Abstract
Background and aims: Fatty liver is a common histological finding in human liver biopsy specimens. It affects 10–24% of the general population and is believed to be a marker of risk of later chronic liver disease. The present study examined the risk of development of cirrhotic liver disease and the risk of death in a cohort diagnosed with pure fatty liver without inflammation.
Methods: A total of 215 patients who had a liver biopsy performed during the period 1976–1987 were included in the study. The population consisted of 109 non-alcoholic and 106 alcoholic fatty liver patients. Median follow up time was 16.7 (0.2–21.9) years in the non-alcoholic and 9.2 (0.6–23.1) years in the alcoholic group. Systematic data collection was carried out by review of all medical records. All members of the study cohort were linked through their unique personal identification number to the National Registry of Patients and the nationwide Registry of Causes of Death, and all admissions, discharge diagnoses, and causes of death were obtained.
Results: In the non-alcoholic fatty liver group, one patient developed cirrhosis during the follow up period compared with 22 patients in the alcoholic group. Survival estimates were significantly (p<0.01) different between the two groups, for men as well as for women, with a higher death rate in the alcoholic fatty liver group. Survival estimates in the non-alcoholic fatty liver group were not different from the Danish population.
Conclusions: This study revealed that patients with type 1 non-alcoholic fatty liver disease have a benign clinical course without excess mortality.
Keywords: fatty liver, epidemiology, cirrhosis, mortality, liver biopsy
Fatty liver or steatosis hepatitis, the accumulation of lipid within hepatocytes, is a common histological finding in human liver biopsy specimens and affects 10–24% of the general population.1–3
In routine clinical practice, most cases are attributable to alcohol excess; however, it can also occur in association with a wide range of toxins, drugs, and diseases, such as morbid obesity, type 2 diabetes, hyperlipidaemia, and after jejunoileal bypass surgery and debilitating diseases with cachexia.4,5 Non-alcoholic fatty liver disease (NAFLD) is often histologically and clinically indistinguishable from the liver damage resulting from alcohol excess.5
The pathophysiology of NAFLD is believed to involve two steps.6 The first step involves insulin resistance and obesity and causes the development of steatosis; the second step is oxidative stress, activating an inflammatory response and causing non-alcoholic steatohepatitis (NASH).
The prognosis of NAFLD is uncertain. Only a few patients have been followed prospectively in order to describe the natural history of the disease. Published follow up studies include small numbers of patients with histological diagnoses varying from simple fatty liver to NASH and cirrhosis. Teli and colleagues7 found that the non-alcoholic fatty liver had a benign long term prognosis but only 40 patients were followed in this study. Studies involving predominantly NASH patients show a much more aggressive natural history, with development of cirrhosis in up to 26% of patients.8–13
Predictive factors that may distinguish between pure fatty liver patients with a good prognosis and those developing NASH have not yet been identified. Patients with alcoholic fatty liver disease who continue to consume large amounts of alcohol daily have been found to have a risk of 8–30% of developing fibrosis or cirrhosis after 10 years.14,15
The aim of this study was to examine the risk of developing cirrhosis and the risk of death in patients histologically diagnosed with pure fatty liver without inflammation (type 1 NAFLD) and without other known chronic liver diseases.
PATIENTS AND METHODS
Patient population
Liver biopsy specimens obtained in 243 patients during the period 1976–1987 with a histological diagnosis of pure fatty liver without inflammation were identified (the index liver biopsy) through a computerised pathology register at the Department of Pathology, Hvidovre Hospital, University of Copenhagen, Denmark.
The 243 patients were examined with respect to the following exclusion criteria: (1) presence of acute or chronic liver disease during follow up, modifying the index liver biopsy: hepatitis B, hepatitis C, primary biliary cirrhosis, autoimmune hepatitis, α1 antitrypsin deficiency, haemochromatosis, other types of infectious hepatitis, and human immunodeficiency virus; (2) jejunoileal bypass operation during the follow up period; (3) total parenteral nutrition at the time of the index liver biopsy; (4) use of methotrexate, amiodarone, tamoxifen, or high doses of corticosteroids; and (5) malignancy at the time of the index liver biopsy.
