Abstract
Background: We and others have reported the prophylactic efficacy of oral consumption of probiotic lactobacilli in the interleukin 10 knockout (IL-10 KO) model of colitis. It has not been demonstrated that the oral route is essential for probiotic efficacy.
Aims: (i) To determine the effect of parenteral administration (subcutaneous) of Lactobacillus salivarius 118 on colitis of IL-10 KO mice; and (ii) to determine if observed responses are disease specific.
Methods: (i) IL-10 KO mice were injected subcutaneously with L salivarius 118 or saline over 19 weeks. At sacrifice, the bowels were histologically scored. Isolated splenocytes were cultured in vitro and cytokine levels measured. (ii) In the collagen induced arthritis model, DBA/1 mice were injected subcutaneously with the probiotic or saline. At sacrifice, paw thickness was measured and joints were histologically scored.
Results: (i) Colonic inflammatory scores were significantly decreased in IL-10 KO mice injected with L salivarius 118 compared with controls (p<0.05). Proinflammatory cytokine production from stimulated splenocytes was significantly lower for the probiotic group whereas stimulated transforming growth factor β (TGF-β) levels were significantly increased (p<0.05). (ii) Scoring of arthritis and paw thickness were significantly improved in the group of mice injected with L salivarius 118 compared with controls.
Conclusions: (1) Subcutaneous administration of L salivarius 118 significantly attenuated colitis in the IL-10 KO model and suppressed collagen induced arthritis, suggesting that the oral route may not be essential for probiotic anti-inflammatory effects and that responses are not disease specific. (2) The probiotic effect was associated with reduced production of proinflammatory (T helper 1) cytokines and maintained production of anti-TGF-β.
Keywords: colitis, probiotics, arthritis, cytokines
Probiotics are live microorganisms that confer health benefits through a number of mechanisms.1–4 Probiotic consumption has shown prophylactic efficacy against colitis in murine models of inflammatory bowel disease and effects have also been seen in human trials of the disease.5–7 We have previously shown that oral consumption of the probiotic Lactobacillus salivarius 118 can attenuate colitis seen in interleukin 10 knockout (IL-10 KO) mice.8,9 This model develops spontaneous colitis similar to that of inflammatory bowel disease, and the influence of the enteric flora on gut inflammatory activity has been demonstrated as germ free animals do not develop disease.10–12
The effect of probiotics on the systemic immune response is less well understood. In particular, little is known of their possible effects on extraintestinal inflammatory states, such as arthritis. Lactobacillus casei strain Shirota, when administered orally, has shown a reductive effect on the development of collagen induced arthritis.13 This is a widely used experimental murine model of polyarthritis. Collagen induced arthritis can be induced in susceptible strains of mice by immunisation with type II collagen, the major component of articular cartilage.14–17 Indeed, it is not yet known whether oral feeding and colonisation of the gut are necessary for probiotic effects. Bacterial DNA has been demonstrated to have anti-inflammatory and immunomodulatory properties when administered subcutaneously in a number of animal models of colitis.18
The purpose of the present study was: (i) to determine the effect of systemically administered L salivarius 118 on colitis of IL-10 KO mice; and (ii) to determine the effect of subcutaneous administration of L salivarius 118 on the collagen induced arthritis murine model of arthritis.
METHODS
Animals
IL-10 KO mice
Twenty 129 Ola×C57BL/6-IL 10 KO female mice, aged 7–9 weeks, were used in this study (B&K Universal Ltd, East Yorkshire, UK; The Jackson Laboratory, Bar Harbor, Maine, USA). These mice were maintained on a homozygous background and were housed under specific pathogen free conditions, with a maintained temperature of 20±2°C and a 12 hour light/dark cycle. Following initiation of this study, all mice consumed a standard non-sterile diet. Mice consumed food and water ad libitum.
DBA/1 mice
Twenty six male DBA/1 mice, aged 6–8 weeks, were used. (Harlan UK Ltd, Oxon OX23 ITP, UK). These mice were housed on a homozygous background, under specific pathogen free conditions, with a maintained temperature of 20±2°C and a 12 hour light/dark cycle. Following initiation of this study, food and water were provided ad libitum.
Probiotic strains
L salivarius subspecies salivarius UCC118 was originally isolated from the ileocaecal region of a human adult undergoing reconstructive surgery. This probiotic strain was isolated on the basis of having desirable probiotic properties. Briefly, these properties include being of human origin, non-pathogenic, resistant to intestinal acid and bile, ability to adhere to human epithelial cells, and ability to temporarily colonise and be metabolically active within the human gastrointestinal tract.19L salivarius 118 was routinely cultured in Man, Rogosa, Sharpe (MRS) broth (Oxoid, UK) at 37°C in an anaerobic environment for 24 hours. A spontaneous rifampicin resistant variant of the strain was isolated, prior to initiation of this study, in order to facilitate uncomplicated identification of this bacteria from all other lactobacilli.
