The frequency of Crohn’s disease (CD) has increased substantially over the last 50 years. It is most prevalent in highly industrialised temperate regions. CD and ulcerative colitis (UC) are rare in less developed countries. This suggests that critical environmental factors affect the worldwide distribution of inflammatory bowel disease (IBD). The “IBD hygiene hypothesis” states that raising children in extremely hygienic environments negatively affects immune development which predisposes them to immunological diseases such as IBD.1 It is also postulated that the modern day lack of exposure to helminths due to our hygienic practices is an important environmental factor contributing to IBD. Until modern times, nearly all children and most adults harboured intestinal helminths. Helminths and the immune system of Homo sapiens co-evolved in close proximity over many 1000s of years. Helminths regulate their host’s immune system and prevent excessive inflammatory responses, which could underlie the mechanism of protection. Moreels and colleague2 now lend further support for this hypothesis by reporting in this issue of Gut that infection with the helminth Schistosoma mansoni protects rats from trinitrobenzene sulphonic acid (TNBS) induced colitis [see page 99].
Approximately two million people in the USA and Europe have CD or UC, which usually begins during the second to third decade of life. IBD probably results from an inappropriately vigorous immune response to contents of the intestinal lumen. Evidence supporting this contention includes the effectiveness of immune suppressants at controlling the disease and experimental data derived from mice prone to IBD because of defects in immune regulation.3 In most of these murine models, the inflammation is driven by T helper 1 (Th1) circuitry and by substances in the intestinal lumen.
THE CASE FOR GENETICS IN IBD
UC and CD are disorders of complex derivation caused by the interplay of poorly defined environmental exposures and, at least in some instances, the inheritance of susceptibility genes. Often cited as evidence for genetic predisposition for IBD is the higher than expected occurrence of IBD in family members of patients with this condition and the high prevalence of the disease in Jewish populations of Western countries.4 Yet IBD is much less prevalent in the Jewish population of Israel5 with similar ethnic origin.6 Twin studies provide evidence of genetic predisposition for at least CD.7 A genetic defect in CARD15/NOD2, an intracellular protein that senses the bacterial product muramyl dipeptide,8,9 leaves some people more susceptible to CD. Various other genetic alterations are proposed as IBD risk factors. Yet genetic predispositions do not explain the rapidly increasing incidence of disease.
THE CASE FOR ENVIRONMENT IN IBD
There certainly are important environmental factors that affect the regional frequency of these diseases worldwide. Smoking is a risk factor for CD.10,11 Appendectomy for appendicitis under the age of 20 years decreases the incidence of UC.12–14 The risk for IBD varies according to geography and occupation. There is a North-South gradient of IBD in the USA and Europe, with IBD being more common in people raised in the North.15,16 US military veterans are at low risk for this disease if they were raised in the rural South,17 were prisoners of war, or served in combat in tropical regions.18 People with blue collar jobs exposing them to dirt and physical exercise are less prone to IBD.19 IBD is more common in urban versus rural areas.20 CD and UC are rare in South America,21 Central America, Africa,22,23 and Asia24 with the White population of South Africa being the exception.25 Migration studies show that children of people from regions of low CD or UC frequency acquire a greater risk for IBD if they move to areas of high disease prevalence.26–28
THE HABITAT OF HELMINTHS
Helminths are parasitic animals (worms) which, depending on species, live in locations such as the intestinal lumen, blood stream, or muscles of the host. These organisms colonise more than one third of the world population. Helminth colonisation is most common in children living in warm climates and subject to poor sanitation. The infective forms of these organisms are spread through contact with contaminated soil, food, or water. Before the 1940s, many children and adults in the USA carried helminths. Worm carriage was particularly common in rural areas of the South and in indigent populations of major cities.1 In the USA and Europe, helminthic colonisation has steadily declined. They are found in recent immigrants from less developed countries29 and in economically disadvantaged populations living in underserved regions of the USA such as some Indian reservations.30 These groups are at low risk for IBD. There is an inverse relationship between the frequency of worm colonisation and the prevalence of CD. There is more CD in urban versus rural populations, in northern versus southern regions of the USA and Europe, and developed versus less developed countries. The opposite is true for worm carriage.
