Abstract
Background: Id2, an inhibitor of basic helix-loop-helix transcription factors, regulates cell differentiation. Id2−/− mice exhibit a variety of phenotypes in the immune system.
Aims: In this study we investigated whether Id2 plays a role in intestinal intraepithelial lymphocytes (IELs), which constitute the main defence against pathogens in the intestinal tract.
Methods: Flow cytometry and bone marrow transplantation were used to analyse and characterise subsets of IELs of Id2−/− mice. Gene expression was analysed by real-time polymerase chain reaction. Intestinal barrier function was evaluated by treating mice with 5-fluorouracil (5-FU).
Results: Among the four members of the Id gene family, Id2 was selectively expressed in all T cell subsets in the small intestinal IELs. Id2−/− mice showed alteration in the proportions of T cell subsets and a substantial reduction in the number of IELs, especially those of the CD4+ and CD8αβ+ T cell subsets, indicating a more pronounced effect on thymus derived IELs. Expression of αE integrin was reduced in CD4+ and CD8αβ+ T cell subsets in IELs of Id2−/− mice. IELs isolated from C57BL/6 mice reconstituted with Id2−/− bone marrow cells showed a similar phenotype to that of Id2−/− mice, indicating that the defects are intrinsic to bone marrow derived cells. Expression of genes encoding intestinal epithelial cell derived cytokines was reduced in Id2−/− mice. The 5-FU treatment revealed impaired intestinal barrier function of Id2−/− mice.
Conclusions: The Id2 gene is essential for constituting the intestinal mucosal barrier, particularly with respect to IELs. Id2 null mutant mice may provide a good experimental model for studying the ontogeny of IELs and intestinal inflammation and infection.
Keywords: Id2 mice, intestinal intraepithelial lymphocytes, lamina propria lymphocytes, mucosal immunity, small intestine
The intestinal mucosal barrier, which consists of intestinal epithelial cells (IECs) and intestinal intraepithelial lymphocytes (IELs), is the first line of the host immune defence in the gut. IELs constitute a major lymphocyte population residing in close proximity to the intestinal lumen,1–3 and the size of the IEL population is equivalent to, or larger than, the population of peripheral lymphocytes in the spleen.1 These cells have been considered to comprise several ontogenetically and phenotypically distinct T cell subpopulations that can be identified by their expression of T cell receptor (TCR), CD4 and CD8: TCRαβ+CD4+ T cells, CD8αβ+ T cells expressing TCRαβ, and CD8αα+ T cells expressing TCRαβ or TCRγδ, together with minor subpopulations of TCRαβ+CD4+CD8αα+ and TCRγδ+CD4−CD8− T cells.2 Among these T cells, CD8αα+ T cells are unique to the IELs as they are not detected in conventional peripheral lymphoid organs such as the spleen, lymph nodes, and Peyer’s patches.2–4 In addition, CD8αα+ IELs do not recirculate in the periphery whereas TCRαβ+CD4+ and TCRαβ+CD8αβ+ T cells may represent circulating CD4 and CD8αβ T cells.5 Thus IELs are developmentally categorised into two groups: thymically derived TCRαβ+CD4+ and TCRαβ+CD8αβ+ T cells, and extrathymically derived CD8αα+ T cells, which may differ with respect to their use of different cytokine signalling pathways to regulate their differentiation.3–5
IELs have a number of important immunological functions, such as cytotoxic activity, secretion of cytokines, including interleukin (IL)-2, IL-3, IL-5, tumour necrosis factor α, transforming growth factor β (TGF-β), and interferon γ (IFN-γ), and modulation of epithelial cell death and regeneration.6 Previous studies have demonstrated an essential role for IELs against infections caused by certain microorganisms and parasites. CD8αβ+ T cells are the major population active against Toxoplasma gondii infection7 and markedly contribute to elimination of virus infected intestinal mucosa cells.8 Other T cell subsets are also known to contribute to the defence system in the intestinal tract.9 On the other hand, IECs can produce various cytokines and play an important role in trafficking and maintenance of IELs.10–12 For example, αEβ7 is one of the adhesion molecules that mediate homing of lymphocytes to the gut, and upregulation of its expression has been shown to be completely dependent on epithelial derived TGF-β.11 Studies with cytokine receptor deficient mice have also pointed to the importance of IEC derived IL-2, IL-7, and IL-15 for IEL development.13,14 These observations imply that the IEL-IEC interaction is required to maintain intestinal immunity.
Id proteins are negative regulators of the functions of basic helix-loop-helix (bHLH) transcription factors, which contain a helix-loop-helix domain for protein-protein interaction and a basic region for DNA binding.15 Id proteins are involved in many aspects of the immune system, reflecting the central role of bHLH factors (exemplified by E2A gene products E47 and E12) in the differentiation, proliferation, and functions of immune cells.15–21 Id2−/− mice lack lymph nodes, Peyer’s patches,17 and nasopharyngeal lymphoid tissue18 due to a developmental defect of lymphotoxin producing cells that are essential for secondary lymphoid organ development. In addition, Id2 is required for the development of natural killer cells,19 CD8α+ dendritic cells,20 and Langerhans cells in the skin.20 Furthermore, we have shown that Id2 functions as a safeguard against inappropriately increased class switching to IgE that is induced by E2A gene products in response to B cell activation.21
Given these findings, we speculated that Id2 might be involved in the development and/or activation of intestinal lymphocytes. By analysing intestinal lymphocytes of Id2−/− mice, we found impairments in their number and in the proportions of their subsets. Alteration of the ability of IECs to produce various cytokines was also noted in Id2−/− mice. Id2−/− mice exhibited impaired barrier function of the small intestine. Our data provide evidence that Id2 is an important molecule for the organisation of the mucosal defence in the intestine.
METHODS
Mice
Male Id2 deficient mice, 12−16 weeks of age, of mixed genetic background (129/Sv × NMRI) were used in this study. All mice were maintained under specific pathogen free conditions and all experimental procedures followed the guidelines of School of Medicine, University of Fukui for animal experiments.