IL-10 KO colitis model
Twenty IL-10 KO mice were randomised to one of two groups, with 10 mice per group. L salivarius 118 was administered subcutaneously to the study group while sterile phosphate buffered saline (PBS) was administered subcutaneously to the control group. L salivarius 118 was initially grown to a 10 ml volume in MRS broth by incubating overnight at 37°C under anaerobic conditions. The bacteria was washed twice and resuspended in sterile PBS to a final concentration of 1×109 per ml. A dose of 1×108 bacteria/mouse was then injected subcutaneously. These innoculations were performed at weeks 2, 4, 6, 10, 14, and 18 and mice were sacrificed after 19 weeks.
An additional group of controls (n = 20) receiving heat treated L salivarius 118 was also studied. L salivarius 118 was heat treated at 80°C for 10 minutes. Cells were then immediately placed on ice. Control mice received PBS, which had undergone the same heating treatment.
Murine faecal samples were collected at weekly intervals over the trial period. The trial was completed after 19 weeks of feeding, at which time all surviving mice were sacrificed by cervical dislocation. Blood samples were obtained by cardiac puncture for serological analysis. The caecum and colon were fixed in formaldehyde for histopathological analysis. Spleens were removed from each mouse at sacrifice and splenocytes isolated for in vitro culturing.
Histopathology
At sacrifice, the caecum, proximal colon (ascending and transverse colon), and distal colon (descending colon, rectum, and anal canal) of all mice were fixed in 10% formalin and assessed by two histopathologists. Two blinded independent observers, using a histological index ranging from 0 to 4, graded the severity of inflammation at each site within the murine gastrointestinal tract. This index was based on the degree of epithelial layer erosion, goblet cell depletion, and inflammatory cell infiltrate (0 = normal; 1 = minimal evidence of inflammatory infiltrate; 2 = significant evidence of inflammatory infiltrate (cryptitis, crypt abscesses); 3 = significant evidence of inflammatory infiltrate with goblet cell depletion; and 4 = significant evidence of inflammatory infiltrate with erosion of mucosa).
Splenocyte cultures
The spleens of all mice were removed at the time of sacrifice. Each spleen was immediately placed into serum free Dulbecco’s modified Eagle’s medium (DMEM). The spleen was sieved through a sterilised metal filter into 5 ml of 0.87% ammonium chloride to lyse red blood cells. The cell suspension was centrifuged twice at 100 g for 10 minutes. Cells were resuspended in DMEM (10% fetal calf serum) at a concentration of 1×106 cells/ml for in vitro culturing. The isolated lymphocytes were cocultured with the proinflammatory bacterium Salmonella typhimurium (1×106 cells/ml) for 72 hours at 37°C. Cell supernatants were isolated and stored at −80°C. Cytokine analysis was performed on the supernatants using ELISA (BD Pharmingen, Oxford, UK). Cytokines analysed were tumour necrosis factor α (TNF-α), interleukin 12 (IL-12), and transforming growth factor β (TGF-β).
Microbial analysis
Faecal samples were collected weekly, weighed, and dispersed in 10 ml of PBS. Microbial analysis of the faecal samples involved enumeration of L salivarius subspecies salivarius UCC118, total lactobacilli, total bifidobacteria, coliforms, and Clostridia perfringens. This analysis was performed by pour plating or spread plating onto MRS agar (pH 5.5) plus rifampicin; MRS agar supplemented with 0.05% cysteine hydrochloride (Sigma, St Louis, Missouri, USA) plus rifampicin; MRS agar; MRS agar supplemented with 5% sheep blood, 0.2% lithium chloride (BDH Laboratory Supplies, Poole, UK), 0.3% sodium propionate (Sigma), and 0.05% cysteine hydrochloride (Sigma); violet red bile agar; and Clostridia perfringes selective medium OPSP with supplements A SR 76 and B SR 77, respectively (all Oxoid, UK unless otherwise stated). Violet red bile agar plates were incubated aerobically for 24 hours while all other plates were incubated anaerobically for 48 hours at 37°C. Anaerobic environments were created using CO2 generating kits (Anaerocult A; Merck Darmstadt, Germany) in sealed gas jars (BBL BD, Dublin, Ireland).
Collagen induced arthritis model
Twenty six DBA/1 mice were randomised to three groups. Mice in the first group were injected subcutaneously with L salivarius UCC118 at a dose of 1×108 bacteria/mouse (preparation as for the IL-10 KO trial.) The second group received an equal volume of sterile PBS administered subcutaneously. Innoculations were administered at weeks 1, 4, and 8. A final group (n = 6) did not receive any of the above treatments.
