Abstract
Background and aims: Hydrophobic bile acids contribute to hepatocellular injury in cholestasis and rapidly induce apoptosis in vitro; however, unlike Fas agonists, cholestasis does not cause extensive hepatocyte apoptosis. As antioxidants provide protection against bile acid induced liver injury, our premise was that bilirubin, a free radical scavenger with increased plasma levels in the presence of liver disease, could protect hepatocytes against bile acid induced apoptosis.
Methods: Freshly isolated rat hepatocytes were incubated for four hours with 100 μmol/l glycochenodeoxycholate (GCDC) alone or with increasing concentrations of unconjugated (UCB) or conjugated (CB) bilirubin.
Results: Both UCB and CB inhibited GCDC induced apoptosis in a dose dependent fashion and suppressed the generation of reactive oxygen species by hepatocytes.
Conclusions: The antiapoptotic effect of bilirubin associated with its antioxidant properties indicates that hyperbilirubinaemia may have a protective role in liver disease.
Keywords: apoptosis, bile acids, bilirubin, cholestasis, rat hepatocytes
In patients with cholestasis, accumulation of bile acids within the liver contributes to hepatocellular damage. While high bile acid concentrations can induce hepatocyte necrosis, lower concentrations of these compounds are associated with apoptosis1,2 which can be triggered by specific cell surface “death” receptors (extrinsic pathway) or by mitochondrial dysfunction induced by internal stress (intrinsic pathway),3 such as the generation of reactive oxygen species (ROS).4–6
Despite an increasing body of evidence demonstrating that bile acid mediated hepatocyte apoptosis plays an important role in hepatobiliary diseases,3,7 the incidence of parenchymal liver cell apoptosis in cholestatic disorders is lower than expected on the basis of in vitro studies.8 Possible explanations for this discrepancy include activation of antiapoptotic pathways8,9 and the presence of protective humoral factors. Free radical scavengers such as lazaroids,10 α-tocopherol, and ebselen4 inhibit bile acid induced hepatocyte apoptosis in vitro. Bilirubin, a yellow tetrapyrrole derived from the enzymatic degradation of haeme, also accumulates in the plasma of patients with cholestasis due to impaired biliary excretion.11 This lipid soluble pigment is commonly considered merely a toxic waste compound because neonatal hyperbilirubinaemia can lead to brain damage (“kernicterus”).1 However, the pigment acts as an efficient free radical scavenger in vitro at micromolar concentrations.13–15 Prior to biliary excretion, bilirubin is conjugated with one or two molecules of glucuronic acid, yielding a water soluble compound. Unconjugated (UCB) and conjugated (CB) bilirubin accumulate in tissues and plasma during cholestasis.11,16
In the present work, we demonstrate that both UCB and CB effectively inhibit bile acid induced apoptosis in freshly isolated hepatocytes, an effect associated with suppression of ROS generation, suggesting that these pigments may play a protective role in cholestatic disorders.
MATERIALS AND METHODS
Materials
CD rats were purchased from Charles River (Kisslegg, Germany). William’s E medium, fetal bovine serum, penicillin/streptomycin, glutamine, and collagenase were from Life Technologies Inc. (Grand Island, New York, USA). Trypan blue, dexamethasone, glucagon, glycochenodeoxycholate (GCDC), diamidino-2-phenylindole dihydrochloride (DAPI), and propidium iodide were supplied by Sigma Chemical Co. (St. Louis, Missouri, USA). UCB (Sigma Chemical Co) was recrystallised before use.17 CB ditaurate (a commercially available surrogate for bilirubin glucuronate) was from Porphyrin Products Inc. (Logan, Utah, USA). According to the manufacturer, the pigment contains approximately 10–12% monotaurate and less than 2% of UCB.