During the inclusion period, several obesity research projects were performed at the Department of Endocrinology, Hvidovre University Hospital. Seventy five (35%) patients described in this study had their index liver biopsy performed as part of these research projects and all were morbidly obese. The indication for the index liver biopsy in the rest of the cohort was either incidental finding of abnormalities in liver function tests, mainly elevated serum aspartate aminotransferase, and/or hepatomegaly, or suspicion of alcoholic liver disease.
Data collection
The unique personal identification number assigned to all inhabitants in Denmark was found through record linkage to the pathology register at Hvidovre Hospital. Systematic data collection was carried out by review of all 243 medical records. Age, height, and weight were documented at baseline (time of index liver biopsy). Body mass index (BMI) was calculated and defined as weight/height2 (kg/m2). History of diabetes mellitus, hyperlipidaemia, liver diseases, malignancy, or other chronic medical conditions was recorded at baseline (time of index liver biopsy) when available, together with data on drug and alcohol intake, as noted in the medical records. However, there was no systematic testing for diabetes or hyperlipidaemia at baseline.
Laboratory data at baseline included the following variables when available: serum aspartate aminotransferase (ASAT), serum lactate dehydrogenase (LDH), serum alkaline phosphates, serum bilirubin, serum albumin, plasma prothrombin time, blood glucose, urine glucose, serum sodium, serum potassium, serum creatinine, platelet count, serum cholesterol, serum triglycerides, and hepatitis B surface antigen.
The Danish National Registry of Patients (LPR)16 contains information on all patients admitted to non-psychiatric hospitals in Denmark since 1977. This includes date of birth, the unique personal identification number, sex, hospital, department, date of admission, and discharge diagnoses. Diagnoses were coded according to the WHO International Classification of Diseases, eighth edition (ICD-8)17 from 1 January 1977 to 31 December 1993, and from 1 January 1994 according to 10th edition (ICD-10).18
All members of the study cohort were linked through their personal identification number to the LPR and the nationwide Registry of Causes of Death,19 and all admissions, discharge diagnoses, and causes of death in the study population were obtained. Patients were followed until death or 31 December 1999 in the LPR and Registry of Causes of Death; information regarding time of death for survival statistics was until 1 May 2001, thus allowing follow up for up to 23 years. Patients were excluded if they were lost to follow up in the registries.
Liver cirrhosis was accepted as present in patients who had a discharge diagnosis, death certificate diagnosis, or a histological finding in the follow up period consistent with cirrhosis. The first registered date of diagnosis was used in the statistical analysis.
Patients with an alcohol intake above the sensible drinking limits set by the Danish National Board of Health (21 drinks per week for men (1 drink = 12 g alcohol) and 14 drinks per week for women) or an alcohol related diagnosis at any time from biopsy to 31 December 1999 were considered to have alcoholic fatty liver. Alcohol related diagnoses used were: alcohol abuse (ICD-8 codes 303.xx + 294.30 + 780.19 + 979.19 + 979.29 + 979.49; ICD-10 codes F10.x + E51.2 + Z72.1), alcoholic polyneuropathy (ICD-8 code 303.91; ICD-10 code G62.1), alcoholic cardiomyopathy (ICD-10 code I42.6), and alcoholic pancreatitis (ICD-10 code K86.0).
The Danish Data Protection Agency and the regional scientific ethics committee approved the study.
Histological assessment
Formalin fixed paraffin embedded index liver biopsies were cut into 4–5 µm sections, approximately 50 sections per biopsy. Sections were stained with haematoxylin and eosin, Van Gieson Hansen, periodic acid, periodic acid with diastase, Pearls iron, and for reticulum.
Two pathologists reviewed the histological slides without knowledge of the patient’s clinical or biochemical data, and morphological findings were recorded in a semi-quantitative manner (0 to +++) regarding steatosis and fibrosis. Furthermore, the location of steatosis (centrilobular, periportal, or diffuse) and fibrosis (periportal or diffuse, perisinusoidal or pericellular) were recorded. The size of the steatotic vesicles (macrovesicular, microvesicular, or mixed) was also recorded.
Statistical analysis
Results are presented as medians (ranges) unless otherwise stated. The Mann-Whitney U test was used to test for differences between groups. A p value of <0.05 was considered statistically significant.
The primary end point was death from all causes and the secondary end point was cirrhosis. Survival curves were constructed based on the Kaplan-Meier method. We performed our statistical analysis using delayed entry, taking into account both the age of the patient and time of entry at diagnosis, and compared survival between the groups in a Cox model. Survival curves for the whole Danish population as a comparison were estimated from data from Statistics Denmark for 1982–83.