IMMUNE REGULATION AND IBD
Inflammation can generate various regulatory agents such as interleukin (IL)-10, transforming growth factor β (TGF-β), IL-4, IL-13, and prostaglandin E2 (PGE2) that help modulate immune responses and limit tissue injury at mucosal surfaces. IL-10 is a mediator with strong immunomodulatory actions. For instance, IL-10 inhibits macrophage and dendritic cell function and suppresses the production of important proinflammatory cytokines such as tumour necrosis factor α, IL-12, IL-1, nitric oxide, and various chemokines. Mice with a disruption of the IL-10 gene develop severe colitis showing the importance of IL-10 for mucosal immune homeostasis.31 TGF-β mediates highly pleiotropic immunoregulatory functions, and transgenic mice with a T cell selective blockade in TGF-β signalling develop colitis.32 PGE2 is another well known factor that influences T helper 1 cell/T helper 2 cell (Th1/Th2) activation. It preferentially downregulates IL-12 receptor expression, inhibits the differentiation of Th1 cells, blocks IL-12 production from antigen presenting cells, and more. Mice deficient in the PGE receptor EP4 are more subject to dextran sodium sulphate induced colitis33 suggesting that PGE2 is important for mucosal protection.
Regulatory T cells can induce peripheral tolerance and limit mucosal reactivity.34 In various animal models, several regulatory T cell phenotypes have been reported. Some express CD4 while others CD8. In some systems, they are distinguished through differential expression of surface molecules, such as CD25, CD45RB, and CTLA-4. This pattern of cell surface protein expression suggests that they may be in a primed effector or memory state. These regulatory cells may mediate some of their affects through production of IL-10 and TGF-β. Described is an anergic regulatory T cell (Tr1) that produces high levels of IL-10 and TFG-β. Another cell called Th3 suppresses induction of experimental autoimmune encephalomyelitis primarily through production of TGF-β. Still others are not dependent on soluble IL-10 or TGF-β but instead express on their surface latency associated peptide, which is the amino terminal domain of the TGF-β precursor peptide.35 These cells can induce suppression via cell-cell contact.
Rag mice reconstituted with CD4+, CD45high T cells can develop severe colitis, which can be prevented by cotransfer of CD4+, CD45low T cells.36 TGF-β and IL-10 are required for protection, suggesting a role for these cytokines in the regulatory process. These studies suggest that regulatory T cells are also important in preventing IBD.
THERE IS AN IMMUNOLOGICAL BASIS FOR HELMINTHIC PROTECTION
Populations experiencing deworming also undergo other socioeconomic alternations that could affect risk for disease. These include changes in diet, housing, and sanitation among others. Yet there is an immunological basis to suspect deworming as a risk factor. People bearing helminths display dampened immune responses to unrelated concurrent antigenic exposures.1,37,38 These changes in immune responsiveness can persist long after elimination of these helminthic exposures.37,39 Mice colonised with helminths have blunted Th1 responses.40–43 Helminths promote Th2 responses associated with production of IL-4 and IL-13.44,45 IL-4 helps impede Th1 cell differentiation. Thus induction of IL-4 could underlie the alternations seen in host immunity. However, the mechanism of protection is not simply “Th2 suppresses Th1” as helminths also appear to protect the host from aberrant Th2 diseases such as asthma and food allergy.46,47 Interactions between these parasites and their hosts are complex and multifaceted as would be expected for such a successful co-evolutionary process that leads to “peaceful” coexistence. Helminths not only trigger Th2 responses, which help to limit worm number in the host, they also promote production of powerful immunomodulatory molecules such as IL-1048 and TGF-β, and “regulatory” T cells.49
HELMINTHS PROTECT
There is now substantial human epidemiological data and several animal studies supporting the hypothesis that helminths protect the host from immunological disease. For instance, people colonised with helminths have high serum levels of IL-10, which may protect them from atopy.50 Helminths protect mice51,52 and rats from TNBS induced colitis, experimental autoimmune encephalomyelitis,53,54 and other diseases of immunity47,55 most likely in part through induction of IL-4. They also reverse ongoing colitis in IL-10KO animals via induction of regulatory T cells (manuscript submitted). Thus natural exposure to helminths may guard people from developing IBD and other immunological diseases through induction of IL-4, IL-10, TGF-β, regulatory T cells, or perhaps by other means. In a preliminary and uncontrolled trial, we have demonstrated that oral administration of Trichuris suis ova to patients with active ulcerative colitis or Crohn’s disease is safe and possibly effective.56 Controlled clinical trials in both disorders are also being conducted and are nearing completion using a similar approach.