Isolation of lymphocytes from intestinal tissues
IELs, lamina propria lymphocytes (LPLs), and IECs were isolated from individual mice by a standard mechanical dissociation method, as described previously.12,22 Briefly, after the Peyer’s patches as well as fatty tissues and the mesentery were removed from the small intestine, the gut was opened longitudinally and cut into 1 cm pieces. These pieces were washed in RPMI 1640 (Sigma, St Louis, Missouri, USA) and incubated with stirring in 40 ml of RPMI 1640 containing 1 mM ethylenediamine tetraacetic acid (EDTA; Wako Pure Chemical Industries, Ltd, Japan) at 37°C for 30 minutes. The supernatants containing epithelial cells and IELs were collected, and the isolation procedure was repeated two more times. Pooled supernatants were then passed through a 70 μm nylon filter to remove cell debris and separated on a discontinuous density gradient of 25%, 40%, and 75% Percoll. IELs at the 40%/75% interface and IECs at the 25%/40% interface were collected and extensively washed with RPMI 1640 containing 10% fetal calf serum (FCS; JRH Biosciences, Kansas, USA).
Flow cytometric analysis and cell sorting
Freshly isolated IELs and LPLs from individual mice were suspended in phosphate buffered saline (PBS) containing 2% FCS at 106 cells/ml and stained in plastic tubes with the following fluorescein isothiocyanate (FITC), phycoerythrin (PE), or biotin conjugated monoclonal antibodies (mAbs) purchased from Pharmingen (San Diego, California, USA): anti-CD3ε (145-2C11), anti-CD4 (RM4-5), anti-CD8α (53−6.7), anti-CD8β (53−5.8), anti-CD45R/B220 (RA3-6B3), anti-49d (R1-2), anti-CD103 (M290), anti-TCRβ (H57.597), and anti-TCRδ (GL3). After incubation on ice for 20 minutes, cells were washed twice in PBS containing 2% FCS and analysed using a FACSCalibur (Becton Dickinson, California, USA) with Cellquest. Forward and side angle scatter were used to exclude dead and aggregated cells. In order to obtain a highly enriched epithelial cell population, Percoll gradient isolated IECs were stained with FITC conjugated anti-I-Ab (AF6-120.1), PE conjugated anti-CD3, anti-CD4, anti-CD8α, anti-CD45R/B220 mAb, and I-Ab+, Lin− cells were sorted by use of an EPICS Elite cell sorter (Coulter Electronics Ltd, Miami, Florida, USA). The purity of the cell population was more than 99%.
RT-PCR and real time PCR analyses of mRNA levels
Total RNA was extracted from IECs using Isogen reagent (Wako) according to the manufacturer’s protocol. RNA samples were reverse transcribed (RT) with 5 μM random hexamers, 1 mM dNTP, 20 U of RNase inhibitor (Promega Biosciences, San Luis Obispo, California, USA), and 100 U of Moloney murine leukaemia virus reverse transcriptase (Wako) in a volume of 20 μl at 42°C for 50 minutes. Ids expression was determined by polymerase chain reaction (PCR) using the following primer sets: Id1, 5′-TCA GGA TCA TGA AGG TCG CCA GTG-3′ and 5′-TGA AGG GCT GGA GTC CAT CTG GT-3′; Id2, 5′-TCT GAG CTT ATG TCG AAT GAT AGC-3′ and 5′-CAC AGC ATT CAG TAG GCT CGT GTC GTC-3′; Id3, 5′-CCTCTC TAT CTC TAC TCT CCA ACA-3′ and 5′-TGA CCA GCG TGT GCT AGC TCT TCA-3′; Id4, 5′-GCG ATA TGA ACG ACT GCT ACA GTC-3′ and 5′-ACT TAG CAG TCT GGT CGA CAA CAC-3′. Real time PCR was performed using the double stranded DNA binding dye SYBR Green I with the ABI Prism 7700 system (Applied Biosystems, Foster City, California, USA). The sequences of primers used in this study were as follows: stem cell factor (SCF), 5′-TCT TCA ACT GCT CCT ATT T-3′ and 5′-ACT GCT ACT GCT GTC ATT-3′; TGF-β1, 5′-CGG GAG GCC AGC CGC GGG AC-3′ and 5′-GTA ACG CCA GGA ATT GTT GC-3′; IL-6, 5′-GAA CAA CGA TGA TGC ACT TGC AG-3′ and 5′-TCC TTA GCC ACT CCT TCT GTG ACT-3′; IL-7, 5′-GCC TGT CAC ATC ATC TGA GTG CC-3′ and 5′-CAG GAG GCA TCC AGG AAC TTC TG-3′; IL-15, 5′-CTT CTG TCC AGC TAC TCT TCC CCA-3′ and 5′-CCA AAC ACA GCA GGA TCC CGT CTT-3′; β-actin, 5′-CAA CCG TGA AAA GAT GAC CCA GAT C -3′ and 5′-AGT CCA TCA CAA TGC CTG TGG TAC-3′.
Bone marrow transplantation
Male C57BL/6 mice (Ly9.2) at eight weeks of age were lethally irradiated (9.0 Gy) and injected intravenously with 5×106 bone marrow cells isolated from Id2+/+ or Id2−/− (129/Sv, Ly9.1) mice. Eight weeks after cell transfer, three colour fluorescence cytometry was used to detect donor derived lymphocytes based on expression of Ly9.1.
5-fluorouracil (5-FU) treatment and counting of endogenous bacterial colonies
Mice were injected with 800 mg/kg 5-fluorouracil (5-FU) (Sigma) intraperitoneally and mortality was monitored everyday. The livers and spleens were removed and homogenised with 5 ml PBS. Homogenates were serially diluted and spread on agar plates containing the MacConkey medium to detect enterobacteria (Kyokuto, Tokyo, Japan). Colony numbers were counted after incubation for 24 hours at 37°C.
