Abstract
Background: Microsatellite instability (MSI) has been identified as a factor with good prognosis and chemosensitivity in stage III C colon cancer. The purpose of this study was to evaluate the routine use of immunohistochemical analysis (immunohistochemical staining of MSH2 and MLH1) to identify T3N0M0 (stage II) colon cancer with MSI and assess the prognostic value of this analysis. The study was conducted in a large cohort of patients in a single institution who had a curatively resected T3N0M0 colon cancer and were not receiving adjuvant therapy.
Methods: Between June 1995 and December 2001, 142 patients (77 females) with a mean age of 68 years, suffering from T3N0M0 colon cancer curatively resected and not receiving adjuvant therapy, were checked in terms of their follow up status. The results of colonoscopy, hepatic ultrasonography, chest x ray, and blood carcinoembryological antigen were noted. All tumours were immunohistochemically stained for MSH2 and MLH1. Perineural invasion, lymphovascular invasion, and the presence of vascular neoplastic emboli were assessed.
Results: Twenty four patients (17%) had MSI tumours. Patients with MSI and microsatellite stable (MSS) tumours did not differ in terms of age, perineural or lymphovascular invasion, or the presence of vascular neoplastic emboli. Patients with MSI tumours were more frequently female (18/24 v 60/118; p = 0.001) and more frequently suffered from right sided cancer (19/24 v 58/118; p<0.001). Patients with MSI tumours exhibited significantly better recurrence free survival than those with MSS tumours (p = 0.02). Cox analysis identified age and MSI determined by immunohistochemistry as independent predictive factors of good prognosis (p = 0.009, odds ratio 1.04 (1.01–1.08); p = 0.04, odds ratio 7.9 (1.05–59.6)).
Conclusions: MSI determined by immunohistochemistry is an independent predictive factor of good prognosis in T3N0M0 colon cancer. The prognosis for MSI T3N0M0 colon cancer is excellent and chemotherapy should not be proposed in these patients as immunohistochemical analysis produces rapid results.
Keywords: colon cancer, microsatellite instability, MSH2, MLH1, prognosis
Colorectal cancer remains the second most common cause of cancer related death in Western countries. An assessment of prognosis, based on features of the resected tumour, is of value to the triage of patients who may benefit from adjuvant therapy. Currently, the TNM1 and Astler-Coller2 classifications are the only tools used during the selection process to determine which patients might be eligible for adjuvant therapy. Patients with stage I disease are usually treated with surgery alone because of the 90% five year survival rate following surgical resection. Patients with stage IV disease generally receive chemotherapy as adjuvant, neoadjuvant,3 or palliative treatment. Since the publication of Moertel and colleagues,4 patients with stage III colon cancer have been treated with surgery and adjuvant chemotherapy. However, there is still controversy as to whether adjuvant therapy benefits patients with stage II colon cancer.5 Identification of additional tumour characteristics to supplement standard clinical and pathological staging may make it possible to subdivide patients with stage II disease, thus highlighting those at highest risk of recurrence following surgery who would benefit most from adjuvant therapy.
Colorectal cancer has been shown to arise through at least two distinct genetic pathways: one involving chromosomal instability and the other involving microsatellite instability (MSI).6–8 Initial studies suggested that MSI was associated with an improved prognosis.9,10 Since then, numerous studies have confirmed the better prognosis of patients with stage III colon cancer and MSI,11–15 and suggested the greater chemosensitivity of MSI colon cancer.14–16 However, this latter point remains controversial, with a recent report17 demonstrating that patients with stage II or III colon cancer and an MSI phenotype experienced a poorer outcome if they received adjuvant chemotherapy compared with those who had not. Moreover, studies11,12 which also considered the prognostic significance of MSI in stage II colon cancer were unable to demonstrate a difference.