At week 6, arthritis was induced as follows: bovine type II collagen (Chondrex) was dissolved in 0.05 M acetic acid to a concentration of 2 mg/ml by stirring overnight at 4°C. This was then emulsified in equal volumes of Freund’s complete adjuvant (2 mg/ml of M tuberculosis strain H37Ra (Difco BD, Dublin, Ireland)). Groups 1and 2 were immunised subcutaneously, in the tail, with 100 µl at week 6. At week 9, a booster immunisation of 50 µl of collagen emulsified in Freund’s incomplete adjuvant (Difco) was administered to all three groups. From week 10 onwards, mice were assessed on a daily basis for visual appearance of arthritis in the peripheral joints.20,21 Visual signs were assessed using the following index: 0, normal; 1, mild but definite redness and swelling of the ankle or wrist, or apparent redness and swelling limited to individual digits, regardless of the number of affected digits; 2, moderate redness or swelling of the ankle or wrist; 3, severe redness and swelling of the entire paw including digits; and 4, maximally inflamed limb with involvement of multiple joints.20,21 The trial was completed after 12 weeks at which time all mice were sacrificed by cervical dislocation. At sacrifice, the thickness of each paw was measured using a spring loaded calliper.
Histopathology
At sacrifice, limbs were removed and fixed in formaldehyde for histopathological analysis by an independent blinded observer. Following paraffin embedding, limbs were surface decalcified using Calbonex (KB Scientific, Togher, Cork, Ireland). Tissue sections (7 μm) were stained with haemolysin and eosin. Histological analysis of cartilage and bone destruction by pannus formation and mononuclear cell infiltration in synovial tissues was undertaken using the following scoring system: cartilage and bone destruction by pannus formation: 0, no change; 1, mild change (pannus formation within cartilage); 2, moderate change (pannus invasion into cartilage/subchondral bone); and 3, severe change (pannus invasion into the subchondral bone); mononuclear cell infiltration: 0, no infiltration; 1, mild infiltration; 2, moderate infiltration; and 3, severe infiltration.22
Statistical analysis
Analysis of variance, for differences between groups in inflammatory activity, was carried out using ANOVA analysis while differences between groups in microbial numbers were estimated using area under the curve analysis.
RESULTS
IL-10 KO colitis model
Histopathology
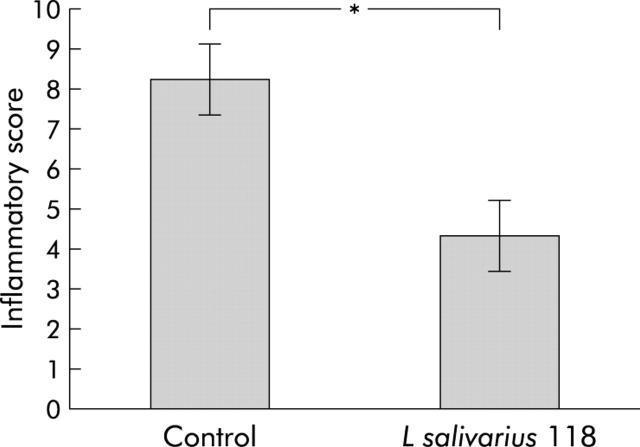
Post sacrifice, a score of 0–4 was awarded to each examined area of bowel; caecum, proximal colon (ascending and transverse colon), and distal colon (descending colon, rectum, and anal canal) with a total possible score of 12 to be awarded to each mouse gut. When all sections had been examined, the mean score for each group was calculated. Mean inflammatory score for the control group was 8.25 (0.94), and mean inflammatory score for the group given L salivarius UCC118 was 4.35 (0.9). Thus the probiotic group showed a significant reduction in inflammatory score compared with the control group (p<0.05) (fig 1 ▶). In addition, four mice in the PBS control group were found to have severe pancolitis, with all of the proximal and distal colon equally affected. No case of pancolitis was seen in the probiotic group.
Figure 1.
Post sacrifice, a histological score of 0–4 was awarded to each examined area of bowel; caecum, proximal colon (ascending and transverse colon), and distal colon (descending colon, rectum and anal canal) with a total possible score of 12 to be awarded to each mouse gut. Gastrointestinal inflammatory score in the probiotic and control fed groups is shown. *p<0.05, n = 10 per group. Results are expressed as mean (SEM) inflammatory score.
The group receiving heat treated L salivarius 118 had 100% mortality rate by week 10 of the trial and were not included in the analysis. These mice had an obvious and rapid response to administration of the killed bacteria, becoming shocked within hours. This effect is likely to be related to the method used to kill the bacteria, and studies are now planned to incorporate a range of alternative strategies.