Experimental methods
Preparation of stock solutions of UCB and CB
Stock solutions of 42.75, 21.38, and 10.69 mmol/l UCB were prepared by dissolving 25, 12.5, and 6.25 mg of pigment, respectively, in 1 ml of 0.1 N NaOH. After complete dissolution, 1.750 ml of albumin solution (70 mg bovine serum albumin (BSA) in 1.75 ml of William’s E medium) were added to each UCB solution. The solutions, constantly protected from light, were diluted with incubation medium containing 5% fetal bovine serum to the desired pigment concentrations (200, 100, and 50 μmol/l) just before use. As the albumin content of 5% fetal bovine serum corresponded to 13.44 μmol/l, the final albumin concentration in incubation medium (4.73 μmol/l from added BSA+13.44 μmol/l from fetal bovine serum) was 18.17 μmol/l. The bilirubin/albumin molar ratios in the 200, 100, and 50 μmol/l solutions were therefore 11, 5.5, and 2.75, respectively. As bile salts are also bound to albumin, the albumin solution without bilirubin was diluted with incubation medium to obtain the same final concentration of BSA in experiments not including bile pigments. The GCDC/albumin molar ratio was 5.5. UCB solution was centrifuged and the bilirubin concentration assessed in duplicate by the diazo method before and after centrifugation. No change in bilirubin concentration was observed after centrifugation, nor were pigment aggregates found at microscopic examination (400×) of the solution. The pH of the culture medium containing BSA-UCB (10 ml aliquots) was adjusted to 7.4 by adding 10–25 μl of 1.2 M HCl. CB was dissolved directly in William’s E medium containing BSA and fetal bovine serum as described above, and no pH correction was necessary.
Hepatocyte isolation and culture
Rat primary hepatocytes were isolated from male CD rats (150–200 g) by collagenase perfusion of the liver.18 Cell viability, determined by trypan blue exclusion, was 85–93%. After isolation, hepatocytes were resuspended in William’s E medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 U/ml streptomycin, 3 mM glutamine, 0.16 U/ml insulin, 9.6 μg/ml dexamethasone, and 0.014 μg/ml glucagon. A total of 5×105 cells/well, seeded onto uncoated plastic tissue culture plates, were maintained at 37°C in a 5% CO2 humidified atmosphere for three hours. Plates were then washed with the medium to remove unattached cells and incubated with a medium containing 5% fetal bovine serum, 100 U/ml penicillin, 100 U/ml streptomycin, 3 mM glutamine, 0.16 U/ml insulin, and 100 μmol/l GCDC, alone or in combination with 50, 100, or 200 μmol/l UCB or CB for four hours at 37°C in 5% CO2 humidified atmosphere. Elsewhere, we observed that approximately 1% of UCB added to the incubation medium is converted by cultured rat hepatocytes to CB after four hours.19
Assessment of nuclear fragmentation (apoptosis)
Isolated rat hepatocytes were plated onto uncoated plastic tissue culture plates (5×105 cells/well) and incubated for four hours with 100 μmol/l GCDC alone or in combination with UCB or CB. Nuclear changes indicating apoptosis were quantified by staining rat hepatocytes with DAPI, a membrane permeant fluorescent DNA binding dye to label the nucleus of hepatocytes. Propidium iodide (1 μM) was also added to identify necrotic cells. For morphological evaluation of apoptosis at fluorescence microscopy, cultured rat hepatocytes were incubated with DAPI (1 μg/ml) for 10 minutes at 37°C. Cells were considered apoptotic if the classic features of nuclear margination/condensation and nuclear fragmentation were present. Fluorescent stained nuclei were considered fragmented if at least three separate fragments of condensate chromatin were identified in a cell. At least 300 cells in four high power fields were counted, and nuclear fragmentation was expressed as a percentage of total cells, excluding propidium iodide.20,21
Measurement of reactive oxygen species (ROS) generation
Isolated hepatocytes (5×105 cells/well) were preloaded with 10 μmol/l 2.79-dichlorofluorescein (DCF) at 37°C for 30 minutes, washed twice, and resuspended in the incubation medium with or without 100 μmol/l GCDC and in the presence or absence of 50, 100, and 200 μmol/l UCB or CB. Aliquots of cells, removed at 0, 60, 120, 180, and 240 minutes, were analysed for fluorescence in real time at 490 nm excitation and 520 nm emission wavelengths in a 1420 Victor 2 (EG&G Wallac, Turku, Finland) fluorescence spectrophotometer.
