial cell differentiation on the crypt-villus axis seems to be impaired.39 The action of TGF-βs on the IgA switch also may be altered in genetically susceptible individuals who may also benefit from early recognition and treatment of coeliac disease in terms of IgA production and related pathology.
In conclusion, IgG anti-tTG antibodies measured with human tTG are highly reliable serum markers of coeliac disease in IgA deficient subjects. To ensure the detection of these patients, IgG anti-tTG measurements should be integrated into diagnostic and screening strategies and coeliac disease should be considered in all IgA deficient individuals.
Acknowledgments
The authors thank Drs Margit Lörincz, Katalin Szabados, Ágnes Horváth, and Márta Balogh for their work in the care of the patients. The study was supported by the Medical Research Fund of Tampere University Hospital, the Päivikki and Sakari Sohlberg Foundation, the Foundation of the Friends of the University Children’s Hospital in Finland, and the Finnish Coeliac Society.
Abbreviations
AGA, antigliadin antibodies
ARA, R1-type reticulin antibodies
ELISA, enzyme linked immunosorbent assay
EMA, endomysial antibodies
ROC, receiver operating characteristics
TGF-β, transforming growth factor beta
tTG, tissue or type 2 transglutaminase
REFERENCES
- 1.Cunningham-Rundles C. Physiology of IgA and IgA deficiency. J Clin Immunol 2001;21:303–9. [DOI] [PubMed] [Google Scholar]
- 2.Koistinen J. Selective IgA deficiency in blood donors. Vox Sang 1975;29:192–202. [DOI] [PubMed] [Google Scholar]
- 3.Vorechovsky I, Cullen M, Carrington M, et al. Fine mapping of IGAD1 in IgA deficiency and common variable immunodeficiency: identification and characterization of haplotypes shared by affected members of 101 multiple-case families. J Immunol 2000;164:4408–16. [DOI] [PubMed] [Google Scholar]
- 4.Koskinen S. Long-term follow-up of health in blood donors with primary selective IgA deficiency. J Clin Immunol 1996;16:165–70. [DOI] [PubMed] [Google Scholar]
- 5.Savilahti E, Pelkonen P, Visakorpi JK. IgA deficiency in children. A clinical study with special reference to intestinal findings. Arch Dis Child 1971;46:665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collin P, Mäki M, Keyrilainen O, et al. Selective IgA deficiency and coeliac disease. Scand J Gastroenterol 1992;27:367–71. [DOI] [PubMed] [Google Scholar]
- 7.Meini A, Pillan NM, Villanacci V, et al. Prevalence and diagnosis of celiac disease in IgA-deficient children. Ann Allergy Asthma Immunol 1996;77:333–6. [DOI] [PubMed] [Google Scholar]
- 8.Heneghan MA, Stevens FM, Cryan EM, et al. Celiac sprue and immunodeficiency states: a 25-year review. J Clin Gastroenterol 1997;25:421–5. [DOI] [PubMed] [Google Scholar]
- 9.Cataldo F, Marino V, Ventura A, et al. Prevalence and clinical features of selective immunoglobulin A deficiency in coeliac disease: an Italian multicentre study. Gut 1998;42:362–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catassi C, Fanciulli G, D’Appello AR, et al. Antiendomysium versus antigliadin antibodies in screening the general population for coeliac disease. Scand J Gastroenterol 2000;35:732–6. [DOI] [PubMed] [Google Scholar]
- 11.Schober E, Bittmann B, Granditsch G, et al. Screening by anti-endomysium antibody for celiac disease in diabetic children and adolescents in Austria. J Pediatr Gastroenterol Nutr 2000;30:391–6. [DOI] [PubMed] [Google Scholar]
- 12.Beutner EH, Kumar V, Chorzelski TP, et al. IgG endomysial antibodies in IgA-deficient patient with coeliac disease. Lancet 1989;1:1261–2. [DOI] [PubMed] [Google Scholar]
- 13.Mäki M, Hällström O, Vesikari T, et al. Evaluation of a serum IgA-class reticulin antibody test for the detection of childhood celiac disease. J Pediatr 1984;105:901–5. [DOI] [PubMed] [Google Scholar]
- 14.Korponay-Szabó IR, Kovács JB, Czinner A, et al. High prevalence of silent celiac disease in preschool children screened with IgA/IgG antiendomysium antibodies. J Pediatr Gastroenterol Nutr 1999;28:26–30. [DOI] [PubMed] [Google Scholar]
- 15.Sulkanen S, Halttunen T, Laurila K, et al. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology 1998;115:1322–8. [DOI] [PubMed] [Google Scholar]
- 16.Bazzigaluppi E, Lampasona V, Barera G, et al. Comparison of tissue transglutaminase-specific antibody assays with established antibody measurements for coeliac disease. J Autoimmun 1999;12:51–6. [DOI] [PubMed] [Google Scholar]