Microbial analysis
Faecal samples from all mice were analysed to assess changes in the microflora and to detect transit of the probiotic strain. There was no significant difference in culturable bacteria when total lactobacilli, total bifidobacteria, coliforms, and Clostridia perfringens were enumerated in faecal samples (data not shown). The L salivarius 118 strain was not isolated from any of the mice in the control or probiotic group (data not shown).
Immunological assessment
Cytokine analysis was performed on the splenocyte supernatants by ELISA following stimulation in vitro with the proinflammatory bacterium Salmonella typhimurium. Cytokines analysed were TNF-α, IL-12, and TGF-β.
TNF-α levels were significantly reduced in the group given L salivarius 118 following stimulation with the proinflammatory stimulus (TNF-α levels in the control group 1522.4 (112.2), TNF-α levels in the L salivarius UCC118 group 872.8 (143.8); p<0.05) (fig 2A ▶). TNF-α levels were undetectable in non-stimulated cultures.
Figure 2.
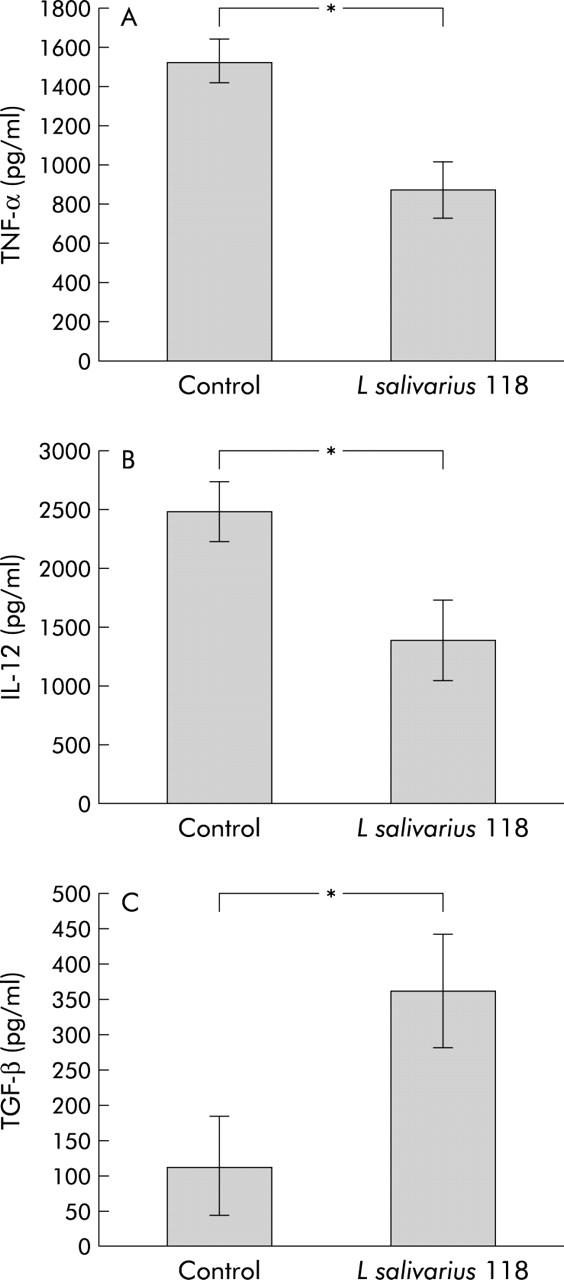
Cytokine production by stimulated splenocytes in control and probiotic interleukin 10 knockout mice. Results are expressed as mean (SEM) cytokine level per group. There was a reduction in the proinflammatory cytokines tumour necrosis factor α (TNF) (A) and interleukin 12 p40 (IL-12) (B), with an increase in the anti-inflammatory cytokine transforming growth factor β (TGF) (C). *p<0.05; n = 10 per group.
IL-12 levels following stimulation with Salmonella typhimurium showed a significant reduction in the probiotic group (p<0.05). IL-12 levels in the control group were 2471.2 (256) compared with 1386.4 (347.2) in the L salivarius 118 group (p<0.05) (fig 2B ▶). IL-12 levels recorded in non-stimulated splenocyte supernatants were reduced in the probiotic group compared with the control group, although this did not reach statistical significance.
Unstimulated TGF-β levels were increased in the probiotic group compared with the control group (TGF-β levels in the control group 114.4 (71.6); TGF-β levels in the L salivarius UCC118 group 362.3 (79.6); p<0.05) (fig 2C ▶). There was no statistically significant difference in levels of TGF-β between the two groups following stimulation with salmonella.
Cytokine levels in serum samples were below detectable limits in all groups on analysis by ELISA.