Enzyme release
During the four hour incubation of hepatocytes with or without GCDC, UCB, and CB, cellular release of lactate dehydrogenase (LDH), glutamate dehydrogenase (GLDH), and aspartate aminotransferase (AST) was measured hourly, and expressed as a percentage of enzyme released into the buffer of the total activity present in hepatocytes (sum of the activities measured after cell lysis and in the medium). Measurement of GLDH, LDH, and AST was achieved using enzymatic method kits (Randox, Crumlin, UK, Roche Diagnostica SpA, Monza, Italy, and Roche Diagnostica SpA, Monza, Italy, respectively). All measurements were performed using an automatic analyser (Hitachi 912, Tokyo, Japan).
Statistics
Values are expressed as mean (SD). A GraphPad InStat microcomputer program (GraphPad Software, Inc. San Diego, California, USA) was used to evaluate differences between groups with the Mann-Whitney rank sum test and regression analyses. A p value <0.05 was considered statistically significant.
RESULTS
Morphological features of apoptosis
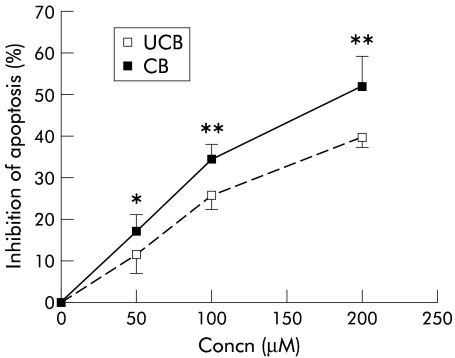
After four hours of treatment with 100 μmol/l GCDC, 63.32% (1.84%) of hepatocytes were apoptotic whereas <1% of cells were apoptotic in the absence of the bile acid, as assessed by nuclear fragmentation after DAPI staining (table 1 ▶, fig 1 ▶). Both UCB and CB inhibited GCDC induced apoptosis in a concentration dependent fashion. The inhibitory effect of CB on GCDC induced apoptosis was significantly stronger compared with UCB (fig 2 ▶).
Table 1.
Effect of unconjugated (UCB) and conjugated (CB) bilirubin on glycochenodeoxycholate (GCDC) induced apoptosis. Rat primary hepatocytes in culture were incubated for four hours with incubation medium alone or with 100 µmol/l GCDC and with increasing concentrations of UCB and CB
| Experimental group (n = 6) | % Apoptosis |
| Incubation medium | 0.66 (0.51) |
| UCB 50 µM | 0.67 (0.52) |
| UCB 100 µM | 0.83 (0.41) |
| UCB 200 µM | 0.66 (0.51) |
| CB 50 µM | 0.83 (0.41) |
| CB 100 µM | 0.70 (0.54) |
| CB 200 µM | 0.70 (0.55) |
| GCDCA 100 µM | 63.32 (1.84) |
| GCDCA 100 µM+UCB 50 µM | 55.97 (3.28)** |
| GCDCA 100 µM+UCB 100 µM | 42.92 (2.91)** |
| GCDCA 100 µM+UCB 200 µM | 38.21 (2.15)** |
| GCDCA 100 µM+CB 50 µM | 52.50 (2.43)** |
| GCDCA 100 µM+CB 100 µM | 35.50 (3.08) |
| GCDCA 100 µM+CB 200 µM | 30.33 (4.27) |
Results are expressed as mean (SD) per cent apoptosis occurring in GCDC treated cells.