In order to identify these MSI carcinomas, an MSI test based on the polymerase chain reaction (PCR) can be used. However, this requires DNA extraction and specialised equipment, is time consuming, and is not ideally suited to all histopathology laboratories. Routine use of this test in all cases of colon cancer is therefore not cost effective and not feasible. Since the report of Cawkwell and colleagues,18 several publications19–23 have shown the high correlation between MSI phenotype determined by PCR and complete absence of MSH2 or MLH1 expression using immunohistochemical analysis.
The purpose of this study was to evaluate the routine use of immunohistochemical analysis (immunohistochemical staining of MSH2 and MLH1) in identifying T3N0M0 colon cancer with MSI and to assess the prognostic value of this analysis. The study was conducted in a large cohort of patients in a single institution who had a curatively resected T3N0M0 colon cancer and were not receiving adjuvant therapy. Stage II colon cancer includes T3 and T4 N0M0 tumours. Only T3N0M0 colon cancers were included in our study as extension of tumours to the serosa or adjacent organs, defining T4 tumours, represents a prognostic factor which is difficult to stratify.
PATIENTS AND METHODS
Patients
Patients who had undergone tumour resection in our department between June 1995 and December 2000 and who were classified as having T3N0M0 colon cancer according to the TNM classification1 were included in the study. T4N0M0 tumours were excluded from this study because extension of the tumour to the serosa or adjacent organs is a prognostic factor which is difficult to stratify. Histological analysis was performed on haematoxylin-eosin stained slides prepared using routine histological methods. Information on disease specific survival was obtained from family doctors and local death registers if it was not accessible through our follow up protocol.
The follow up protocol consisted of clinical evaluation with hepatic ultrasonography, pulmonary x ray, and carcinoembryological antigen determinations every four months during the first two years after surgery, then every six months for two years, and then annually. Endoscopic follow up was performed one year after resection and then once every three years if normal.
Pathology
All tumours were reassessed by a pathologist (NM) who looked for the following histopathological features: perineural invasion, lymphovascular invasion, and the presence of vascular neoplastic emboli.
Immunohistochemistry
One block of formalin fixed paraffin wax embedded adenocarcinoma tissue was selected in each case. In all cases, this block comprised an area of normal colonic mucosa adjacent to the tumour. Sections (4 µm) were affixed to Superfrost plus slides (CML, Nemours, France) and dried overnight at 37°C. After dewaxing and rehydration with distilled water, endogenous peroxidase activity was quenched by incubating the slides in 3% hydrogen peroxide in methanol for 2× 15 minutes. Sections were subjected to heat antigen retrieval by autoclaving in a citrate buffer at pH 6.0 for 15 minutes at 750 W and 15 minutes at 150 W. For the MLH1 antibody (G168-728; Pharmingen, San Diego, California, USA), dilution was 1/70 and the incubation time was one hour; for the MSH2 antibody (FE11; Calbiochem, Cambridge, USA), dilution was 1/100 and the incubation time was 30 minutes. All incubations were performed at room temperature. The following methods were used: the avidin-biotin complex method for MLH1 and indirect immunoperoxidase for MSH2. Immunohistochemical reactions were performed with the Optimax plus system (Biogenex, San Ramon, USA). Colour was developed with amino-ethyl-carbazole (Vector, Burlingame, USA) as the chromogen. Sections were washed under running tap water and then lightly counterstained in Mayer’s haematoxylin. Loss of expression was recorded when nuclear staining was absent from all malignant cells but preserved in normal epithelial and stroma cells. Two observers (NM, JFF) assessed all cases independently. Three cases with discrepant scores were re-evaluated jointly on a second occasion and agreement was finally reached (no loss of expression).
The pathologists were unaware of patient outcomes when reassessing the histological features or determining the MSI phenotype by immunohistochemistry analysis.
Because the aim of our study was to assess the value of MSI, as determined by immunohistochemical staining, and because we were confident in using this technique based on our previous results,23 PCR analysis was not performed to confirm the immunohistochemical results obtained during this study.