Collagen induced arthritis model
Daily clinical assessment of mice for visual signs of arthritis
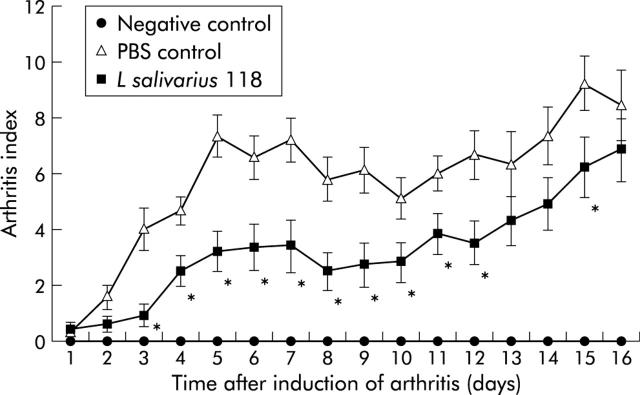
Daily macroscopic scoring of visual signs of arthritis was carried out from week 10 to the time of sacrifice (week 12) using an established scoring system.20,21L salivarius 118 had a suppressive effect on disease development. From week 10, day 3 to week 11, day 12, there was a significant reduction in arthritic score in the L salivarius 118 group (p<0.01). The reduction in arthritis was also significant at week 12, day 15 (p = 0.05) (fig 3 ▶).
Figure 3.
Comparison of daily clinical scores of mice subcutaneously inoculated with L salivarius 118 and phosphate buffered saline (PBS) following induction of arthritis. Mice were examined daily from week 10 (day 1) to week 12 (day 16) for visual signs of arthritis using an established scoring system on a scale of 0–4. The macroscopic score (mean (SEM)) was expressed as a cumulative value for all paws, with a maximum possible score of 16 (n = 10 per group, except unaffected negative control group, n = 6). The L salivarius 118 group had a significantly reduced daily clinical score compared with the PBS control on days 3–day 9 (p = 0.01), on days 10–12, and on day 15 (p = 0.05).
Paw thickness
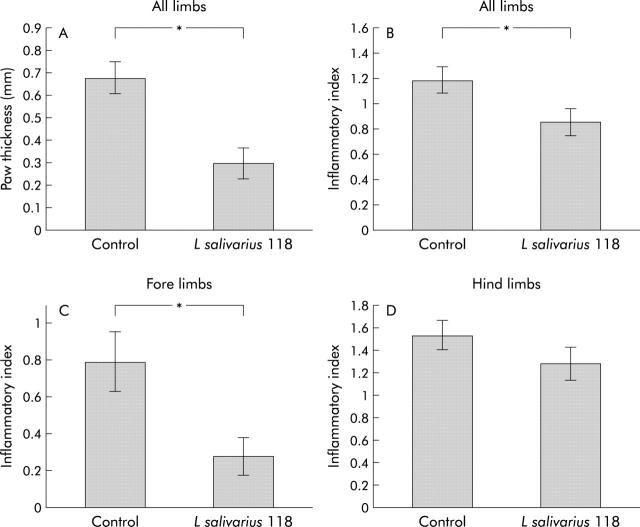
The thickness of each paw was measured using spring loaded callipers at the time of sacrifice. Results were described as paw thickness above normal (mean thickness for the unaffected/negative control group was 1.62 (0.051) mm). The L salivarius 118 group showed statistically significant reduction in thickness compared with the control group (p = 0.0002) (L salivarius 118 group paw thickness 0.292 (0.071); control group paw thickness 0.677 (0.071)) (fig 4A ▶).
Figure 4.
Effect of subcutaneous inoculation of L salivarius 118 and phosphate buffered saline (PBS) on paw thickness (A), total inflammation in all limbs (B), inflammation in the fore limbs (C), and inflammation in the hind limbs (D), in collagen induced arthritis. The thickness of each paw was measured with spring loaded callipers. Total paw swelling for each mouse was calculated by adding the thickness of the individual paws. Paw thickness of the unaffected/negative control group was taken as 0. Results (A–D) are expressed as mean (SEM). The L salivarius 118 group showed a significant reduction in paw thickness compared with controls (n = 10 per group).
Histopathology
Following sacrifice, histopathological changes were scored using a scale of 0–3, based on cartilage and bone destruction by pannus formation (fig 5 ▶). Infiltration of inflammatory cells was also scored on a scale of 0–3, ranging from no cell infiltration to severe infiltration. When all sections had been examined, the mean score for each group was calculated with a maximum possible score of 6. While no significant reduction was observed between the placebo vaccinated and L salivarius 118 groups for total histopathological changes, a significant reduction in inflammatory cell infiltration was observed in the L salivarius 118 group compared with controls (mean inflammatory cell score for the control group 1.18 (0.11); L salivarius 118 group 0.85 (0.11); p = 0.04) (fig 4B ▶). However, on further analysis, a significant reduction in cartilage and bone destruction, as well as inflammatory cell infiltration, was observed in the fore limbs of mice given the probiotic bacteria (mean histological score for the control group 1.30 (0.46); L salivarius 118 group 0.46 (0.19); p = 0.03) (fig 4C ▶). There was no significant differences in the hind limbs of these groups (mean histological score for the control group 2.55 (0.26), L salivarius 118 group 2.33 (0.29)) (fig 4D ▶).