Statistical tests were performed using SPSS software (version 10.1 for Windows) and SAS software (version 8 for Windows).
RESULTS
Clinical and biochemical results
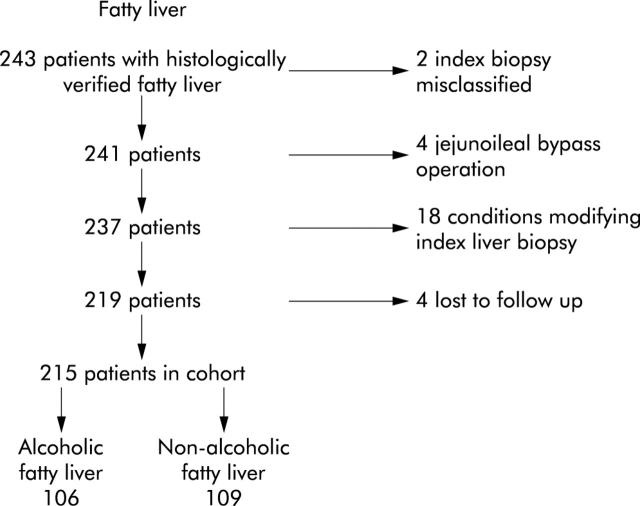
Numbers of patients and reasons for exclusion are shown in fig 1 ▶. The medical records of the 243 patients initially identified as having fatty liver were traced and the biopsies reviewed. Two index biopsies were misclassified; four patients had an jejunoileal bypass operation performed in the follow up period due to obesity; 13 were excluded owing to specific hepatic diseases diagnosed in the follow up period that could have modified the index biopsy (three biopsies shortly after acute hepatitis B, one hepatitis C, four primary biliary cirrhosis, two haemochromatosis, one α1 antitrypsin deficiency, one autoimmune hepatitis, one histologically verified cirrhosis diagnosed prior to the index liver biopsy); two received methotrexate treatment; three had malignant disease at the time of biopsy; and four were lost to follow up.
Figure 1.
Details of patients studied, showing reasons for exclusions.
No patient was receiving drugs known to be associated with the development of steatosis. All biopsies were without histological signs of viral hepatitis.
Table 1 ▶ shows the clinical characteristics at the time of the index liver biopsy in 215 patients in the two groups. Median follow up time was 16.7 (0.2–21.9) years in the non-alcoholic and 9.2 (0.6–23.1) years in the alcoholic group.
Table 1.
Clinical and biochemical data
| Non-alcoholic fatty liver (n = 109) | Alcoholic fatty liver (n = 106) | |
| Sex (F/M) | 76/33 | 31/75 |
| Age (y) | 39 (19–80) | 50 (26–72)*** |
| Obesity (%) | 81 (n = 101) | 30** (n = 76) |
| BMI (kg/m2) | 42 (19–72) (n = 82) | 26 (18–50)*** (n = 31) |
| Daily alcohol intake drinks† | 0 (0–3) (n = 95) | 10 (0–50)*** (n = 95) |
| ASAT (10–40 U/l)‡ | 25 (9–201) | 43 (10–576)*** |
| LDH (200–450 U/l) | 393 (165–948) | 361 (177–747) |
| Bilirubin (5–17 U/l) | 7 (2–49) | 10 (3–57)** |
| Alkaline phosphatases (80–275 U/l) | 212 (100–6360) | 226 (99–1037) |
| Prothrombin (0.70–1.30) | 1.09 (0.62–2.01) | 1.15 (0.49–1.99) |
| Albumin (540–800 µmol/l) | 630 (407–754) | 584 (303–726)*** |
| Platelets (135–400 109/l) | 277 (121–638) | 255 (102–624) |
| Cholesterol (3.5–8.0 mmol/l) | 5.13 (2.22–8.84) | 5.35 (3.32–7.97) |
†1 Drink = 12 g alcohol.
‡Normal range.
Values are median (range).
**p<0.01; ***p<0.001.
BMI, body mass index; ASAT, aspartate aminotransferase; LDH, lactate dehydrogenase.