Statistical analysis
The Fisher exact test and Mann-Whitney test were used to compare frequencies and medians. The cumulative probabilities of survival and recurrence free survival were calculated using the method developed by Kaplan and Meier. The prognostic factors studied were as follows: age at tumour diagnosis, sex, tumour site, perineural invasion, lymphovascular invasion, presence of vascular neoplastic emboli, and MSI status determined by immunohistochemical analysis. Univariate analysis of survival was performed by computing survival curves according to the Kaplan-Meier method. Curves were compared using the log rank test. Independent prognostic factors for survival were assessed using the Cox regression model. Significance was set at p<0.05. Analyses were performed using Statview software (1992–1998; SAS Institute Inc., Cary, North Carolina, USA).
RESULTS
Baseline characteristics of patients
During the period of the study, 648 patients underwent resection of colon cancer in our institution. Of these 648 patients, 142 (23%) were classified as having curatively resected T3N0M0 colon cancer. The mean number of lymph nodes analysed in surgical specimens was 26 (SD 3). Over the same period of time, seven patients underwent palliative resection (no lymphadenectomy performed) of tumours that were then classified as T3N0M0 colon cancer; these patients were not included in the present study. The 142 curatively resected patients comprised 77 females and 65 males with a median age at diagnosis of 67.7 (13.69) (range 29–98) years. Defining right sided tumours as those originating before the splenic flexure and left sided tumours as those distal to this site, 75 patients had right sided tumours and 67 left sided tumours. Twenty six tumours had vascular neoplastic emboli (18%), 21 lymphovascular invasion (15%), and 17 perineural invasion (12%). Paraffin embedded tumour and normal tissue specimens suitable for immunohistochemical analysis were obtained from all of these patients. Twenty four patients (17%) with colon cancer had an MSI phenotype (table 1 ▶). Of these MSI tumours, six had a total lack of expression for MSH2 and 18 a total lack of expression for MLH1 in the tumour. The remaining 118 tumours with normal nuclear expression of both proteins were considered microsatellite stable (MSS).
Table 1.
Characteristics and follow up of patients according to microsatellite instability status
| MSI (n = 24) | MSS (n = 118) | p Value* | |
| Age (y)† | 74 [29–94] | 69 [35–93] | 0.56 |
| Follow up (months)† | 50 [26–82] | 43 [2.5–86] | 0.02 |
| Sex (female/male) | 19 (79%)/5 (212%) | 59 (50%)/59 (50%) | 0.0123 |
| Perineural invasion, lymphovascular invasion, or presence of vascular neoplastic emboli (Y/N) | 4 (17%)/20 (83%) | 45 (38%)/73 (62%) | 0.058 |
| Perineural invasion (Y/N) | 2 (8%)/22 (92%) | 24 (20%)/94 (80%) | 0.24 |
| Lymphovascular invasion (Y/N) | 1 (4%)/23 (96%) | 20 (17%)/98 (83%) | 0.20 |
| Presence of vascular neoplastic emboli (Y/N) | 1 (4%)/23 (96%) | 16 (14%)/102 (86%) | 0.30 |
| Right sided tumour (Y/N) | 19 (79%)/5 (21%) | 59 (50%)/59 (50%) | 0.012 |
| Recurrence (Y/N) | 0 (0%)/24 (100%) | 15 (13%)/103 (87%) | 0.07 |
MSI, microsatellite instability; MSS, microsatellite stable;
†Values are median [minimum−maximum].
*Mann-Whitney test or Fisher’s exact test.