**p<0.005 compared with GCDC.
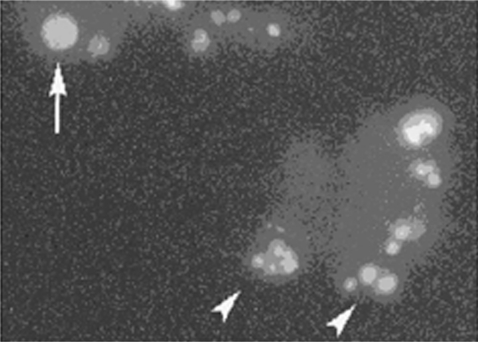
Figure 1.
Morphological features of glycochenodeoxycholate (GCDC) induced apoptosis of rat hepatocytes. Fluorescence photomicrograph of cells stained with the DNA binding dye diamidino-2-phenylindole dihydrochloride (DAPI). Cells undergoing apoptosis after four hours of incubation with 100 μmol/l GCDC showed nuclear fragmentation (arrowheads). A cell with a normal nucleus is visible on the upper left corner (arrow) (original magnification 400×).
Figure 2.
Inhibition of glycochenodeoxycholate (GCDC) induced apoptosis by unconjugated (UCB) and conjugated (CB) bilirubin. Rat primary hepatocytes in culture were incubated for four hours with 100 μmol/l GCDC and with increasing concentrations of UCB or CB. Both the unconjugated and conjugated pigment inhibited GCDC induced apoptosis but CB exhibited a stronger effect (mean (SD), n = 6). *p<0.05, **p<0.005 compared with UCB.
GCDC induced ROS generation
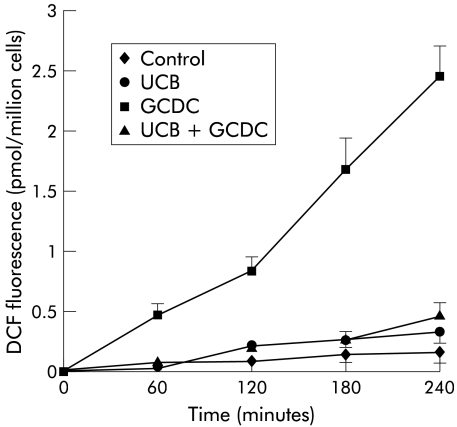
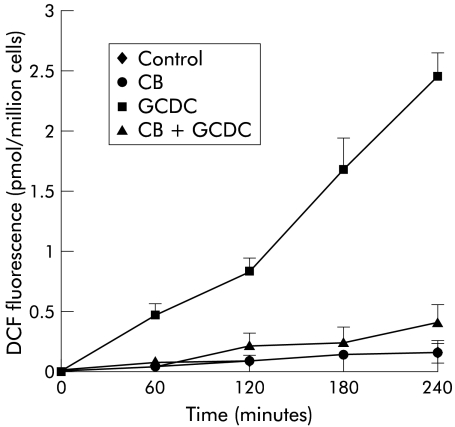
In order to investigate any relationship between bilirubin inhibition of GCDC induced apoptosis and the antioxidant properties of the pigment, ROS generation was assessed in rat hepatocytes treated with 100 μmol/l GCDC in the presence or absence of 50, 100, and 200 μmol/l UCB or CB, as described above. Addition of both pigments strongly suppressed the increase in GCDC stimulated DCF fluorescence, thus indicating strong suppression of ROS generation (figs 3 ▶, 4 ▶; only data obtained at a concentration of 100 μmol/l are shown). Inhibition of ROS generation was not significantly different with UCB or CB at any concentration tested.
Figure 3.
Effect of unconjugated bilirubin (UCB) on glycochenodeoxycholate (GCDC) induced hydroperoxide generation. Rat hepatocytes were incubated for four hours with control culture medium, or with medium containing 100 μmol/l UCB, 100 μmol/l GCDC, or a combination of both. UCB strongly suppressed GCDC induced 2.79-dichlorofluorescein (DCF) fluorescence, a measurement of hydroperoxide generation (mean (SD), n = 6).
Figure 4.