Information on BMI was available in 53% of the total population; 75% in the non-alcoholic and 29% in the alcoholic fatty liver group. The non-alcoholic fatty liver group had a significantly higher BMI which in part reflected the fact that they comprised a selected group from the ongoing obesity research projects at that time. If, in the present context, obesity was defined as BMI ⩾30 or a clinical description of obesity in the patient record by the physician, 81% were obese in the non-alcoholic group and 30% in the alcoholic group. Four patients (of 106 patients with alcoholic fatty liver) had their initial index liver biopsy performed due to participation in an obesity research project but were later classified as having alcoholic fatty liver because of excessive alcohol intake at the time of the index liver biopsy. None developed cirrhosis or had an alcohol related diagnosis registered during the follow up period.
There were no correlations between the prevalence of cirrhosis and obesity in the alcoholic group. Only four of 22 patients diagnosed with alcoholic cirrhosis were also characterised as obese. There was no difference in the amount of alcohol consumed in the obese alcoholic patients diagnosed with cirrhosis compared with non-obese alcoholic patients with cirrhosis.
At the time of the index liver biopsy, 2% and 1% of patients had known type 1 diabetes in the non-alcoholic and alcoholic groups, respectively. Type 2 diabetes was present in 7% and 2% in the non-alcoholic and alcoholic groups, respectively.
ASAT and bilirubin were significantly higher, and albumin significantly lower, in the alcoholic group at the time of the index liver biopsy. Other biochemical results were not significantly different (table 1 ▶). Serological markers for hepatitis B were examined in 27% of the cohort at the time of the index liver biopsy and none was positive for hepatitis B surface antigen. No patient was diagnosed with hepatitis B after the index liver biopsy. None was tested for hepatitis C at the time of the index liver biopsy but one patient had a diagnosis of hepatitis C registered in the follow up period and was excluded from the study.
Histological end points
In the non-alcoholic fatty liver group, one patient (1%) developed cirrhosis during the follow up period compared with 22 (21%) in the alcoholic fatty liver group (table 2 ▶). Two of 22 patients diagnosed with alcoholic cirrhosis denied alcohol intake at the time of the index liver biopsy but had alcohol related diagnoses registered during the follow up period.
Table 2.
Development of chronic liver diseases and death
| Non-alcoholic fatty liver (n = 109) | Alcoholic fatty liver (n = 106) | |
| Cirrhosis | 1 (1) | 22 (21) |
| Death | 27 (25) | 79 (74) |
Values are number (%).
Of 23 patients diagnosed with cirrhosis in our cohort, seven had a histologically verified diagnosis, either by means of a second liver biopsy during the follow up period or at autopsy, 12 had a diagnosis registered in the LPR, and four patients were classified as having cirrhosis based only on the information on the death certificate. In eight of the 12 patients, the diagnosis in the LPR was based on the following clinical signs: bleeding oesophageal varices (n = 1); oesophageal varices and ascites (n = 1); hepatic coma (n = 1); hepatic coma and ascites (n = 1); hepatic coma and coagulopathy (n = 3); and compensated cirrhosis (n = 1). The last four patients were admitted to other hospitals and we do not know whether the diagnosis was based on histology or clinical criteria alone.
A total of 13 patients in the alcoholic and six patients in the non-alcoholic group were autopsied. Another 19 in the alcoholic and 20 patients in the non-alcoholic group had at least one follow up biopsy during the study period.
Two patients were diagnosed with NASH in a second liver biopsy less than one year after the index liver biopsy. Both were obese women (BMI >38 kg/m2) and one had diabetes at the time of the index liver biopsy. None died in the follow up period. The four patients with alcoholic steatohepatitis diagnosed in another liver biopsy during the follow up period were all men. Two died of causes unrelated to liver disease.
Mortality
A total of 106 patients died during the follow up period; 44 women and 62 men (table 2 ▶). Of these, 20 were liver related in the alcoholic group while 59 died from other causes. The only patient in the non-alcoholic group diagnosed with cirrhosis died of liver disease; 26 died of causes unrelated to liver disease. Comparing the two groups in a Cox model, survival was found to be significantly higher (p<0.01) in the non-alcoholic fatty liver group for men as well as for women. Survival estimates of those with non-alcoholic fatty liver were not different from the Danish population (according to the confidence intervals for the survival curve for patients) (fig 2 ▶).
Figure 2.