Statistical analysis
The Student’s t test was used to determine the significance of differences. A p value of less than 0.05 was taken as significant.
RESULTS
Small intestinal IELs express Id2 mRNA
As mice deficient in Id genes show various defects in the immune system, we considered whether Id2 plays some role in small intestinal lymphocytes which constitute a large part of the immune system in mammals. To address this question, we first used RT-PCR to examine expression of Id genes in IELs and found selective expression of Id2 in these cells (fig 1A ▶). We further sorted various T cell subsets from IELs and LPLs of wild-type mice and examined mRNA expression of Id genes in these subsets. As shown in fig 1B ▶, all subsets of IELs (CD8αα+, CD8αβ+, and CD4+ T cells) mainly expressed Id2 mRNA, and also weakly expressed Id3. In addition, although T cell subsets of LPLs (CD4+ and CD8+ T cells) expressed Id2 and Id3, expression was barely detectable. These results strongly suggest that Id2 is profoundly involved in the development, survival, and/or activation of IELs.
Figure 1.
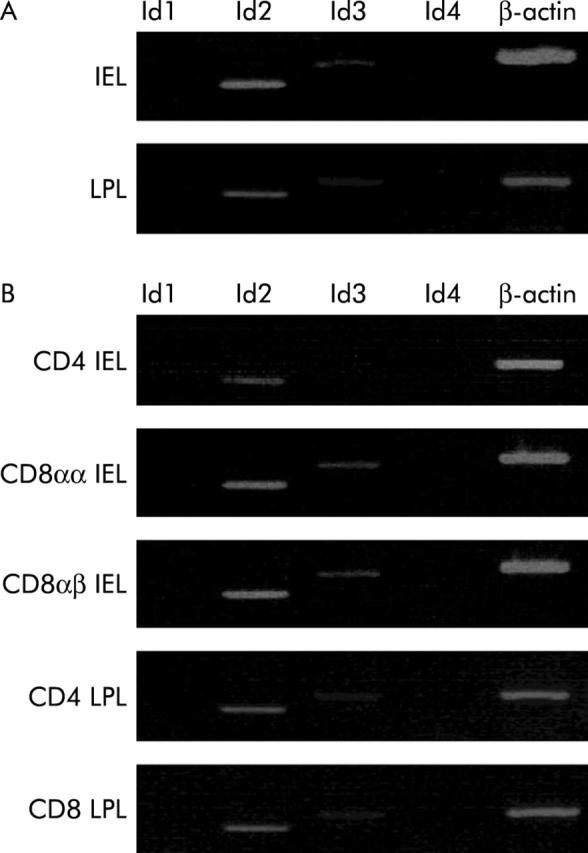
Reverse transcription-polymerase chain reaction (RT-PCR) analysis of gene expression of Ids in intestinal intraepithelial lymphocytes (IELs). (A) Id gene expression in IELs and (B) lamina propria lymphocytes (LPLs) and in their subsets. RNA was prepared from the respective cells as indicated on the left and subjected to RT followed by PCR. Genes analysed are indicated on the top. β-actin served as an internal loading control. Representative data of three independent experiments are shown.
Id2−/− mice have decreased numbers of CD8+ and CD4+ T lymphocytes in IELs
We next determined the total number and proportions of IEL subsets in Id2−/− mice and found dramatic alterations. The absolute number of small intestinal IELs in Id2−/− mice was 0.36 (0.10)×106 cells while that in Id2+/− mice was 2.29 (0.57)×106 cells, indicating a sevenfold reduction in Id null mice. Population analysis using cell surface markers showed that the percentages of cells positive for CD4, CD8β, and TCRβ were decreased by 3.2-, 5.6-, and 2.0-fold in Id2−/− mice, respectively, indicating that the proportions of thymus dependent CD4+ and CD8αβ+ subsets were reduced (fig 2A ▶). The absolute numbers of various subsets of IELs of Id2−/− mice were 0.02 (0.02), 0.16 (0.06), 0.15 (0.05), and 0.02 (0.01)×106 cells/intestine for CD4+, CD8+, CD8αα+, and CD8αβ+ T cell subsets, respectively (fig 2C ▶). These data reveal that the subset cell numbers were decreased by 12.0-, 8.5-, 4.9-, and 38.0-fold compared with those of control Id2+/− littermates (0.24 (0.16), 1.36 (0.42), 0.74 (0.37), and 0.66 (0.21)×106 cells/intestine for CD4+, CD8+, CD8αα+, and CD8αβ+ subsets, respectively). A similar tendency was also observed for LPLs, along with a decrease in the number of B220+ cells, although the CD4+ T cell subset of LPLs showed only a slight tendency to decrease (fig 2B ▶, 2D ▶, and data not shown). These results indicate that Id2 deficiency affects IELs more severely than LPLs, and on thymus dependent subsets than on thymus independent subsets.
Figure 2.