Survival
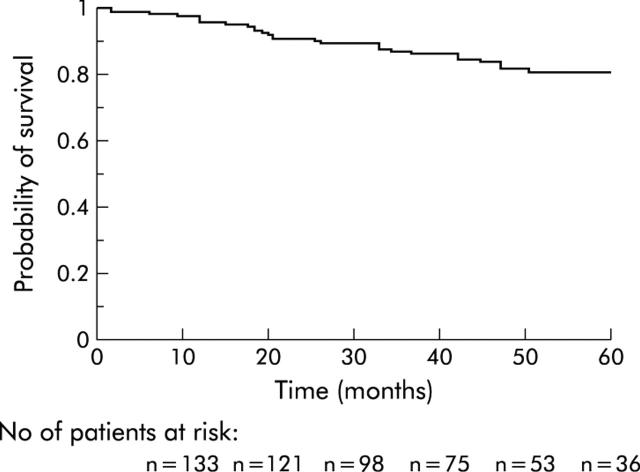
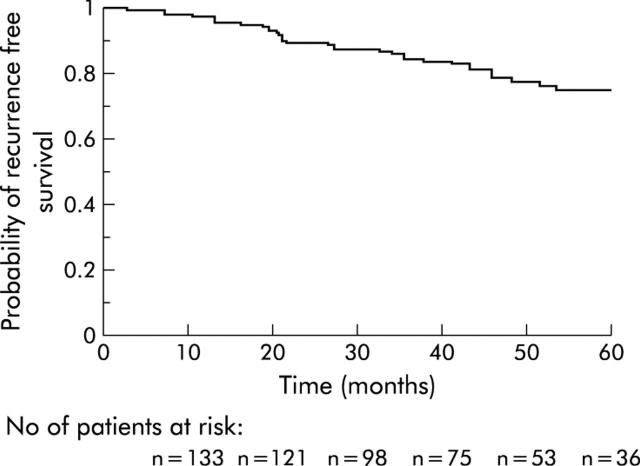
None of these patients received adjuvant chemotherapy, as is the case in our institution for all colon cancer patients without node invasion or metastases. The median follow up period for all patients included in this study was 42 months (range 0.2–86) while the median follow up of survivors was 46 months (range 0.2–86). Nine patients were lost to follow up but were not excluded from the study, and their data were included in the survival analysis. These were all MSS patients. Twenty one patients died a mean of 25 (SD 14) (median 21; range 2.4–51.4) months after surgery. The causes of death were: recurrence of cancer (n = 9), death from unspecified causes other than recurrence (n = 4), myocardial infarction (n = 3), dementia (n = 1), bladder cancer (n = 1), hepatocarcinoma developed on liver cirrhosis (n = 1), myeloma (n = 1), and postoperative peritonitis (n = 1). Fifteen patients developed recurrence after a mean follow up of 33 (SD 14) (median 32; range 12.8–64.5) months, all in the MSS group. The sites of recurrence were: liver (n = 8), lung (n = 5), peritoneum (n = 5), and brain (n = 1). The cumulative probabilities of overall survival and recurrence free survival are shown in figs 1 ▶ and 2 ▶.
Figure 1.
Probability of survival in the overall sample.
Figure 2.
Probability of recurrence free survival in the overall sample.
Prognostic factors for survival
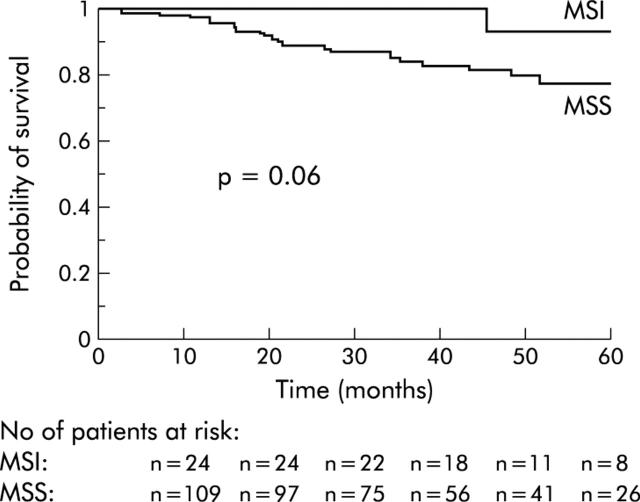
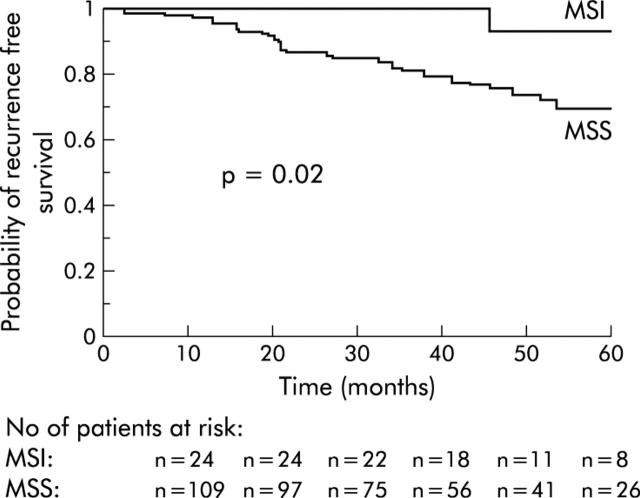
In the univariate analysis, only age (older or younger than 75 years) was significantly associated with survival (table 2 ▶). Patients with MSI tumours determined by immunohistochemical analysis had a higher probability of survival but the difference did not reach statistical significance (table 2 ▶, fig 3 ▶). The probability of recurrence free survival was significantly higher in patients with MSI tumours than in those with MSS tumours (table 3 ▶, fig 4 ▶). Age (older or younger than 75 years) and the presence of perineural invasion, lymphovascular invasion, or vascular neoplastic emboli were also associated with recurrence free survival but without reaching statistical significance. In the multivariate analysis, age at surgery and MSI phenotype determined by immunochemistry were independent predictors of recurrence free survival (p = 0.009, odds ratio1.04 (range: 1.01–1.08); and p = 0.04, odds ratio 7.9 (range 1.05–59.6), respectively).