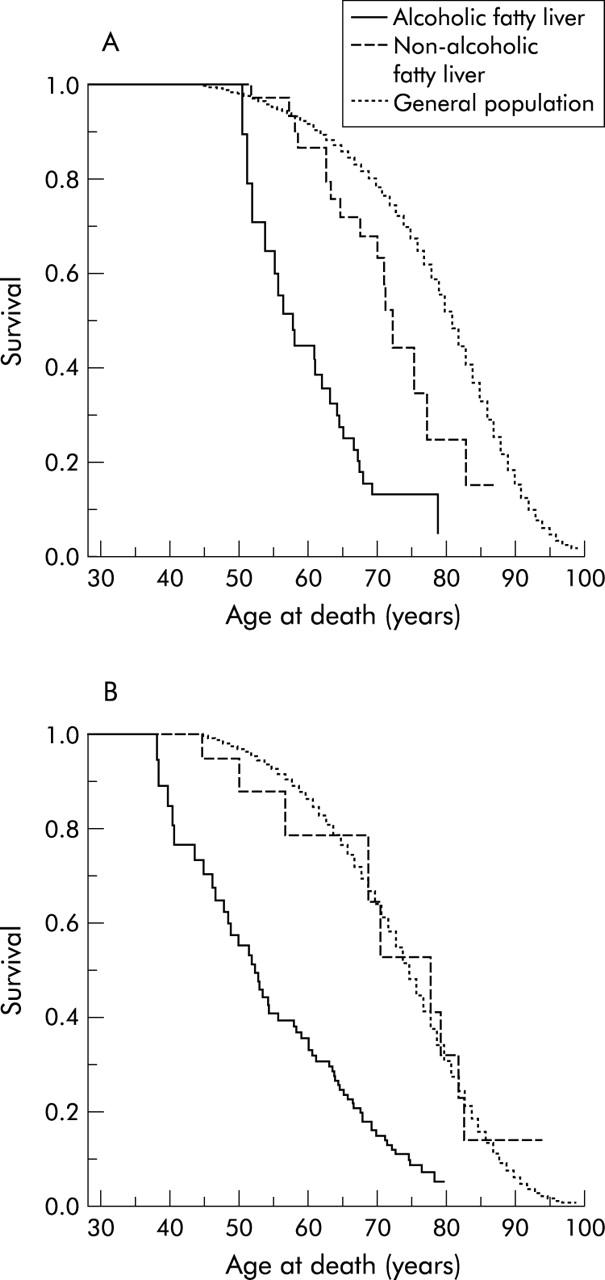
Survival probability for women (A) and men (B) with histologically verified non-alcoholic and alcoholic fatty liver in comparison with the general population.
At the time of the index biopsy, nine patients had an alcohol consumption lower than the limits defined by the Danish National Board of Health but had an alcohol related diagnosis registered in the LPR during the follow up period and were characterised as having alcoholic fatty liver. Seven of these died; two from cirrhosis and two from alcohol related causes.
DISCUSSION
In this large cohort study, we found that patients diagnosed with alcohol induced fatty liver disease had a high risk of developing cirrhosis and premature death, for both men and women. In contrast, patients with type 1 NAFLD9 seemed to have the same life expectancy as the average normal population and the risk of progressing to end stage liver disease was small.
The study was based on register, clinical, and histological data. The LPR is a unique source of data for monitoring long term outcome, as in this cohort of patients, and record linkage using the unique personal identification number ensures complete follow up.
The discharge diagnosis in the LPR may vary in validity but is generally high.20–22 We minimised this problem further by a thorough examination of the medical records and a search in the pathology register supplementing the diagnostic information available in the LPR. The index diagnosis, alcoholic versus non-alcoholic, was made from the information on alcohol intake in the medical record at the time of the index biopsy and before we requested data from the LPR and Registry of Causes of Death. However, if the patient had an alcohol related diagnosis at any time during the follow up period, they were characterised as having alcoholic steatosis, regardless of alcohol intake. The ICD diagnoses from the registries were not altered or interpreted.
The majority of patients had not moved from the uptake area of Hvidovre Hospital and medical records from the follow up period were available. It cannot be excluded that some patients with clinical cirrhosis were treated by their general practitioner and thereby not registered in the LPR. However, the structure of the Danish health service makes it likely that patients with clinically significant liver disease are admitted to hospital and the long follow up period makes this even more likely.