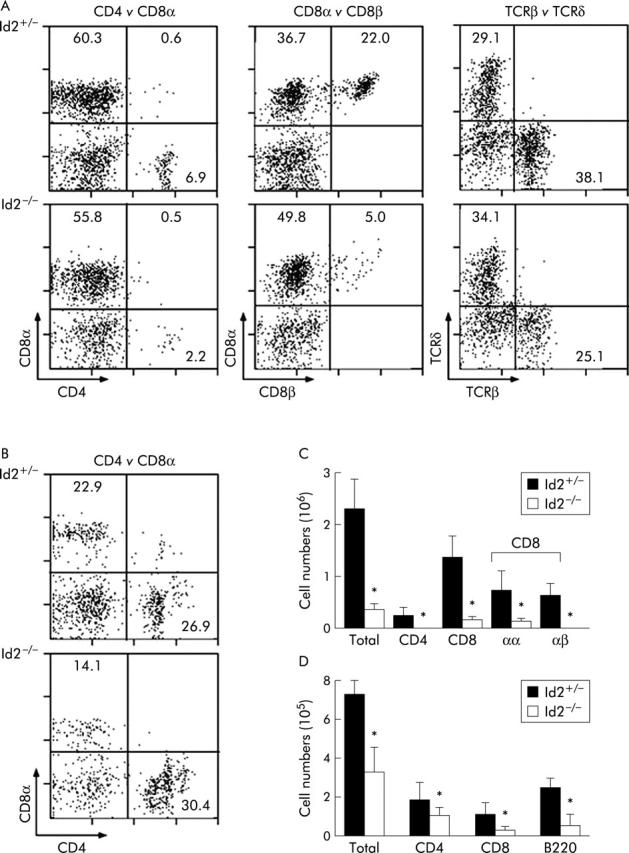
Decreased numbers and aberrant proportions of intestinal lymphocytes in Id2−/− mice. (A) Flow cytometric analysis of intestinal T cell subsets of intestinal intraepithelial lymphocytes (IELs). Representative data from 4–6 independent experiments are shown. Intestinal lymphocytes were isolated and analysed for the proportion of each T cell subset. The cell surface markers used are indicated at the bottom left corners. Percentages of subsets are shown in the respective fractions. Genotypes are indicated on the left. (B) Flow cytometric analysis of intestinal T cell subsets of lamina propria lymphocytes (LPLs). Details as described in (A). (C) Cell numbers of the respective IEL subsets. The absolute number of each subset was obtained by multiplying the percentage of cells by the total number of cells in the respective populations. Results are represented as mean (SEM) number. Each number represents the mean of 4–6 animals per group. *p<0.05, as determined by the Student’s two tailed t test. (D) Cell numbers of the respective LPL subsets. Details as described in (C).
Impairment of IELs of Id2−/− mice is intrinsic to bone marrow derived cells
To obtain insight into the cause of impairment of IELs in Id2−/− mice, we next performed bone marrow transplantation experiments and analysed the T cell subsets in the intestinal mucosa. Transplantation was conducted from 129/Sv mice (Ly9.1) to C57/BL/6 mice (Ly9.2), and donor derived cells were discriminated from recipient cells by utilising the Ly9 isotype system.17 In mice that received bone marrow cells from Id2−/− mice, there was a fourfold reduction in the total number of IELs compared with that in mice that received Id2+/+ bone marrow cells (fig 3 ▶). In addition, the proportions of the various T cell subsets in IELs recapitulated the phenotype found in Id2−/− mice (fig 3A ▶). Taken together, these results demonstrate that the defects found in IELs of Id2−/− mice are intrinsic to bone marrow derived cells.
Figure 3.
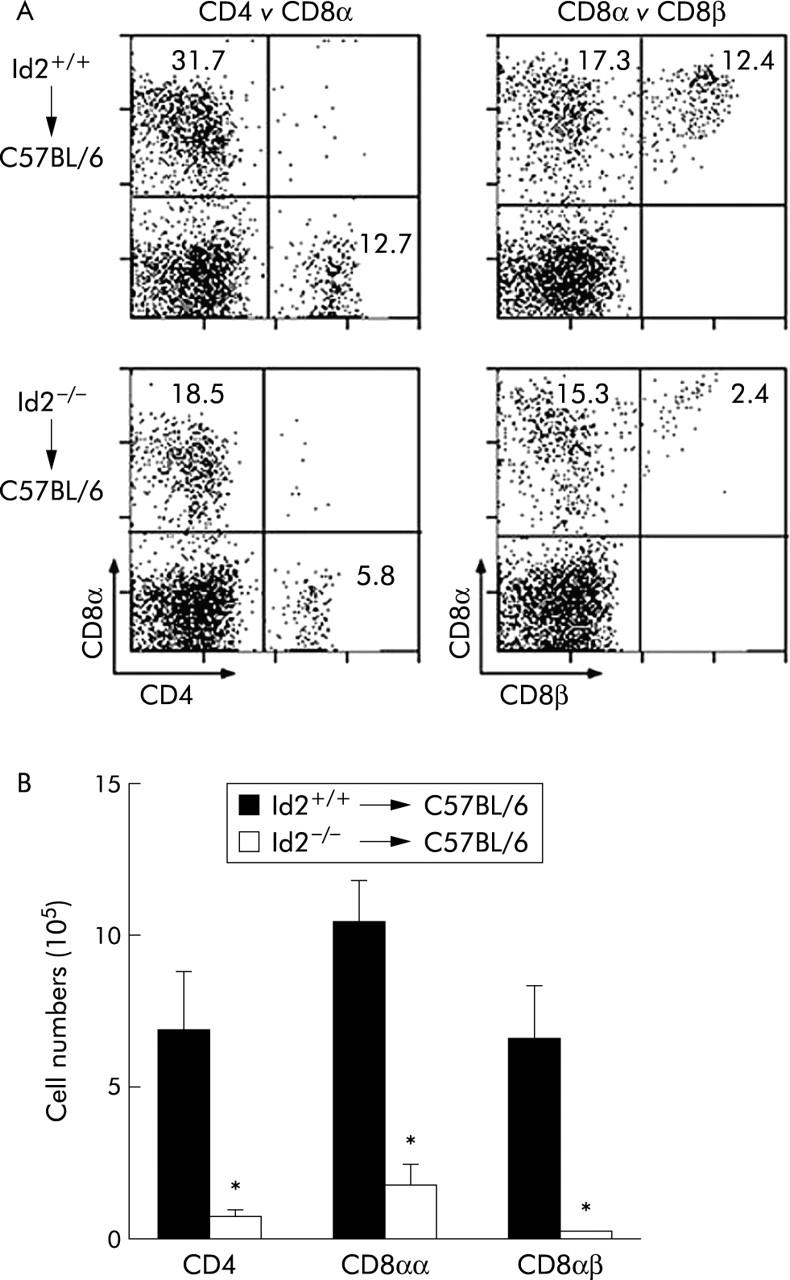
Phenotypic analysis of intestinal intraepithelial lymphocytes (IELs) after bone marrow transplantation. (A) Flow cytometric analysis of IELs of C57BL/6 mice (Ly 9.2) mice that were reconstituted with bone marrow cells of Id2+/+ or Id2−/− mice (Ly 9.1). IELs of donor origin were analysed for cell surface marker expression, as indicated by gating Ly 9.1+ cells. Percentages of cells with a given phenotype are shown. Representative data of four mice per group are shown. (B) Absolute number of each subset was obtained by multiplying the percentage of each subpopulation by the total number of viable cells. Results are expressed as mean (SEM) number. Each number represents the mean of four animals per group. *p<0.05, as determined by the Student’s two tailed t test.