Effect of conjugated bilirubin (CB) on glycochenodeoxycholate (GCDC) induced hydroperoxide generation. Rat hepatocytes were incubated for four hours with control culture medium, or with medium containing 100 μmol/l CB, 100 μmol/l GCDC, or a combination of both. CB strongly suppressed GCDC induced 2.79-dichlorofluorescein (DCF) fluorescence, a measurement of hydroperoxide generation (mean (SD), n = 6).
Enzyme release
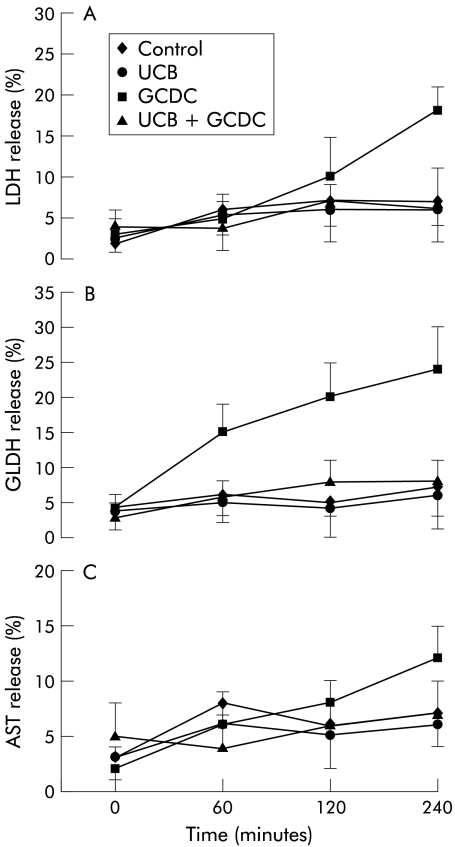
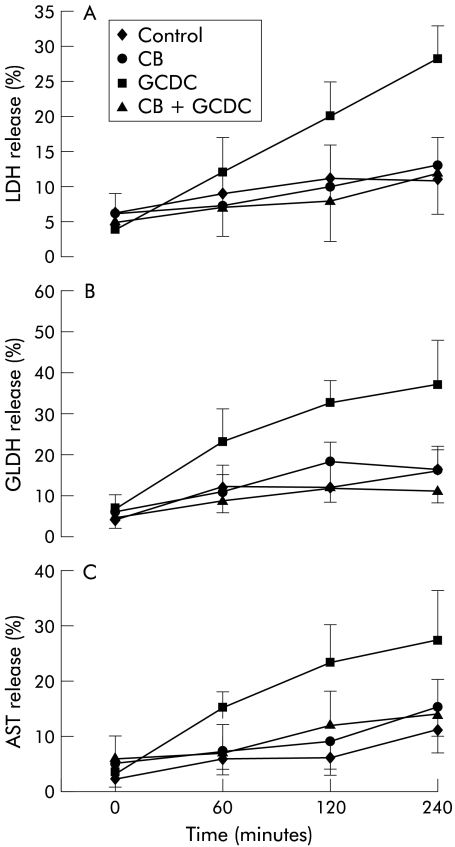
Incubation with 100 μmol/l GCDC was followed by increased release of LDH, GLDH, and AST with respect to control samples. Coincubation with 100 μmol/l UCB or CB strongly reduced the release of all enzymes tested (figs 5 ▶, 6 ▶).
Figure 5.
Bile acid stimulated enzyme release from hepatocytes was inhibited by unconjugated bilirubin (UCB). Hepatocytes were incubated with 100 μmol/l glycochenodeoxycholate (GCDC) with or without 100 μmol/l UCB. Enzyme activity released in the incubation medium was expressed as per cent of total enzyme activity measured on cell lysates at the end of each time point. Release of lactate dehydrogenase (LDH) (A), glutamate dehydrogenase (GLDH) (B), and aspartate aminotransferase (AST) (C) to control medium and after incubation with UCB, GCDC, and GCDC+UCB for four hours (mean (SD), n = 6).
Figure 6.