Figure 5.

Joint histology in collagen induced arthritis. Following sacrifice, histopathological changes were scored using a scale of 0–3, based on cartilage and bone destruction by pannus formation. Infiltration of inflammatory cells was also scored on a scale of 0–3, ranging from no cell infiltration to severe infiltration. The range of the histological grading system used in the control and L salivarius groups is illustrated. (A) Example of a normal joint with no signs of arthritis. (B) A joint with mild inflammation and synovial hyperplasia. (C) A joint with moderate inflammation and pannus invasion of cartilage. (D) A joint with severe inflammation and invasion of pannus to underlying bone.
DISCUSSION
The results of this study confirm that systemic administration of L salivarius 118 had an anti-inflammatory effect on colitis in IL-10 KO mice, demonstrating that the oral route is not mandatory for its effect. It is noteworthy that the anti-inflammatory effect was of comparable, but not superior, magnitude to that previously reported by us using the oral route of administration.9 Dosing and frequency of administration were arbitrary, with a compromise between exposure to the microbes while minimising stress to the animals. Whether alternative regimens would have a greater or lesser impact requires study. The results are not specific to this model, nor to intestinal inflammation, because the anti-inflammatory effect was also seen in a murine model of arthritis. This further supports an earlier observation that probiotic effects are not solely local, but may include systemic anti-inflammatory activity.9 The systemic nature of the probiotic activity is reflected in a decrease in proinflammatory cytokines and increase in the regulatory cytokine TGF-β.
In this study, the probiotics were given as a prophylactic measure for a number of weeks before disease development. While they did not completely prevent disease occurrence, they were successful in ameliorating the severity of colitis and collagen induced arthritis, which are both aggressive models of inflammation. This is not the first time the influence of bacteria has been demonstrated in the setting of experimental induced arthritis; a previous study demonstrated both suppression and induction of arthritis by various fed bacteria.23 Other studies of experimental colitis demonstrated contrasting responses for different strains of lactobacilli, suggesting that different lactobacilli strains may have variable host specificity or different efficacy in various inflammatory conditions.24 These results demonstrate the complex nature of the large ecosystem that is the intestinal microflora and the variety of responses that can be expected when this is manipulated.
Overall, the mechanism of action of probiotics is not well understood. Whether the effects rely on alteration of the gut flora, adherence to human epithelial cells, or production of an antimicrobial compound following ingestion of probiotics depends on the clinical setting and desired effect.25 The intestinal microflora has been shown to have an impact on the development and functioning of the immune system and it is believed that the microflora influences, among other intestinal functions, the mucosal immune response through signalling with the gut epithelium.26–28 Interactions with Toll-like receptors and dendritic cells in the gut are believed to be involved in this communication.29,30 Dendritic cells inhabiting the gut mucosa are mostly immature and potentially prone to modulation by the environment, containing microorganisms. It has been demonstrated that the Th1/Th2/Th3 cytokine profiles induced by gut dendritic cells can be modulated by administration of lactobacilli.31 Indeed, probiotics have been seen to alter cytokine production by immunocompetent cells in vitro.32 Our results suggest that although these are possible mechanisms for the probiotic effect by the oral route, there appear to be other mechanisms that allow a probiotic effect distant from the site of administration. It has previously been shown that bacterial DNA has anti-inflammatory and immunostimulatory properties when administered subcutaneously in a number of animal models of colitis.18 While the effect in our study is likely to be direct at the level of regulatory dendritic/T cells, immunisation to bacterial components may also occur.
The IL-10 KO colitis model and the collagen induced arthritis model are associated with dysregulated Th1 proinflammatory cytokines. In the IL-10 KO model, IL-12 is a key mediator responsible for inducing colitis and is necessary for sustaining proliferation of chronically activated Th1 cells.33–35 In collagen induced arthritis, it has been shown that anti-IL-12 and anti-TNF treatment reduced joint damage.36 This suggests a role for IL-12 as well as TNF in arthritis, and modulation of these cytokines provides a therapeutic target in this disease. The associated downregulation of IL-12 and TNF-α levels seen in our study suggest that the probiotic effect may be mediated by alteration of these cytokines.