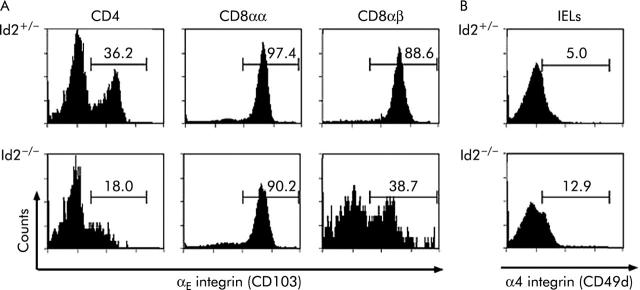
Expression of αE integrin in CD8αβ+ IELs is impaired in Id2−/− mice
We next evaluated expression of various adhesion molecules, including β2 integrin (CD18), α4 integrin (CD49d), ICAM-1 (CD54), and αE integrin (CD103) as they are known to be involved in the development and trafficking of CD8+ T cells in the intestinal epithelium.23,24 As shown in fig 4A ▶, the CD4+ T cell subset of IELs isolated from Id2−/− mice showed a significantly reduced level of αE integrin expression. In addition, the CD8αβ+T cell subset of IELs also showed a reduction of αE integrin expression in a biphasic manner while CD8αα+ IELs of Id2−/− mice showed little alteration in expression. On the other hand, we could not find any significant differences in expression of α4 integrin (CD49d), β2 integrin (CD18), or ICAM-1 (CD54) in T cell subsets between wild-type and Id2−/− mice (fig 4B ▶, and data not shown). These results suggest that αE integrin expression is selectively impaired in thymus dependent IELs of Id2−/− mice which is consistent with the observation that impairment of IELs of Id2−/− mice was more severe in thymus dependent IELs than in thymus independent IELs.
Figure 4.
Reduced expression of αE integrin on thymus derived intestinal intraepithelial lymphocytes (IELs). (A) IELs were isolated and stained with phycoerythrin conjugated antimouse CD4, antimouse CD8α, fluorescein isothiocyanate (FITC) conjugated antimouse CD8β, or biotin conjugated antimouse CD103 monoclonal antibody (mAb). After gating of cells positive for the respective mAbs, the percentages of αE integrin positive cells in the histogram were calculated. (B) Isolated IELs were stained with FITC conjugated antimouse CD49d mAb. Cell populations are indicated on the top of each panel. Representative data of 4–6 independent experiments are shown.
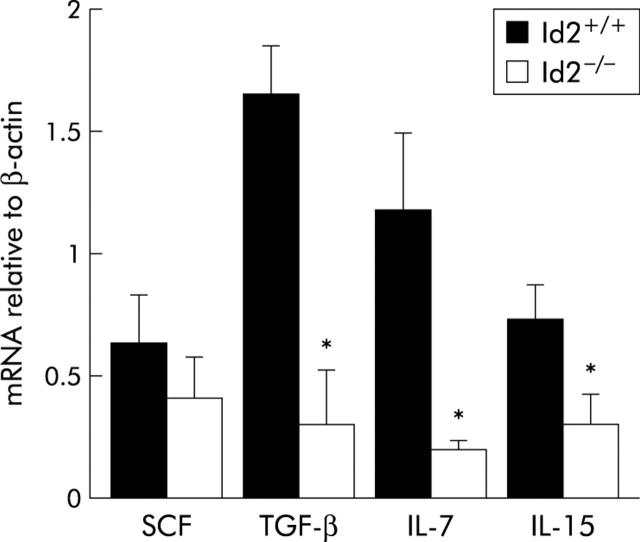
mRNA expression of IEC derived cytokines is decreased in Id2−/− mice
IECs have been shown to produce several cytokines10,12 and it was important to examine whether the decrease in IELs affected the cytokine production ability of IELs in Id2−/− mice. To compare cytokine mRNA expression in wild-type and Id2−/− mice, we isolated IECs and carried out cytokine specific real time PCR. As shown in fig 5 ▶, expression of IL-7, IL-15, and TGF-β1 mRNAs in isolated IECs was decreased in Id2−/− mice compared with those in IECs of control littermates. There was no significant difference in expression of SCF between the two groups of mice.
Figure 5.
Real time polymerase chain reaction (PCR) analysis of cytokine expression in intestinal epithelial cells (IECs). RNA was prepared from the IECs of Id2+/+ or Id2−/− mice and subjected to reverse transcription followed by real time PCR, as described in the methods section. Results are expressed as mean (SEM) number. Each bar represents the mean of three animals per group. *p<0.05, as determined by the Student’s two tailed t test. SCF, stem cell factor; IL, interleukin; TGF-β, transforming growth factor β.
High susceptibility of 5-FU treatment in Id2−/− mice
The impaired number of IELs and cytokine production by IECs of Id2−/− mice prompted us to examine intestinal barrier function of Id2−/− mice. To this end, we utilised 5-FU treatment of mice. It is well known that the cytotoxicity of 5-FU disrupts the epithelial barrier and allows intestinal bacteria to invade, which can lead to translocation of intestinal bacteria to the liver and lethal infection.25–28 This system allowed us to evaluate intestinal barrier function against invading bacteria. After 5-FU treatment, all of wild-type control mice survived during the experimental period. In contrast, only 37.5% of Id2−/− mice survived to day 12 after treatment (fig 6A ▶). In accordance with these observations, the numbers of enterobacteria in the liver and spleen of Id2−/− mice were substantially increased compared with wild-type control mice after administration of 5-FU (fig 6B ▶). These results demonstrate that Id2−/− mice are susceptible to 5-FU treatment and have impaired intestinal barrier function.