SUMMARY
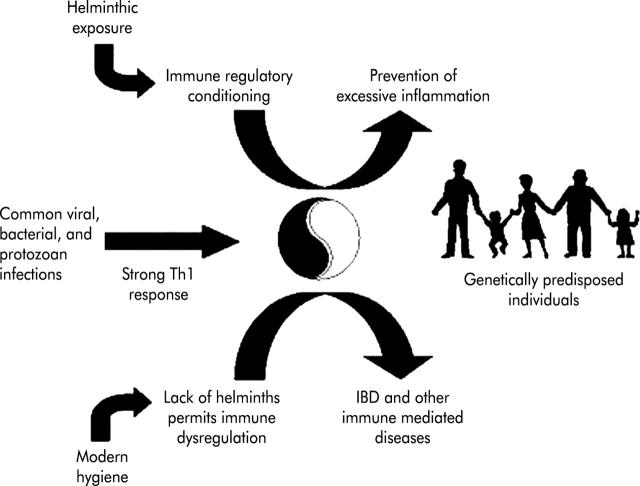
Environmental factors affect the worldwide distribution of IBD. Supported by a growing volume of both epidemiological and experimental data, it appears plausible that exposure to helminths is a factor that protects people from IBD (fig 1 ▶). As reported by Moreels and colleagues2 in this issue of Gut, helminths protect mice from experimental colitis. Many factors help initiate and maintain immunological diseases. Targeting one or just a few cytokines in most cases may not prove sufficient to permanently suppress disease activity. Helminths have broad immunoregulatory properties that evolved as part of the successful host-parasite interaction. Studying helminths and how they alter the host’s immune response could lead to new and highly effective therapeutic strategies for human IBD. Such studies may also provide new insight into the pathogenesis of CD, UC, and other emerging immunological diseases.
Figure 1.
The inflammatory bowel disease (IBD) hygiene hypothesis.
Acknowledgments
The study was supported by grants from the National Institutes of Health (DK38327, DK58755, DK02428, DK25295), and the Crohn’s and Colitis Foundation of America, Inc.
REFERENCES
- 1.Elliott DE, Urban JF jr, Argo CF, et al. Does the failure to acquire helminthic parasites predispose to Crohn’s disease? FASEB J 2000;14:1848–55. [DOI] [PubMed] [Google Scholar]
- 2.Moreels TG, Nieuwendijk RJ, De Man JG, et al. Concurrent infection with Schistosoma mansoni attenuates inflammation induced changes in colonic morphology, cytokine levels, and smooth muscle contractility of trinitrobenzene sulphonic acid induced colitis in rats. Gut 2004;53:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boismenu R, Chen Y. Insights from mouse models of colitis. J Leukoc Biol 2000;67:267–78. [DOI] [PubMed] [Google Scholar]
- 4.Roth MP, Petersen GM, McElree C, et al. Geographic origins of Jewish patients with inflammatory bowel disease. Gastroenterology 1989;97:900–4. [DOI] [PubMed] [Google Scholar]
- 5.Fireman Z, Grossman A, Lilos P, et al. Epidemiology of Crohn’s disease in the Jewish population of central Israel, 1970–1980. Am J Gastroenterol 1989;84:255–8. [PubMed] [Google Scholar]
- 6.Grossman A, Fireman Z, Lilos P, et al. Epidemiology of ulcerative colitis in the Jewish population of central Israel 1970–1980. Hepatogastroenterology 1989;36:193–7. [PubMed] [Google Scholar]
- 7.Halfvarson J, Bodin L, Tysk C, et al. Inflammatory bowel disease in a Swedish twin cohort: a long-term follow-up of concordance and clinical characteristics. Gastroenterology 2003;124:1767–73. [DOI] [PubMed] [Google Scholar]
- 8.Inohara M, Ogura Y, Fontalba A, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2: implications for Crohn’s disease. J Biol Chem 2003;278:5509–12. [DOI] [PubMed] [Google Scholar]
- 9.Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 2003;278:8869–72. [DOI] [PubMed] [Google Scholar]
- 10.Calkins BM. A meta-analysis of the role of smoking in inflammatory bowel disease. Dig Dis Sci 1989;34:1841–54. [DOI] [PubMed] [Google Scholar]
- 11.Cottone M, Rosselli M, Orlando A, et al. Smoking habits and recurrence in Crohn’s disease. Gastroenterology 1994;106:643–8. [DOI] [PubMed] [Google Scholar]
- 12.Andersson RE, Olaison G, Tysk C, et al. Appendectomy and protection against ulcerative colitis. N Engl J Med 2001;344:808–14. [DOI] [PubMed] [Google Scholar]
- 13.Derby LE, Jick H. Appendectomy protects against ulcerative colitis. Epidemiology 1998;9:205–7. [PubMed] [Google Scholar]
- 14.Russel MG, Dorant E, Brummer RJ, et al. Appendectomy and the risk of developing ulcerative colitis or Crohn’s disease: results of a large case-control study. South Limburg Inflammatory Bowel Disease Study Group. Gastroenterology 1997;113:377–82. [DOI] [PubMed] [Google Scholar]