- 17.Cataldo F, Lio D, Marino V, et al. IgG(1) antiendomysium and IgG antitissue transglutaminase (anti-tTG) antibodies in coeliac patients with selective IgA deficiency. Gut 2000;47:366–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar V, Jarzabek-Chorzelska M, Sulej J, et al. Celiac disease and immunoglobulin A deficiency: how effective are the serological methods of diagnosis? Clin Diagn Lab Immunol 2002;9:1295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sulkanen S, Collin P, Laurila K, et al. IgA- and IgG-class antihuman umbilical cord antibody tests in adult coeliac disease. Scand J Gastroenterol 1998;33:251–4. [DOI] [PubMed] [Google Scholar]
- 20.Sblattero D, Berti I, Trevisiol C, et al. Human recombinant tissue transglutaminase ELISA: an innovative diagnostic assay for celiac disease. Am J Gastroenterol 2000;95:1253–7. [DOI] [PubMed] [Google Scholar]
- 21.Bürgin-Wolff A, Dahlbom I, Hadziselimovic F, et al. Antibodies against human tissue transglutaminase and endomysium in diagnosing and monitoring coeliac disease. Scand J Gastroenterol 2002;37:685–91. [DOI] [PubMed] [Google Scholar]
- 22.Csorba S, Karmazsin L. Serum immunoglobulin values in healthy infants and children (Hungarian). Gyermekgyógyászat 1977;28:32–5. [Google Scholar]
- 23.Walker-Smith JA, Guandalini S, Schmitz J, et al. Revised criteria for diagnosis of coeliac disease. Arch Dis Child 1990;65:909–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koskinen S, Tölö H, Hirvonen M, et al. Long-term persistence of selective IgA deficiency in healthy adults. J Clin Immunol 1994;2:116–19. [DOI] [PubMed] [Google Scholar]
- 25.Hansson T, Dahlbom I, Rogberg S, et al. Recombinant human tissue transglutaminase for diagnosis and follow-up of childhood coeliac disease. Pediatr Res 2002;51:700–5. [DOI] [PubMed] [Google Scholar]
- 26.Korponay-Szabó IR, Kovács JB, Lörincz M, et al. Prospective significance of antiendomysium antibody positivity in subsequently verified celiac disease. J Pediatr Gastroenterol Nutr 1997;25:56–63. [DOI] [PubMed] [Google Scholar]
- 27.Hirvonen M, Koskinen S, Tölö H. A sensitive enzyme immunoassay for the measurement of low concentrations of IgA. J Immunol Methods 1993;163:559–65. [DOI] [PubMed] [Google Scholar]
- 28.Kozlowska H, Rowinski J, Bem W, et al. Fibrous connective tissue of rat binds anti-immunoglobulin antibodies. Folia Histochem Cytobiol 1997;35:123–4. [PubMed] [Google Scholar]
- 29.Picarelli A, Sabbatella L, Di Tola M, et al. Celiac disease diagnosis in misdiagnosed children. Pediatr Res 2000;48:590–2. [DOI] [PubMed] [Google Scholar]
- 30.Prince HE, Norman GL, Binder WL. Immunoglobulin A (IgA) deficiency and alternative celiac disease-associated antibodies in sera submitted to a reference laboratory for endomysial IgA testing. Clin Diagn Lab Immunol 2000;7:192–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basso D, Guariso G, Plebani M. Serologic testing for celiac disease. Clin Chem 2002;48:2082–3. [PubMed] [Google Scholar]
- 32.Zhang Y, Pacheco S, Acuna CL, et al. Immunoglobulin A-deficient mice exhibit altered T helper 1-type immune responses but retain mucosal immunity to influenza virus. Immunology 2002;105:286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Ginkel FW, Wahl SM, Kearney JF, et al. Partial IgA-deficiency with increased Th2-type cytokines in TGF-beta 1 knockout mice. J Immunol 1999;163:1951–7. [PubMed] [Google Scholar]
- 34.Price P, Witt C, Allcock R, et al. The genetic basis for the association of the 8.1 ancestral haplotype (A1,B8,DR3) with multiple immunopathological diseases. Immunol Rev 1999;167:257–74. [DOI] [PubMed] [Google Scholar]
- 35.Marconi M, Plebani A, Avanzini MA, et al. IL-10 and IL-4 co-operate to normalize in vitro IgA production in IgA-deficient (IgAD) patients. Clin Exp Immunol 1998;112:528–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ratnaike RN, Wangel AG. Immunological abnormalities in coeliac disease and their response to dietary restriction. I. Serum immunoglobulins, antibodies and complement. Aust N Z J Med 1977;7:349–52. [DOI] [PubMed] [Google Scholar]
- 37.Cazac BB, Roes J. TGF-beta receptor controls B cell responsiveness and induction of IgA in vivo. Immunity 2000;13:443–51. [DOI] [PubMed] [Google Scholar]
- 38.Hansson T, Ulfgren AK, Lindroos E, et al. Transforming growth factor-beta (TGF-beta) and tissue transglutaminase expression in the small intestine in children with coeliac disease. Scand J Immunol 2002;56:530–7. [DOI] [PubMed] [Google Scholar]
- 39.Halttunen T, Mäki M. Serum immunoglobulin A from patients with celiac disease inhibits human T84 intestinal crypt epithelial cell differentiation. Gastroenterology 1999;116:566–72. [DOI] [PubMed] [Google Scholar]