Figure 6.
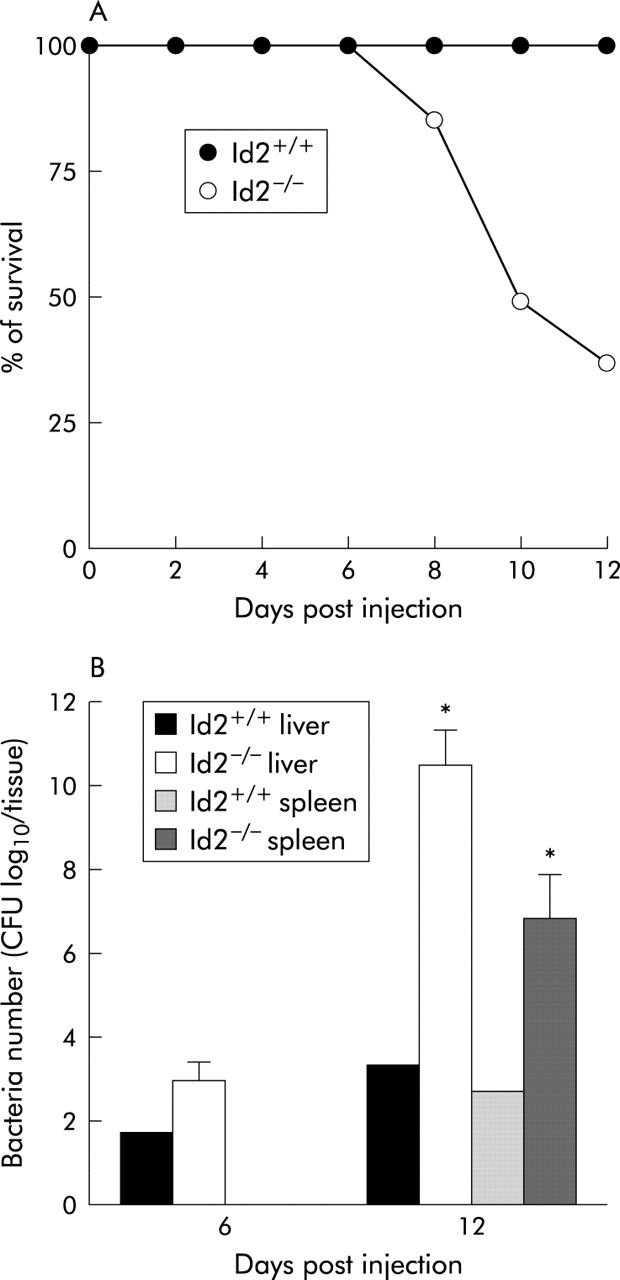
Increased susceptibility of Id2 deficient mice after 5-fluorouracil (5-FU) treatment. (A) Survival rate of mice after 5-FU treatment. Id2+/+ and Id2−/− mice were treated intraperitoneally with 800 mg/kg 5-FU. Eight mice were used in each group. (B) Translocation of endogenous bacteria from the intestine to the liver and spleen in mice treated with 5-FU. Numbers of bacteria in the liver and spleen of Id2+/+ and Id2−/− mice were determined on the indicated days after intraperitoneally injection of 800 mg/kg 5-FU. Values represent mean (SEM) of three animals. *p<0.05, as determined by the Student’s two tailed t test.
DISCUSSION
In this study, we have shown that Id2 is required for IELs to constitute the normal mucosal barrier system in the intestine and thus demonstrated a novel aspect of the in vivo function of Id2, which has been shown to be required for differentiation of various cell types in the immune system. Consistent with our finding of Id2 expression in IELs, we found a generalised reduction in the number of IELs in Id2−/− mice. The reduction was more pronounced in thymus derived CD4+ and CD8αβ+ T cell subsets, leading to alteration of the subset profile. These two subsets of IELs in Id2−/− mice were also found to express decreased levels of αE integrin. Furthermore, bone marrow transplantation experiments demonstrated that the defects were intrinsic to bone marrow derived cells of Id2−/− mice. In addition to the phenotypes found for IELs, IECs of Id2−-/− mice were found to have an impaired ability to produce cytokines that are thought to play an important role in maintaining the mucosal defence in the intestinal tract. Thus our findings strengthen the idea that Id2 is an essential molecule for establishment of the immune system that protects our bodies from invading microorganisms.
The decrease in the number of lymphocytes observed in Id2−/− mice is a phenomenon that selectively affects IELs because there is no such impairment in splenocytes or thymocytes of Id2−/− mice.17 It is currently not known why IELs of Id2−/− mice are decreased as a whole, irrespective of the T cell subset, although it is clear that the phenotypes of IELs in Id2−/− mice are derived from bone marrow cells. The fact that Id2 is the predominantly expressed member of the Id gene family, expressed in each T cell subset of IELs, implies a distinct role for Id2 in the development, survival, and/or proliferation of lymphocytes in the intestine. As Id proteins negatively regulate the function of E proteins, type A bHLH transcription factor,29 the balance between the activities of Id2 and E proteins may be important for the development and/or maintenance of IELs.
We observed selectively diminished expression of αE integrin in CD4+ and CD8αβ+ IELs of Id2−/− compared with other adhesion molecules analysed. Adhesion molecule play an indispensable role in maintaining the intestinal immune cells, and αEβ7 integrin mediates the binding of IELs to IECs through interaction with E-cadherin, a membrane protein previously known for its role in the homophilic interactions of adherent junctions.30 Moreover, αE integrin deficient mice show a substantially reduced number of IELs.23,24 As E-cadherin expression in IECs of Id2−/− mice is normal (unpublished observations), impaired expression of αE integrin in thymus derived IEL subsets can explain, at least in part, why the number of thymus derived IELs is more severely reduced than that of extrathymically derived subsets, and supports the notion that αEβ7 integrin is important for IELs. Id2 may be involved in regulating αE integrin expression by inhibiting the activity of some bHLH factor that suppresses expression in these cell types, although the detailed mechanism remains unclear.