- 15.Sonnenberg A, McCarty DJ, Jacobsen SJ. Geographic variation of inflammatory bowel disease within the United States. Gastroenterology 1991;100:143–9. [DOI] [PubMed] [Google Scholar]
- 16.Shivananda S, Lennard-Jones J, Logan R, et al. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD). Gut 1996;39:690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonnenberg A, Wasserman IH. Epidemiology of inflammatory bowel disease among U.S. military veterans. Gastroenterology 1991;101:122–30. [DOI] [PubMed] [Google Scholar]
- 18.Delco F, Sonnenberg A. Military history of patients with inflammatory bowel disease: an epidemiological study among U.S. veterans. Am J Gastroenterol 1998;93:1457–62. [DOI] [PubMed] [Google Scholar]
- 19.Sonnenberg A. Occupational distribution of inflammatory bowel disease among German employees. Gut 1990;31:1037–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ekbom A, Helmick C, Zack M, et al. The epidemiology of inflammatory bowel disease: a large, population-based study in Sweden. Gastroenterology 1991;100:350–8. [DOI] [PubMed] [Google Scholar]
- 21.Rolon PA. Gastrointestinal pathology in South America. Isr J Med Sci 1979;15:318–21. [PubMed] [Google Scholar]
- 22.Hutt MS. Epidemiology of chronic intestinal disease in middle Africa. Isr J Med Sci 1979;15:314–17. [PubMed] [Google Scholar]
- 23.Segal I. Ulcerative colitis in a developing country of Africa: the Baragwanath experience of the first 46 patients. Int J Colorectal Dis 1988;3:222–5. [DOI] [PubMed] [Google Scholar]
- 24.Yang SK, Loftus EV jr, Sandborn WJ. Epidemiology of inflammatory bowel disease in Asia. Inflamm Bowel Dis 2001;7:260–70. [DOI] [PubMed] [Google Scholar]
- 25.Wright JP, Marks IN, Jameson C, et al. Inflammatory bowel disease in Cape Town, 1975–1980. Part II. Crohn’s disease. S Afr Med J 1983;63:226–9. [PubMed] [Google Scholar]
- 26.Carr I, Mayberry JF. The effects of migration on ulcerative colitis: a three-year prospective study among Europeans and first- and second-generation South Asians in Leicester (1991–1994). Am J Gastroenterol 1999;94:2918–22. [DOI] [PubMed] [Google Scholar]
- 27.Jayanthi V, Probert CS, Pinder D, et al. Epidemiology of Crohn’s disease in Indian migrants and the indigenous population in Leicestershire. Q J Med 1992;82:125–38. [PubMed] [Google Scholar]
- 28.Probert CS, Jayanthi V, Hughes AO, et al. Prevalence and family risk of ulcerative colitis and Crohn’s disease: an epidemiological study among Europeans and south Asians in Leicestershire. Gut 1993;34:1547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salas SD, Heifetz R, Barrett-Connor E. Intestinal parasites in Central American immigrants in the United States. Arch Intern Med 1990;150:1514–16. [PubMed] [Google Scholar]
- 30.Healy GR, Gleason NN, Bokat R, et al. Prevalence of ascariasis and amebiasis in Cherokee Indian school children. Public Health Rep 1969;84:907–14. [PMC free article] [PubMed] [Google Scholar]
- 31.Rennick DM, Fort MM. Lessons from genetically engineered animal models. XII. IL-10-deficient (IL-10(−/−) mice and intestinal inflammation. Am J Physiol Gastrointest Liver Physiol 2000;278:G829–33. [DOI] [PubMed] [Google Scholar]
- 32.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 2000;12:171–81. [DOI] [PubMed] [Google Scholar]
- 33.Kabashima K, Saji T, Murata T, et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest 2002;109:883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGuirk P, Mills KH. Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol 2002;23:450–5. [DOI] [PubMed] [Google Scholar]
- 35.Oida T, Zhang X, Goto M, et al. CD4+CD25-T cells that express latency-associated peptide on the surface suppress CD4+CD45RBhigh-induced colitis by a TGF-beta-dependent mechanism. J Immunol 2003;170:2516–22. [DOI] [PubMed] [Google Scholar]
- 36.Annacker O, Powrie F. Homeostasis of intestinal immune regulation. Microbes Infect 2002;4:567–74. [DOI] [PubMed] [Google Scholar]