Table 2.
Prognostic factors for survival in the univariate analysis
| Variable | No of patients | Probability of survival | p Value* | |
| 2 y (SE) | 5 y (SE) | |||
| Age | 0.02 | |||
| Age >75 y | 45 | 0.9 (0.05) | 0.7 (0.1) | |
| Age<75 y | 97 | 0.92 (0.03) | 0.87 (0.04) | |
| Sex | 0.62 | |||
| Female | 78 | 0.92 (0.03) | 0.82 (0.05) | |
| Male | 64 | 0.9 (0.04) | 0.8 (0.1) | |
| Perineural invasion, lymphovascular invasion or presence of vascular neoplastic emboli | 0.8 | |||
| Yes | 49 | 0.87 (0.05) | 0.80 (0.07) | |
| No | 93 | 0.93 (0.03) | 0.80 (0.05) | |
| Tumour site | 0.72 | |||
| Right sided tumours | 77 | 0.90 (0.03) | 0.80 (0.1) | |
| Left sided tumours | 65 | 0.90 (0.03) | 0.81 (0.05) | |
| MSI phenotype | 0.06 | |||
| MSI | 24 | 1 (0.00) | 0.9 (0.1) | |
| MSS | 118 | 0.89 (0.03) | 0.77 (0.05) | |
MSI, microsatellite instability; MSS, microsatellite stable; SE, standard error.
*Log rank test.
Figure 3.
Probability of survival according to MSI phenotype. MSI, microsatellite instability; MSS, microsatellite stable.
Table 3.
Prognostic factors for recurrence free survival in the univariate analysis
| Variable | No of patients | Probability of survival | p Value* | |
| 2 y (SE) | 5 y (SE) | |||
| Age | 0.053 | |||
| Age >75 y | 45 | 0.92 (0.03) | 0.80 (0.05) | |
| Age<75 y | 97 | 0.8 (0.1) | 0.6 (0.1) | |
| Sex | 0.19 | |||
| Female | 77 | 0.90 (0.03) | 0.81 (0.05) | |
| Male | 65 | 0.90 (0.04) | 0.70 (0.1) | |
| Perineural invasion, lymphovascular invasion or presence of vascular neoplastic emboli | 0.08 | |||
| Yes | 49 | 0.92 (0.03) | 0.79 (0.05) | |
| No | 93 | 0.9 (0.1) | 0.7 (0.1) | |
| Tumour site | 0.72 | |||
| Right sided tumours | 77 | 0.90 (0.04) | 0.80 (0.1) | |
| Left sided tumours | 65 | 0.90. (0.04) | 0.74 (0.07) | |
| MSI Phenotype | 0.02 | |||
| MSI | 24 | 1 (0.00) | 0.9 (0.1) | |
| MSS | 118 | 0.87 (0.03) | 0.70 (0.05) | |
MSI, microsatellite instability; MSS, microsatellite stable; SE, standard error.