Bile acid stimulated enzyme release from hepatocytes was inhibited by conjugated bilirubin (CB). Hepatocytes were incubated with 100 μmol/l glycochenodeoxycholate (GCDC) with or without 100 μmol/l CB. Enzyme activity released in the incubation medium was expressed as per cent of total enzyme activity measured on cell lysates at the end of each time point. Release of lactate dehydrogenase (LDH) (A), glutamate dehydrogenase (GLDH) (B), and aspartate aminotransferase (AST) (C) to control medium and after incubation with CB, GCDC, and GCDC+CB for four hours (mean (SD), n = 6).
DISCUSSION
Although our understanding of the pathogenesis of cholestatic liver disease is incomplete, it is generally believed that accumulation of toxic hydrophobic bile acids, such as deoxycholic acid conjugates, within the hepatocyte can contribute to liver injury by inducing hepatocyte apoptosis.7,3 Antioxidants, such as α-tocopherol or lazaroid, reduce both the generation of ROS and cell injury in freshly isolated hepatocytes treated with GCDC4,10 as well as in the intact rat infused with taurochenodeoxycholic acid.22 Bilirubin, the yellow pigment which accumulates in the plasma of patients with cholestasis, was recognised as an antioxidant of possible physiological importance approximately 15 years ago, and its activity as a free radical scavenger was demonstrated in model membrane systems, being equal to or even surpassing that of α-tocopherol.13,14 As bilirubin interacts with biomembranes, it was postulated that this pigment could prevent lipid peroxidation associated with alterations in the physicochemical properties of the membranes leading to cell dysfunction and death.13 The protection against oxidative stress provided by the pigment has been demonstrated in several in vitro and in vivo studies.15,23,24
The findings in the present study demonstrate that bilirubin inhibits GCDC induced apoptosis in rat hepatocytes, and that this effect is associated with inhibition of ROS generation in the same culture system, thus suggesting a link between the antioxidant properties of the pigment and its ability to prevent bile acid induced apoptosis. Four hour incubation of hepatocytes with GCDC led to a slight increase in LDH, GLDH, and AST, which was prevented by addition of bile pigments to the medium (figs 5 ▶, 6 ▶), further suggesting that the membrane protective activity of bilirubin could help to reduce bile acid induced cell death.
The range of bilirubin concentrations tested in the in vitro assays are commonly found in the plasma of patients with cholestatic disorders. In these patients, plasma concentrations of bile acids and bilirubin are about equimolar.25 The final BSA concentration in incubation medium, corresponding to 18.17 μmol/l, was well below both physiological levels (approximately 600 μmol/l) and levels observed in patients with end stage cholestatic liver disease (approximately 300 μmol/l). However, low albumin concentrations have been used in all published studies involving apoptosis induced in vitro by toxic albumin bound compounds in order to achieve, in short incubation periods, significant interaction of the test compound with cultured cells, thus mimicking the conditions of a prolonged exposure in vivo (such as in cholestasis).1–2,4–6,26 Moreover CB, which is not bound to albumin and is the most abundant pigment fraction in the blood of cholestatic patients, inhibited bile acid induced apoptosis to an even greater extent than UCB (fig 2 ▶). Preliminary results showed that CB also behaves as a free radical scavenger.14 In the present experimental setup, suppression of ROS generation was similar with the two pigments. However, further studies are needed to compare the relative antioxidant effect of UCB and CB.
The above findings indicate that bilirubin may prevent the bile acid induced liver injury associated with cholestatic disorders, thus supporting the hypothesis that the pigment plays a “beneficial” role as a powerful biological antioxidant.13,14 In our incubation system with freshly isolated hepatocytes, addition of 100 μmol/l UCB or CB almost completely prevented GCDC induced ROS formation (figs 3 ▶, 4 ▶), but the average inhibition of apoptosis at the same pigment concentration was only 25.9% and 34.5%, respectively. These data lend support to the hypothesis that oxidative stress is not the main trigger of apoptosis but is a secondary phenomenon, amplifying the toxic effect of bile acids.3
According to Silva and colleagues,26 UCB induced apoptosis in cultured neuronal cells, and this effect could be prevented by ursochenodeoxycholic acid. These results seem opposite to our findings. However, our experimental conditions are not comparable with those of Silva et al because while UCB is toxic to neurones it has not been shown to have any adverse effects on hepatocytes. Moreover, ursodeoxycholic acid is an antiapoptotic hydrophilic bile acid27 whereas GCDC is a detergent toxic compound.