- 37.Borkow G, Leng Q, Weisman Z, et al. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. J Clin Invest 2000;106:1053–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabin EA, Araujo MI, Carvalho EM, et al. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis 1996;173:269–72. [DOI] [PubMed] [Google Scholar]
- 39.Bentwich Z, Weisman Z, Moroz C, et al. Immune dysregulation in Ethiopian immigrants in Israel: relevance to helminth infections? Clin Exp Immunol 1996;103:239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kullberg MC, Pearce EJ, Hieny SE, et al. Infection with Schistosoma mansoni alters Th1/Th2 cytokine responses to a non-parasite antigen. J Immunol 1992;148:3264–70. [PubMed] [Google Scholar]
- 41.Actor JK, Shirai M, Kullberg MC, et al. Helminth infection results in decreased virus-specific CD8+ cytotoxic T-cell and Th1 cytokine responses as well as delayed virus clearance. Proc Natl Acad Sci U S A 1993;90:948–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pearlman E, Kazura JW, Hazlett FE jr, et al. Modulation of murine cytokine responses to mycobacterial antigens by helminth-induced T helper 2 cell responses. J Immunol 1993;151:4857–64. [PubMed] [Google Scholar]
- 43.Sacco R, Hagen M, Sandor M, et al. Established T(H1) granulomatous responses induced by active Mycobacterium avium infection switch to T(H2) following challenge with Schistosoma mansoni. Clin Immunol 2002;104:274–81. [DOI] [PubMed] [Google Scholar]
- 44.Finkelman FD, Wynn TA, Donaldson DD, et al. The role of IL-13 in helminth-induced inflammation and protective immunity against nematode infections. Curr Opin Immunol 1999;11:420–6. [DOI] [PubMed] [Google Scholar]
- 45.Urban JF jr, Madden KB, Svetic A, et al. The importance of Th2 cytokines in protective immunity to nematodes. Immunol Rev 1992;127:205–20. [DOI] [PubMed] [Google Scholar]
- 46.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science 2002;296:490–4. [DOI] [PubMed] [Google Scholar]
- 47.Bashir ME, Andersen P, Fuss IJ, et al. An enteric helminth infection protects against an allergic response to dietary antigen. J Immunol 2002;169:3284–92. [DOI] [PubMed] [Google Scholar]
- 48.van den Biggelaar AH, Lopuhaa C, van Ree R, et al. The prevalence of parasite infestation and house dust mite sensitization in Gabonese schoolchildren. Int Arch Allergy Immunol 2001;126:231–8. [DOI] [PubMed] [Google Scholar]
- 49.Doetze A, Satoguina J, Burchard G, et al. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h)3/T(r)1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h)1 to T(h)2 shift. Int Immunol 2000;12:623–30. [DOI] [PubMed] [Google Scholar]
- 50.van den Biggelaar AH, van Ree R, Rodrigues LC, et al. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet 2000;356:1723–7. [DOI] [PubMed] [Google Scholar]
- 51.Elliott DE, Li J, Blum A, et al. Exposure to schistosome eggs protects mice from TNBS colitis. Am J Physiol 2003;284:G385–91. [DOI] [PubMed] [Google Scholar]
- 52.Khan WI, Blennerhasset PA, Varghese AK, et al. Intestinal nematode infection ameliorates experimental colitis in mice. Infect Immun 2002;70:5931–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sewell D, Qing Z, Reinke E, et al. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int Immunol 2003;15:59–69. [DOI] [PubMed] [Google Scholar]
- 54.La Flamme AC, Ruddenklau K, Backstrom BT. Schistosomiasis decreases CNS inflammation and alters the progression of EAE. Infect Immun 2003;71:8869–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fox JG, Beck P, Dangler CA, et al. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med 2000;6:536–42. [DOI] [PubMed] [Google Scholar]
- 56.Summers RW, Elliott DE, Qadir K, et al.Trichuris suis appears to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol 2003;98:2034–41. [DOI] [PubMed] [Google Scholar]