IEC derived cytokines, including IL-6, IL-7, IL-15, and TGF-β, are indispensable for the development and survival of lymphocytes in the intestinal tract, showing distinct but partly overlapping effects on subpopulations of intestinal lymphocytes.10,31,32 Thus decreased gene expression of these cytokines in IECs of Id2−/− mice may reflect the generalised impairment of IECs of Id2−/− mice. However, the defective features of IELs of Id2−/− mice were essentially reconstituted in wild-type mice that received bone marrow transplants from Id2−/− mice. These findings suggest that the defect is intrinsic to the immune cells of Id2−/− mice. It is therefore plausible that IECs of Id2−/− mice per se have a defect in producing these cytokines, although we cannot completely rule out the possibility that impaired IELs affect the functions of IECs of Id2−/− mice. In relation to these observations, we analysed the Th1/Th2 balance in IELs of Id2−/− mice because splenic CD4+ T cells of Id2−/− mice are skewed to Th2 cells.33 Although we observed a higher proportion of IL-4 producing CD4+ T cells, we did not see a difference in IFN-γ producing CD4+ T cells in IELs (data not shown). These findings may suggest that differentiation and/or maintenance of CD4+ T cells in the intestine is different from that in the spleen. Currently, it is unclear whether the increased proportion of Th2 cells in the intestine contributes to generation of impaired IECs of Id2−/−- mice. Further investigations are required to characterise IECs of Id2−/− mice.
The gastrointestinal tract serves as a major site for primary infection as well as for the entry of pathogens, and the immune response at the mucosal surface provides the first barrier to invading pathogens. As IELs have a predominant role in excluding pathogens in the intestine through their cytolytic activity and/or inflammatory cytokine production,6–10 we assessed the physiological meaning of impaired number of IELs and cytokine production of IECs in Id2 null mutant mice. Utilising 5-FU treatment, a decreased survival rate and enhanced numbers of enterobacteria in the liver and spleen of Id2−/− mice (fig 6 ▶) strongly suggested that intestinal barrier function is severely impaired in mutant mice. In this regard, Id2 null mutant mice may provide a good model system for studying gastrointestinal infection. In addition, Id2−/− mice are unique among other gene deficient mice that show a defect in intestinal lymphocytes, such as IL-7−/−, IL-7Rα−/−, and IL-2Rβ−/− mice,34–37 in that Id2 null mice show impairment of lymphocytes rather selectively in the intestine, a generalised reduction of IELs, and a stronger effect on thymus derived IEL subsets. Further investigations using Id2−/− mice may disclose in detail the developmental pathway of lymphocytes in the intestine.
Acknowledgments
This work was supported by Grants-in Aid from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, (YY #13470034, #13037016, and #15390102). J-KK was supported by grants from the Japan Society for the Promotion of Science.
Abbreviations
bHLH, basic helix-loop-helix
IELs, intestinal intraepithelial lymphocytes
LPLs, lamina propria lymphocytes
IECs, intestinal epithelial cells
RT-PCR, reverse transcription-polymerase chain reaction
TCR, T cell receptor
IL, interleukin
TGF-β, transforming growth factor β
IFN-γ, interferon γ
FCS, fetal calf serum
PBS, phosphate buffered saline
FITC, fluorescein isothiocyanate
PE, phycoerythrin
mAb, monoclonal antibody
5-FU, 5-fluorouracil
SCF, stem cell factor
REFERENCES
- 1.Kaufmann SH. γ/δ and other unconventional lymphocytes: what do they see and what do they do? Proc Natl Acad Sci U S A 1996;93:2272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocha B, Vassalli P, Guy-Grand D. Thymic and extrathymic origins of gut intraepithelial population in mice. J Exp Med 1994;180:681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poussier P, Julius M. Thymus independent T cell development and selection in the intestinal epithelium. Annu Rev Immunol 1994;12:521–53. [DOI] [PubMed] [Google Scholar]
- 4.Beagley KW, Husband AJ. Intraepithelial lymphocytes: origins, distribution, and function. Crit Rev Immunol 1998;18:237–54. [DOI] [PubMed] [Google Scholar]
- 5.Das G, Janeway CA. MHC specificity of iIELs. Trends Immunol 2003;24:88–93. [DOI] [PubMed] [Google Scholar]
- 6.Fujihashi K, Yamamoto M, McGhee JR, et al. Function of αβ TCR+ intestinal intraepithelial lymphocytes: Th-1 and Th-2 type cytokine production by CD4+CD8− and CD4+CD8+ T cells for helper activity. Int Immunol 1993;5:1473–81. [DOI] [PubMed] [Google Scholar]
- 7.Buzoni-Gatel D, Debbabi H, Moretto M, et al. Intraepithelial lymphocytes traffic to the intestine and enhance resistance to Toxoplasma gondii oral infection. J Immunol 1999;162:5846–52. [PubMed] [Google Scholar]
- 8.Muller S, Buhler-Jungo M, Mueller C. Intestinal intraepithelial lymphocytes exert potent protective cytotoxic activity during an acute virus infection. J Immunol 2000;164:1986–94. [DOI] [PubMed] [Google Scholar]
- 9.McDonald V, Robinson HA, Kelly JP, et al. Immunity to Cryptosporidium muris infection in mice expressed through gut CD4+ intraepithelial lymphocytes. Infect Immun 1996;64:2556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshikai Y. The interaction of intestinal epithelial cells and intraepithelial lymphocytes in host defense. Immunol Res 1999;20:219–35. [DOI] [PubMed] [Google Scholar]