In conclusion, our experimental findings indicate that the antioxidant properties of bilirubin may be relevant in liver disease, suggesting a protective role of the pigment accumulating in plasma and tissues of patients with cholestasis. The present results could have therapeutic implications as removal of bile pigments from plasma by liver support devices28–30 might not be necessarily advantageous in patients with liver disease. As concentrations of UCB were in a similar range as serum total bilirubin levels in physiological jaundice, these findings suggest that physiological jaundice may also have antioxidant properties useful in the newborn. Further studies are needed to assess the clinical relevance of these findings.
Acknowledgments
This work was financed by Regione del Veneto, Giunta Regionale, Ricerca Sanitaria finalizzata n. 03/03/01, Venezia, Italy
Abbreviations
GCDC, glycochenodeoxycholate
UCB, unconjugated bilirubin
CB, conjugated bilirubin
ROS, reactive oxygen species
DAPI, diamidino-2-phenylindole dihydrochloride
BSA, bovine serum albumin
DCF, dichlorofluorescein
LDH, lactate dehydrogenase
GLDH, glutamate dehydrogenase
AST, aspartate aminotransferase
REFERENCES
- 1.Sokol RJ, Winklhofer-Roob BM, Devereaux MW, et al. Generation of hydroperoxides in isolated rat hepatocytes and hepatic mitochondria exposed to hydrophobic bile acids. Gastroenterology 1995;109:1249–56. [DOI] [PubMed] [Google Scholar]
- 2.Galle PR, Theilmann L, Raedsch R, et al. Ursodeoxycholate reduces hepatotoxicity of bile salts in primary human hepatocytes. Hepatology 1990;12:486–91. [DOI] [PubMed] [Google Scholar]
- 3.Guicciardi ME, Gores GJ. Bile acid-mediated hepatocyte apoptosis and cholestatic liver disease. Dig Liver Dis 2002;34:387–92. [DOI] [PubMed] [Google Scholar]
- 4.Yerushalmi B, Dahl R, Devereaux MW, et al. Bile acid-induced rat hepatocyte apoptosis is inhibited by antioxidants and blockers of the mitochondrial permeability transition. Hepatology 2001;33:616–26. [DOI] [PubMed] [Google Scholar]
- 5.Sokol RJ, Straka MS, Dahl R, et al. Role of oxidant stress in the permeability transition induced in rat hepatic mitochondria by hydrophobic bile acids. Pediatr Res 2001;49:519–31. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues CM, Fan G, Wong PY, et al. Ursodeoxycholic acid may inhibit deoxycholic acid-induced apoptosis by modulating mitochondrial transmembrane potential and reactive oxygen species production. Mol Med 1998;4:165–78. [PMC free article] [PubMed] [Google Scholar]
- 7.Patel T, Gores GJ. Apoptosis and hepatobiliary disease. Hepatology 1995;21:1725–41. [DOI] [PubMed] [Google Scholar]
- 8.Kurosawa H, Que FG, Roberts LR, et al. Hepatocytes in the bile duct-ligated rat express Bcl-2. Am J Physiol 1997;72:G1587–93. [DOI] [PubMed] [Google Scholar]
- 9.Miyoshi H, Rust C, Guicciardi ME, et al. NF-kappaB is activated in cholestasis and functions to reduce liver injury. Am J Pathol 2001;158:967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel T, Gores GJ. Inhibition of bile-salt-induced hepatocyte apoptosis by the antioxidant lazaroid U83836E. Toxicol Appl Pharmacol 1997;142:116–22. [DOI] [PubMed] [Google Scholar]
- 11.Muraca M, Fevery J, Blanckaert N. Analytic aspects and clinical interpretation of serum bilirubins. Semin Liver Dis 1988;8:137–47. [DOI] [PubMed] [Google Scholar]