While the definition of probiotics is one of live microorganisms, it is probable that bacterial DNA or bacterial components could themselves be responsible for any observed effect. Curiously, in our study the group given heat treated bacteria had an unacceptably high rate of mortality. This issue of live versus killed bacteria needs to be revisited in a separate study involving different mechanisms of bacterial preparation. The definition of probiotics is likely to undergo continuing modification, particularly in light of the emerging era of genetically modified food grade microbes.37 Irrespective of the mechanism of action, there are reasons which might favour therapeutic usage of live over dead bacteria. Firstly, the oral route of ingestion is likely to be preferable, particularly as the systemic route was not superior in efficacy and live bacteria may be more reliable for enteric transit and occupation of microbial niche. Secondly, live bacteria offer the advantage of elaborating biological molecules other than immunomodulatory DNA that may have relevance in clinical situations other than inflammation. These include the production of well characterised antimicrobial factors.38 Finally, issues such as patient acceptability and bulk manufacturing may determine probiotic product formulations. What is already clear is that there will be an increasing role for bacteria or bacterial products in a therapeutic setting alongside more conventional treatments for inflammatory conditions. Microbial therapeutics is an expanding field of medical microbiology inviting further investigation, and we should not allow ourselves to become captive of the definition of probiotics.
Acknowledgments
The authors are funded, in part, by the Science Foundation of Ireland, the Health Research Board of Ireland, the Higher Education Authority, and the European Union (PROGID QLK-2000-00563).
Abbreviations
IL-10 KO, interleukin 10 knockout
PBS, phosphate buffered saline
MRS broth, Man, Rogosa, Sharpe broth
DMEM, Dulbecco’s modified Eagle’s medium
TNF-α, tumour necrosis factor α
IL-12, interleukin 12
TGF-β, transforming growth factor β
Conflict of interest: LO’M, BK, JKC, and FS are members of a multidepartmental university campus research company (Alimentary Health Ltd) which investigates host-flora interactions and the therapeutic manipulation of these interactions in various human and animal diseases. The content of this article was neither affected nor constrained by this fact.
REFERENCES
- 1.Bengmark S. Ecological control of the gastrointestinal tract. The role of probiotic flora. Gut 1998;42:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunne C, Murphy L, Flynn S, et al. Probiotics from myth to reality. Demonstration of functionality in animal models of disease and human clinical trials. Antonie van Leeuwenhoek 1999;76:279–92. [PubMed] [Google Scholar]
- 3.Kalliomaki M, Salminen S, Arvilommi H, et al. Probiotics in primary prevention of atopic disease: a randomised, placebo-controlled trial. Lancet 2001;357:1076–9. [DOI] [PubMed] [Google Scholar]
- 4.Isolauri E, Kirjavainen PV, Salminen S. Probiotics: a role in the treatment of intestinal infection and inflammation? Gut 2002;50(suppl 3):11154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madsen KL, Doyle JS, Jewell LD, et al. Lactobacillus species prevents development of enterocolitis in interleukin-10 gene-deficient mice. Gastroenterology 1999;116:1107–14. [DOI] [PubMed] [Google Scholar]
- 6.Venturi A, Gionchetti P, Rizzello F, et al. Impact on the composition of the faecal flora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment Pharmacol Ther 1999;13:1103–8. [DOI] [PubMed] [Google Scholar]
- 7.Gionchetti P, Rizzello F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: A double-blind, placebo-controlled trial. Gastroenterology 2000;119:305–9. [DOI] [PubMed] [Google Scholar]
- 8.O’Mahony L, Feeney M, O’Halloran S, et al. Probiotic impact on microbial flora, inflammation, and tumour development in IL-10 knockout mice. Aliment Pharmacol Ther 2001;15:1219–25. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy J, O’Mahony L, O’Callaghan L, et al. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut 2003;52:975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn R, Lohler J, Rennick D, et al. Interleukin-10 deficient mice develop chronic enterocolitis. Cell 1993;75:263–74. [DOI] [PubMed] [Google Scholar]
- 11.Shanahan F. Gene-targeted immunologic knockouts: new models of inflammatory bowel disease. Gastroenterology 1994;17:312–14. [PubMed] [Google Scholar]
- 12.Rennick D, Fort M. Lessons from genetically engineered animal models XII. IL- 10-deficient (IL-10−/−) mice and intestinal inflammation. Am J Physiol Gastrointest Liver Physiol 2000;278:G829–33. [DOI] [PubMed] [Google Scholar]
- 13.Kato Ikuo, Endo-Tanaka Kazuko, Yokokura Teruo. Suppressive effect of the oral administration of Lactobacillus casei on type II collagen-induced arthritis in DBA/1 mice. Life Sci 1998;63:635–44. [DOI] [PubMed] [Google Scholar]
- 14.Trentham DE, Towers AS, Kang AH. Autoimmunity to type II collagen: an experimental model of arthritis. J Exp Med 1977;146:857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Courtenay JS, Dallman MJ, Dayan AB, et al. Immunization against heterologous type II collagen-induced arthritis in mice. Nature 1980;2843:666–8. [DOI] [PubMed] [Google Scholar]
- 16.Wooley PH, Luthra HS, Stuart JM, et al. Type II collagen-induced arthritis in mice: I. major histocompatibility complex (I-region) linkage and antibody correlates. J Exp Med 1981;154:668–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stuart JM, Townes AS, Kang AH. Nature and specificity of immune responses to collagen in type II collagen-induced arthritis in mice. J Clin Invest 1982;69:673–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rachmilewitz D, Karmeli F, Takabayashi K, et al. Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology 2002;122:1428–41. [DOI] [PubMed] [Google Scholar]
- 19.Collins JK, Murphy L, Morrissey D, et al. A randomised controlled trial of a probiotic Lactobacillus strain in healthy adults: assessment of its delivery, transit, and influence on microbial flora and enteric immunity. Microb Ecol Health Dis 2002;14:81–9. [Google Scholar]
- 20.Holmdahl R, Jansson L, Larsson E, et al. Homologous type II collagen induces chronic and progressive arthritis in mice. Arthritis Rheum 1986;29:106. [DOI] [PubMed] [Google Scholar]
- 21.Protocol for the successful induction of collagen-induced arthritis in mice. Redmond, Washington, USA: Chondrex Inc, 2003.