- 11.Shibahara T, Si-Tahar M, Shaw SK, et al. Adhesion molecules expressed on homing lymphocytes in model intestinal epithelia. Gastroenterology 2000;118:289–98. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto M, Fujihashi K, Kawabata K, et al. A mucosal intranet: intestinal epithelial cells down-regulated intraepithelial, but not peripheral T lymphocytes. J Immunol 1998;160:2188–96. [PubMed] [Google Scholar]
- 13.Porter BO, Malek TR. IL-2Rβ/IL-7Rα doubly deficient mice recapitulate the thymic and intraepithelial lymphocyte (IEL) developmental defects of gamma c−/− mice: roles for both IL-2 and IL-15 in CD8αα IEL development. J Immunol 1999;163:5906–12. [PubMed] [Google Scholar]
- 14.Inagaki-Ohara K, Nishimura H, Mitani A, et al. Interleukin-15 preferentially promotes the growth of intestinal intraepithelial lymphocytes bearing γδ T cell receptor in mice. Eur J Immunol 1997;27:2885–91. [DOI] [PubMed] [Google Scholar]
- 15.Yokota Y. Id and development. Oncogene 2001;20:8290–8. [DOI] [PubMed] [Google Scholar]
- 16.Rivera R, Murre C. The regulation and function of the Id proteins in lymphocyte development. Oncogene 2001;20:8308–16. [DOI] [PubMed] [Google Scholar]
- 17.Yokota Y, Mansouri A, Mori S, et al. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature 1999;397:702–6. [DOI] [PubMed] [Google Scholar]
- 18.Fukuyama S, Hiroi T, Yokota Y, et al. Initiation of NALT organogenesis is independent of the IL-7R, LTβR, and NIL signaling pathways but requires the Id2 gene and CD3− CD4+ CD45+ cells. Immunity 2002;17:1–10. [DOI] [PubMed] [Google Scholar]
- 19.Ikawa T, Fujimoto S, Kawamoto H, et al. Commitment to natural killer cells requires the helix-loop-helix inhibitor Id2. Proc Natl Acad Sci U S A 2001;98:5164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hacker C, Kirsch RD, Ju XS, et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol 2003;4:380–6. [DOI] [PubMed] [Google Scholar]
- 21.Sugai M, Gondo H, Kusunoki T, et al. Essential role of Id2 in negative regulation of IgE class switching. Nat Immunol 2003;4:25–9. [DOI] [PubMed] [Google Scholar]
- 22.Kim JK, Takahashi I, Kai Y, et al. Influence of the enterotoxin on mucosal intranet: selective inhibition of extrathymic T cell development in intestinal intraepithelial lymphocyte by oral exposure to heat-labile toxin. Eur J Immunol 2001;31:2960–9. [DOI] [PubMed] [Google Scholar]
- 23.Schon MP, Arya A, Murphy EA, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin αE (CD103)-deficient mice. J Immunol 1999;162:6641–9. [PubMed] [Google Scholar]
- 24.Huleatt JW, Lefrancois L. β2 integrins and ICAM-1 are involved in establishment of the intestinal mucosal T cell compartment. Immunity 1996;5:263–73. [DOI] [PubMed] [Google Scholar]
- 25.Ijiri K, Potten CS. Further studies on the response of intestinal crypt cells of different hierarchical status to eighteen different cytotoxic agents. Br J Cancer 1987;55:113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nomoto K, Yokomura T, Nomoto K. Prevention of 5-fluorouracil-induced infection with indigenous Escherichia coli in tumor-bearing mice by nonspecific immunostimulation. Can J Microbiol 1992;38:774–8. [DOI] [PubMed] [Google Scholar]
- 27.Tancrede CH, Andremont AO. Bacterial translocation and gram-negative bacteria in patients with hematological malignancies. J Infect Dis 1985;152:99–103. [DOI] [PubMed] [Google Scholar]
- 28.Itoh N, Nishimura H, Matsuguchi T, et al. CD8α-deficient mice are highly susceptible to 5-fluorouracil-induced lethality. Clin Diagn Lab Immunol 2002;9:550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massari ME, Rivera RR, Voland JR, et al. Characterization of ABF-1, a novel basic helix-loop-helix transcription factor expressed in activated B lymphocytes. Mol Cell Biol 1998;18:3130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cepak KL, Shaw SK, Parker CM, et al. Adhesion between epithelial cells and T lymphocytes mediated E-cadherin and an integrin αEβ7. Nature 1994;372:190–3. [DOI] [PubMed] [Google Scholar]
- 31.Brunner T, Arnold D, Wasem C, et al. Regulation of cell death and survival in intestinal intraepithelial lymphocytes. Cell Death Differ 2001;8:706–14. [DOI] [PubMed] [Google Scholar]
- 32.Hirose K, Suzuki H, Nishimura H, et al. Interleukin-15 may be responsible for early activation of intestinal intraepithelial lymphocytes after oral infection with Listeria monocytogenes in rats. Infect Immun 1998;66:5677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kusunoki T, Sugai M, Katakai T, et al. TH2 dominance and defective development of a CD8+ dendeitic cell subset in Id2-deficient mice. J Allergy Clin Immunol 2003;111:136–42. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki H, Duncan GS, Takimoto H, et al. Abnormal development of intestinal intraepithelial lymphocytes and peripheral natural killer cells in mice lacking in the IL-2 receptor β chain. J Exp Med 1997;185:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He YW, Malek TR. Interleukin-7 receptor α is essential for the development + T cells, but not natural killer cells. J Exp Med 1996;184:289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maki K, Sunaga S, Komagata Y, et al. Interleukin-7 receptor-deficient mice lack γδ T cells. Proc Natl Acad Sci U S A 1996;93:7172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puddington L, Olson S, Lefrancosis L. Interactions between stem cell factor and c-Kit are required for intestinal immune system homeostasis. Immunity 1994;1:733–9. [DOI] [PubMed] [Google Scholar]