*Log rank test.
Figure 4.
Probability of recurrence free survival according to MSI phenotype. MSI, microsatellite instability; MSS, microsatellite stable.
DISCUSSION
MSI phenotype is now a well known marker of good prognosis in colorectal cancer.11–16 In reports demonstrating a significant difference in prognosis between MSI and MSS stage III colon cancers, MSI phenotype was determined using a PCR test.11–16 However, routine use of this test in all cases of colon cancer is not feasible. For this reason, MSI phenotype remains a tumour characteristic which is not considered in terms of treatment strategy. The purpose of our study was to evaluate the routine use of immunohistochemical MLH1 and MSH2 staining to identify T3N0M0 colon cancer with the MSI phenotype, and to correlate the result of this test with the patient’s prognosis.
Although intensive research into the molecular abnormalities associated with sporadic colorectal cancer has been in progress for many years, no new clinically valid routine tests to complement staging and grading have been introduced. Since the report of Cawkwell and colleagues,18 several publications19–23 have demonstrated the strong correlation between MSI phenotype and total lack of MSH2 or MLH1 expression, as revealed by immunohistochemical analysis. During our study, we tested MSI only using immunohistochemistry analysis, our aim being to assess the usefulness of this test in routine clinical practice. Some cases of MSI colon cancer determined by the PCR test (which remains the gold standard for MSI phenotype determination) might have been missed by immunohistochemical analysis. Other genes of the mismatch repair system may be responsible for MSI colon cancer but without a lack of expression of MSH2 or MLH1. However, such cases are rare.23 Furthermore, inactivation of MLH1 or MSH2 can be functional only, without lack of expression of the protein detected by immunohistochemistry analysis, but no such case has yet been reported. However, our results demonstrate that this test enabled identification of a subgroup of patients with a significantly better prognosis and no recurrence related deaths.
Adjuvant chemotherapy for stage II colon cancer is controversial. Meta-analyses have been performed to determine whether statistical power might be the reason for absence of a significant impact of adjuvant chemotherapy on survival in stage II colon cancers.5,24 Controversy continues as these reports showed no significant advantage of adjuvant chemotherapy on survival. However, guidelines propose adjuvant chemotherapy for only those patients at risk of recurrence (that is, occlusion, perforation, local extension). Further tests are then mandatory to select patients from among those with stage II colon cancer who might benefit from adjuvant therapy. Our results demonstrate that immunohistochemical analysis of MLH1 and MSH2 identifies a subgroup of patients (17% of patients with T3N0M0 (stage II) colon cancer) with a significantly better prognosis. The prognosis of these patients is excellent, with a five year survival rate of over 90% and no recurrence related deaths, thus demonstrating the irrelevance of adjuvant therapy in these patients. With the recent report by Ribic and colleagues17 showing no benefit and a possible deleterious effect of adjuvant chemotherapy on stage II and stage III MSI colon cancers, we suggested that immunohistochemical staining for MSH2 and MLH1 (which is a test that every pathological laboratory can carry out routinely) should be used to assess MSI phenotype. For patients with T3N0M0 colon cancer (a subgroup of patients in whom no benefits from chemotherapy have ever been reported), we also suggest that immunohistochemistry for MSH2 or MLH1 should be used to determine which patients exhibit an MSI phenotype and should not therefore receive fluorouracil based adjuvant chemotherapy.
Acknowledgments
Supported by a grant from “Bourse de Recherche en Colo-proctologie Beaufour Ipsen Pharma, France”.