Death is recorded without errors in the Registry of Causes of Death while the cause of death may be misclassified. Misclassifications in the death certificates may over or underestimate the risk of death from liver cirrhosis. In the group of patients with known excessive alcohol intake, a tendency towards overestimation of cirrhosis as the cause of death might be expected while patients with non-alcoholic fatty liver are less likely to be suspected of chronic liver diseases. This would underestimate the prevalence of the cirrhosis diagnosis for this group of patients both in the LPR and Registry of Causes of Death. In the four patients who did not die in hospital, it is likely that a general practitioner completed the death certificate. In Denmark, it is the patient’s local general practitioner who writes the death certificate in cases of death outside hospital. This makes the certificate more valid because of their knowledge of the patient. In the inclusion period, a high percentage of patients, who died during hospitalisation, had an autopsy performed. We believe that data on liver disease end points in this study are sufficiently valid even though histological verification could not be obtained in all cases.
Patients were classified as having non-alcoholic or alcoholic fatty liver on the information given to the physician on alcohol intake at the time of the index liver biopsy. As this was not a prospective study, the validity of self reported alcohol intake can be questioned and was most likely underreported; potentially patients with high alcohol consumption may be misclassified as belonging to the non-alcoholic group. Because of the lack of a sensitive and specific marker of alcoholism, it is impossible to prove the non-drinking status of patients with NAFLD. We tried to compensate for this misclassification by examining all discharges registered in the LPR. If the patient was discharged from a hospital during the follow up period with an alcohol related diagnosis, they were classified as having alcoholic fatty liver, regardless of when this hospitalisation took place. Nine patients would have been classified as non-alcoholic, based solely on information on alcohol consumption in the medical report at the time of the index liver biopsy but had an alcohol related diagnosis registered during the follow up period and were subsequently reclassified into the alcoholic group. Two of these developed cirrhosis. Thereby, the prevalence of cirrhosis was lowered in the non-alcoholic group and is likely to have increased the validity of the classification.
Patients may have stopped drinking alcohol after the index liver biopsy and these individuals may not have the same risk of developing chronic liver disease as individuals who continue to drink.14,15 However, the design of the study did not allow us to analyse this aspect further. The same method has been used in other follow up studies7,15 but by using the unique information from the registries, we believe classification into the two groups, non-alcoholic and alcoholic, is even more valid.
Not all patients were examined for hepatitis B, and hepatitis C tests were not available at the time of the index liver biopsy. However, none of these patients had any known risk factors for the development of hepatitis C, and the clinical follow up did not suggest viral hepatitis; furthermore, liver biopsies in all patients were consistent with NAFLD or alcoholic fatty liver and did not show the typical findings of chronic hepatitis C infection.23,24 Also, Denmark is a low prevalence area for infectious hepatitis in the general population (0.08%).25,26 However, we recognise that hepatitis C may be present and that we could not control for this potential confounder in the follow up study.
Bouchier and colleagues27 observed a 75% survival rate after 10 years in patients diagnosed with alcoholic fatty liver. Patients with this histological diagnosis had the best survival rate in the spectrum of alcoholic liver diseases. However, they estimated survival in the various groups of patients from the time of diagnosis, regardless of the age at diagnosis. We performed our statistical analysis with delayed entry, taking into account both the age of the patient and time of entry at diagnosis. Thereby the age distribution was not a bias. We found significantly higher mortality in patients with alcoholic fatty liver in comparison with both type 1 NAFLD patients and the general population, as previous observed by Orholm and colleagues28 in patients with alcohol related liver diseases. Patients in our cohort with NAFLD had a benign course as only one of 109 patients developed cirrhosis after the 16.7 year follow up and the survival rate did not appear to differ from that of the general population when survival curves were compared. We did not compare the survival estimate statistically with the general population but the survival curve of the general population was within the confidence interval of the non-alcoholic fatty liver group for both men and women (fig 2 ▶).
Our findings are in contrast with previous reports on the prognosis for NAFLD1,6,9,11,29–33 and the risk of developing chronic liver diseases. This discrepancy may have several explanations. Other studies mainly comprised selected patients with NASH, which accounts for the higher incidence of chronic liver diseases. However, the patient cohort in our study was the same as in other studies, mainly obese women, and we would have expected that the natural history was the same, with development of NASH and a high prevalence of cirrhosis. It seems that factors other than obesity contribute to the development of chronic liver disease in patients with type 1 NAFLD. It may be speculated whether pure non-alcoholic fatty liver predisposes to NASH and the development of chronic liver disease or if NASH develops primarily without the presence of fatty liver, which could explain the apparent different prevalence rates of cirrhosis in the study. Teli and colleagues7 also found a good prognosis for patients with pure non-alcoholic fatty liver. Insulin resistance and hyperlipidaemia are other well known factors that predispose to fatty liver but we have no data to substantiate this hypothesis. The natural history may also be influenced by hitherto unknown genetic or nutritional differences, which could explain the different findings in studies from the USA in particular.