- 22.Taniguchi K, Kohsaka H, Inoue N, et al. Induction of the p16TNK4a senescence gene as a new therapeutic strategy for the treatment of rheumatoid arthritis. Nat Med 1999;5:760–7. [DOI] [PubMed] [Google Scholar]
- 23.Kohashi O, Kohashi Y, Takahashi T, et al. Reverse effect of gram positive bacteria versus gram negative bacteria on adjuvant-induced arthritis in germfree rats. Microbiol Immunol 1985;29:487–97. [DOI] [PubMed] [Google Scholar]
- 24.Dieleman LA, Goerres MS, Arends A, et al. Lactobacillus GG prevents recurrence of colitis in HLA-B27 transgenic rats after antibiotic treatment. Gut 2003;52:370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunne C, O’Mahony L, Murphy L, et al. In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am J Clin Nutr 2001;73:386–92S. [DOI] [PubMed] [Google Scholar]
- 26.Shanahan F. Therapeutic manipulation of gut flora. Science 2000;289:1311–12. [DOI] [PubMed] [Google Scholar]
- 27.Gordon JI, Hooper LV, McNevin MS, et al. Epithelial cell growth and differentiation. III. Promoting diversity in the intestine: conversations between the microflora, epithelium and diffuse GALT. Am J Physiol 1997;273:G565–70. [DOI] [PubMed] [Google Scholar]
- 28.Hooper LV, Wong MH, Thelin A, et al. Molecular analysis of commensal host/microbial relationships in the intestine. Science 2001;291:881–4. [DOI] [PubMed] [Google Scholar]
- 29.Marteau P, Seksik P, Jian R. Probiotics and health: new facts and ideas. Curr Opin Biotechnol 2002;13:486–9. [DOI] [PubMed] [Google Scholar]
- 30.Shanahan F. The host–microbe interface within the gut. Best Pract Res Clin Gastroenterol 16:915–31. [DOI] [PubMed]
- 31.Christensen HR, Frokiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol 2002;168:171–8. [DOI] [PubMed] [Google Scholar]
- 32.Borruel N, Carol M, Casellas F, et al. Increased mucosal TNFα production in Crohn’s disease can be downregulated ex vivo by probiotic bacteria. Gut 2002;51:659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berg DJ, Davidson NJ, Kuhn R, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J Clin Invest 1996;98:1010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davidson NJ, Hudak SA, Lesley RE, et al. IL-12, but not IFN- γ, plays a major role in sustaining the chronic phase of colitis in IL-10-deficient mice. J Immunol 1998;161:3143–9. [PubMed] [Google Scholar]
- 35.Davidson NJ, Fort M, Muller W, et al. Chronic colitis in IL-10−/− mice: Insufficient counter regulation of a Th1 response. Intern Rev Immunol 2000;19:91–121. [DOI] [PubMed] [Google Scholar]
- 36.Butler DM, Malfait AM, Maini RN, et al. Anti-IL-12 and anti-TNF antibodies synergistically suppress the progression of murine collagen-induced arthritis. Eur J Immunol 1999;29:2205–12. [DOI] [PubMed] [Google Scholar]
- 37.Steidler L, Hans W, Schotte L, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 2000;289:1352–5. [DOI] [PubMed] [Google Scholar]
- 38.Flynn S, van Sinderen D, Thornton GM, et al. Characterization of the genetic locus responsible for the production of ABP-118, a novel bacteriocin produced by the probiotic Lactobacillus salivarius subsp. salivarius UCC118. Microbiology 2002;148:973–84. [DOI] [PubMed] [Google Scholar]