- 12.Ostrow JD, Pascolo L, Tiribelli C. Mechanisms of bilirubin neurotoxicity. Hepatology 2002;35:1277–80. [DOI] [PubMed] [Google Scholar]
- 13.Stocker R, Yamamoto Y, McDonagh AF, et al. Bilirubin is an antioxidant of possible physiological importance. Science 1987;235:1043–6. [DOI] [PubMed] [Google Scholar]
- 14.Stocker R, Glazer AN, Ames BN. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci U S A 1987;84:5918–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomaro ML, Batlle AM. Bilirubin: its role in cytoprotection against oxidative stress. Int J Biochem Cell Biol 2002;34:216–20. [DOI] [PubMed] [Google Scholar]
- 16.Van Hootegem P, Fevery J, Blanckaert N. Serum bilirubins in hepatobiliary disease: comparison with other liver function tests and changes in the postobstructive period. Hepatology 1985;5:112–17. [DOI] [PubMed] [Google Scholar]
- 17.Ostrow JD, Hammaker L, Schmid R. The preparation of crystalline bilirubin-C14. J Clin Invest 1961;40:1442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol 1976;113:29–83. [DOI] [PubMed] [Google Scholar]
- 19.Vilei MT, Granato A, Ferraresso C, et al. Comparison of pig, human and rat hepatocytes as a source of liver specific metabolic functions in culture systems—implications for use in bioartificial liver devices. Int J Artif Organs 2001;24:392–6. [PubMed] [Google Scholar]
- 20.Patel T, Bronk SF, Gores GJ. Increases of intracellular magnesium promote glycodeoxycholate-induced apoptosis in rat hepatocytes. J Clin Invest 1994;94:2183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeid IM, Bronk SF, Fesmier PJ, et al. Cytoprotection by fructose and other ketohesoses during bile salt-induced apoptosis of hepatocytes. Hepatology 1997;25:81–6. [DOI] [PubMed] [Google Scholar]
- 22.Sokol RJ, McKim JM Jr, Goff MC, et al. Vitamin E reduces oxidant injury to mitochondria and hepatotoxicity of intravenous taurochenodeoxycholic acid in the rat. Gastroenterology 1998;114:164–74. [DOI] [PubMed] [Google Scholar]
- 23.Meyer M. Association of serum bilirubin concentration with risk of coronary artery disease. Clin Chem 2000;46:1723–7. [PubMed] [Google Scholar]
- 24.Hammerman C, Goldschmidt D, Caplan MS, et al. Protective effect of bilirubin in ischemia-reperfusion injury in the rat intestine. J Pediatr Gastroenterol Nutr 2002;35:344–9. [DOI] [PubMed] [Google Scholar]
- 25.Neale G, Lewis B, Weaver V. Serum bile acids in liver disease. Gut 1971;12:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva RF, Rodrigues CM, Brites D. Bilirubin-induced apoptosis in cultured rat neural cells is aggravated by chenodeoxycholic acid but prevented by ursodeoxycholic acid. J Hepatol 2001;34:402–8. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez CM, Fan G, Ma X, et al. A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation. J Clin Invest 1998;101:2790–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takenaka Y. Bilirubin adsorbent column for plasma perfusion. Ther Apher 1998;2:129–33. [DOI] [PubMed] [Google Scholar]
- 29.Pazzi P, Scagliarini R, Puviani AC, et al. Biochemical assessment and clinical evaluation of a non-ionic adsorbent resin in patients with intractable jaundice. Int J Artif Organs 2000;23:312–18. [PubMed] [Google Scholar]
- 30.Heemann U, Treichel U, Loock J, et al. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatology 2002;36:949–58. [DOI] [PubMed] [Google Scholar]