It has previously been shown that obesity in both alcoholic34 and non-alcoholic patients predisposes to the development of fatty liver and chronic liver disease.35,36 Obesity could be a contributing factor in patients in our cohort with alcoholic fatty liver, even though the observed number of cirrhosis among the 106 patients was comparable and not higher than in other reports on non-obese patients with alcoholic fatty liver.15 Non-alcoholic patients in our cohort had an extremely high median BMI of 42 kg/m2. This makes our population a selected group, but was also a unique opportunity to study the natural history of NAFLD in a large cohort with a long term follow up. Even though they were very obese, only one developed cirrhosis in the 16.7 years of follow up.
In conclusion, in this long term follow up study, we demonstrated a high prevalence of cirrhosis in patients with alcoholic fatty liver, in contrast with a benign clinical course in patients with type 1 NAFLD with no excess mortality. It is important for clinicians to realise that fatty liver is one of the most common causes of liver dysfunction, but few non-alcoholic individuals seem to develop chronic liver disease. However, more information on the natural history of NAFLD in large prospective follow up studies is needed to guide future decisions about diagnostic strategy and identification of subgroups with a risk of developing chronic liver disease and the potential need for future specific treatments.
Acknowledgments
Financial support was from the Liver Foundation at Hvidovre Hospital, University of Copenhagen, Denmark; Gerda and Aage Haenschs Foundation, Denmark; and the Danish Medical Association Research Fund/The Vibe A Linholter Estate. The Danish National Research Foundation supports the Danish Epidemiology Science Centre at the Institute of Preventive Medicine.
Abbreviations
NAFLD, non-alcoholic fatty liver disease
NASH, non-alcoholic steatohepatitis
BMI, body mass index
LPR, the national registry of patients
ICD, International Classification of Diseases
ASAT, aspartate aminotransferase
REFERENCES
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002;346:1221–31. [DOI] [PubMed] [Google Scholar]
- 2.Lonardo A. Fatty liver and nonalcoholic steatohepatitis. Where do we stand and where are we going? Dig Dis 1999;17:80–9. [DOI] [PubMed] [Google Scholar]
- 3.Hilden M, Christoffersen P, Juhl E, et al. Liver histology in a ‘normal’ population—examinations of 503 consecutive fatal traffic casualties. Scand J Gastroenterol 1977;12:593–7. [DOI] [PubMed] [Google Scholar]
- 4.Baddeley RM. An epilogue to jejunoileal bypass. World J Surg 1985;9:842–9. [DOI] [PubMed] [Google Scholar]
- 5.Burt AD, Mutton A, Day CP. Diagnosis and interpretation of steatosis and steatohepatitis. Semin Diagn Pathol 1998;15:246–58. [PubMed] [Google Scholar]
- 6.Day CP. Non-alcoholic steatohepatitis (NASH): where are we now and where are we going? Gut 2002;50:585–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teli MR, James OF, Burt AD, et al. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology 1995;22:1714–19. [PubMed] [Google Scholar]
- 8.Day CP, James OF. Hepatic steatosis: innocent bystander or guilty party? Hepatology 1998;27:1463–6. [DOI] [PubMed] [Google Scholar]
- 9.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116:1413–19. [DOI] [PubMed] [Google Scholar]
- 10.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology 2001;121:91–100. [DOI] [PubMed] [Google Scholar]
- 11.Powell EE, Cooksley WG, Hanson R, et al. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology 1990;11:74–80. [DOI] [PubMed] [Google Scholar]
- 12.Ratziu V, Giral P, Charlotte F, et al. Liver fibrosis in overweight patients. Gastroenterology 2000;118:1117–23. [DOI] [PubMed] [Google Scholar]
- 13.Angulo P, Keach JC, Batts KP, et al. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999;30:1356–62. [DOI] [PubMed] [Google Scholar]
- 14.Sorensen TA, Orholm M, Bentsen KD, et al. Prospective evaluation of alcohol abuse and alcoholic liver injury in men as predictors of development of cirrhosis. Lancet 1984;2:241–4. [DOI] [PubMed] [Google Scholar]
- 15.Teli MR, Day CP, Burt AD, et al. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet 1995;346:987–90. [DOI] [PubMed] [Google Scholar]
- 16.Andersen TF, Madsen M, Jorgensen J, et al. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull 1999;46:263–8. [PubMed] [Google Scholar]
- 17.International Center for Disease Classification. International classification of diseases, 8th revision (ICD-8). Geneva: World Health Organisation, 1974.