Abbreviations
MSI, microsatellite instability
MSS, microsatellite stable
PCR, polymerase chain reaction
REFERENCES
- 1.International Union Against Cancer (UICC). In: Sobin LH, Wittekind Ch, eds. TNM classification of malignant tumours, 5th edn. Wiley-Liss: New York 1997:66–9.
- 2.Astler VB, Coller J. The prognosis significance of direct extension of carcinoma of the colon and rectum. Ann Surg 1954;139:846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bismuth H, Adam R, Levi F, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg 1996;224:509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990;322:352–8. [DOI] [PubMed] [Google Scholar]
- 5.Marsoni S. Efficacy of adjuvant fluorouracil and leucovorin in stage B2 and C colon cancer. International Multicenter Pooled Analysis of Colon Cancer Trials Investigators. Semin Oncol 2001;28(suppl 1):14–19. [DOI] [PubMed] [Google Scholar]
- 6.Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancer. Nature 1997;386:623–7. [DOI] [PubMed] [Google Scholar]
- 7.Dutrillaux R. Pathways of chromosomal alteration in human epithelial cancers. Adv Cancer Res 1995;67:69–82. [DOI] [PubMed] [Google Scholar]
- 8.Sweezy MA, Fishel R. Multiple pathways leading to genomic instability and tumourigenesis. Ann N Y Acad Sci 1994;726:765–77. [DOI] [PubMed] [Google Scholar]
- 9.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science 1993;260:816–19. [DOI] [PubMed] [Google Scholar]
- 10.Lothe RA, Peltomaki P, Meling GI, et al. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res 1993;53:5849–52. [PubMed] [Google Scholar]
- 11.Halling KC, French AJ, McDonnell SK, et al. Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J Natl Cancer Inst 1999;91:1295–303. [DOI] [PubMed] [Google Scholar]
- 12.Gryfe R, Kim H, Hsieh E, et al. Tumour microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000;342:69–77. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe T, Wu TT, Catalano PJ, et al. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med 2001;344:1196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemminki A, Mecklin J-P, Järvinen H, et al. Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterology 2000;119:921–8. [DOI] [PubMed] [Google Scholar]
- 15.Elsaleh H, Joseph D, Grieu F, et al. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet 2000;355:1745–50. [DOI] [PubMed] [Google Scholar]
- 16.Wright CM, Dent OF, Barker M, et al. Prognostic significance of extensive microsatellite instability in sporadic clinicopathological stage C colorectal cancer. Br J Surg 2000;87:1197–202. [DOI] [PubMed] [Google Scholar]
- 17.Ribic C, Sargent D, Moore M, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cawkwell L, Gray S, Murgatroyd H, et al. Choice of management strategy for colorectal cancer based on a diagnostic immunohistochemical test for defective mismatch repair. Gut 1999;45:409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanza G, Gafa R, Maestri I, et al. Immunohistochemical pattern of MLH1/MSH2 expression is related to clinical and pathological features in colorectal adenocarcinomas with microsatellite instability. Mod Pathol 2002;15:741–9. [DOI] [PubMed] [Google Scholar]
- 20.Stone JG, Robertson D, Houlston RS. Immunohistochemistry for MSH2 and MHL1: a method for identifying mismatch repair deficient colorectal cancer. J Clin Pathol 2001;54:484–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcus VA, Madlensky L, Gryfe R, et al. Immunohistochemistry for hMLH1 and hMSH2: a practical test for DNA mismatch repair-deficient tumours. Am J Surg Pathol 1999;23:1248–55. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham JM, Kim C- Y, Christensen ER, et al. The frequency of hereditary defective mismatch repair in a prospective series of unselected colorectal carcinomas. Am J Hum Genet 2001;69:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rigau V, Sebbagh N, Olschwang S, et al. Microsatellite instablity in colorectal carcinoma: the comparison of immunohistochemistry and molecular biology suggests the interest of hMSH6 immunostaining. Arch Pathol Lab Med 2003;127:694–700. [DOI] [PubMed] [Google Scholar]
- 24.International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. J Clin Oncol 1999;17:1356–63. [PubMed] [Google Scholar]