- 18.International Center for Disease Classification. International classification of diseases, 10th revision (ICD-10). Geneva: World Health Organisation, 1994.
- 19.Juel K, Helweg-Larsen K. The Danish registers of causes of death. Dan Med Bull 1999;46:354–7. [PubMed] [Google Scholar]
- 20.Vestberg K, Thulstrup AM, Sorensen HT, et al. Data quality of administratively collected hospital discharge data for liver cirrhosis epidemiology. J Med Syst 1997;21:11–20. [DOI] [PubMed] [Google Scholar]
- 21.Nickelsen TN. Data validity and coverage in the Danish National Health Registry. A literature review. Ugeskr Laeger 2001;164:33–7. [PubMed] [Google Scholar]
- 22.Becker U, Deis A, Sorensen TIA, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology 1996;23:1025–9. [DOI] [PubMed] [Google Scholar]
- 23.Scheuer PJ, Ashrafzadeh P, Sherlock S, et al. The pathology of hepatitis C. Hepatology 1992;15:567–71. [DOI] [PubMed] [Google Scholar]
- 24.Goodman ZD, Ishak KG. Histopathology of hepatitis C virus infection. Semin Liver Dis 1995;15:70–81. [DOI] [PubMed] [Google Scholar]
- 25.Wantzin PS, Krogsgaard K, Dickmeiss E. Screening of Danish blood donors for hepatitis C virus antibodies. Ugeskr Laeger 1990;152:2846–8. [PubMed] [Google Scholar]
- 26.Weis N, Krogsgaard K, Kjærgaard LL, et al. Kronisk hepatitis C. Kombinationsbehandling med alfa-interferon og ribavirin. Medicinsk Teknologivurdering-puljeprojekter 2002;2:47–55. [Google Scholar]
- 27.Bouchier IA, Hislop WS, Prescott RJ. A prospective study of alcoholic liver disease and mortality. J Hepatol 1992;16:290–7. [DOI] [PubMed] [Google Scholar]
- 28.Orholm M, Sorensen TIA, Bentsen K, et al. Mortality of alcohol abusing men prospectively assessed in relation to history of abuse and degree of liver injury. Liver 1985;5:253–60. [DOI] [PubMed] [Google Scholar]
- 29.Bugianesi E, Leone N, Vanni E, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002;123:134–40. [DOI] [PubMed] [Google Scholar]
- 30.Marchesini G, Forlani G. NASH: from liver diseases to metabolic disorders and back to clinical hepatology. Hepatology 2002;35:497–9. [DOI] [PubMed] [Google Scholar]
- 31.Charlton M, Kasparova P, Weston S, et al. Frequency of nonalcoholic steatohepatitis as a cause of advanced liver disease. Liver Transpl 2001;7:608–14. [DOI] [PubMed] [Google Scholar]
- 32.Bacon BR, Farahvash MJ, Janney CG, et al. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology 1994;107:1103–9. [DOI] [PubMed] [Google Scholar]
- 33.Lee RG. Nonalcoholic steatohepatitis: a study of 49 patients. Hum Pathol 1989;20:594–8. [DOI] [PubMed] [Google Scholar]
- 34.Naveau S, Giraud V, Borotto E, et al. Excess weight risk factor for alcoholic liver disease. Hepatology 1997;25:108–11. [DOI] [PubMed] [Google Scholar]
- 35.Andersen T, Gluud C. Liver morphology in morbid obesity: a literature study. Int J Obes 1984;8:97–106. [PubMed] [Google Scholar]
- 36.Bellentani S, Saccoccio G, Masutti F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med 2000;132:112–17. [DOI] [PubMed] [Google Scholar]