Abstract
Background: Probiotic bacteria have a beneficial effect on intestinal inflammation. In this study, we have examined the effect of lactic acid and commensal Gram positive (+) bacteria conditioned media (CM) on tumour necrosis factor α (TNF-α) release and the mechanisms involved.
Methods: Lipopolysaccharide (LPS) induced TNF-α secretion by peripheral blood mononuclear cells or the THP-1 cell line was monitored in the presence or absence of bacteria CM obtained from two probiotic strains, Bifidobacterium breve (Bb) and Streptococcus thermophilus (St), and three commensal bacterial strains (Bifidobacterium bifidum, Ruminococcus gnavus, and unidentified Streptococcus). Bb and St bacteria CM were allowed to cross filter grown intestinal epithelial cell monolayers (HT29-19A) to assess intestinal transport of active bacterial products. These products were characterised and their effect on LPS binding to THP-1 cells and nuclear factor κB (NFκB) activation assessed.
Results: Dose dependent inhibition of LPS induced TNF-α secretion was noted for both probiotic bacteria CM (64% and 71% inhibition for Bb and St, respectively) and to a lesser extent commensal bacteria CM (21–32% inhibition). Active products from Bb and St were resistant to digestive enzymes and had a molecular mass <3000 Da. Their inhibitory effect was preserved after transepithelial transport across intestinal cell monolayers, mainly in inflammatory conditions. LPS-FITC binding to THP-1 cells and NFκB activation were significantly inhibited by Bb and St CM.
Conclusion: B breve and S thermophilus release metabolites exerting an anti-TNF-α effect capable of crossing the intestinal barrier. Commensal bacteria also display a TNF-α inhibitory capacity but to a lesser extent. These results underline the beneficial effect of commensal bacteria in intestinal homeostasis and may explain the role of some probiotic bacteria in alleviating digestive inflammation.
Keywords: probiotics, inflammatory cytokines, intestinal barrier
Various beneficial effects to human health have been attributed to probiotic bacteria, mainly at the intestinal level.1 There is convergent evidence suggesting that probiotics may have anti-inflammatory properties. Allergic inflammation can benefit from the use of probiotics as an adjunct to conventional treatment. Indeed, it was reported that cow’s milk allergic infants, treated with extensively hydrolysed milk formula supplemented with Lactobacillus rhamnosus GG, had a better clinical score and less tumour necrosis factor α (TNF-α) in their faeces than infants treated with the hydrolysed formula only.2 Gastric inflammation associated with Helicobacter pylori infection was improved by supplementing conventional treatment with probiotic bacteria.3 Interestingly, different lactobacilli, bifidobacteria, or probiotic mixtures (VSL#3) have been shown to alleviate digestive diseases in experimental animals4,5and in inflammatory bowel diseases in humans.6–8 In neonatal rats suffering from stress induced necrotising enterocolitis, preliminary administration of Bifidobacterium infantis was protective towards enterocolitis development.9 Recently, it was also shown that Lactobacillus rhamnosus GG conditioned media decreased TNF-α production by macrophages in vitro10 by a contact independent mechanism. However, clear beneficial effects still remain controversial.11 Other studies have stressed the specificity of bacterial strains in inducing anti- or proinflammatory cytokines. Indeed, some lactic acid bacteria, or their secretion products, orally administered in mice (Lactobacillus reuteri or Lactobacillus brevis), had a stimulatory effect on secretion of proinflammatory cytokines such as interleukin (IL)-1β and TNF-α.12
Probiotics are generally administered via the oral route and the intestinal epithelial barrier restricts their interaction with the local immune system. The present study was aimed at characterising the anti-inflammatory (anti-TNF-α) effect of the metabolites produced by two lactic acid bacteria (Bifidobacterium breve and Streptococcus thermophilus) compared with three commensal bacteria strains. Lactic acid bacteria metabolites were characterised and possible mechanisms for their anti-inflammatory properties were studied, as well as their capacity to cross the intestinal barrier under normal or inflammatory conditions.
METHODS
Preparation of bacteria conditioned media
Two lactic acid bacterial strains, Bifidobacterium breve (strain BbC50) and Streptococcus thermophilus (strain St065), and three bacterial strains isolated from the dominant Gram positive human microflora were used. The commensal bacterial strains provided by G Corthier (INRA, Jouy en Josas, France) comprised Bifidobacterium bifidum strain B536, originally isolated from the faecal flora of a healthy breast fed infant, Ruminococcus gnavus (FRE1), isolated from the predominant faecal flora of a healthy adult,13 and a non-identified streptococcus strain isolated from the faecal flora of a one month old infant (named here neonatal streptococcus). All bacterial strains were first cultured on brain-heart medium (bifidobacteria and ruminococcus) or lactose enriched M20 medium (streptococcus). Bacteria were then collected by centrifugation and cultured over 48 hours in RPMI medium containing 20% fetal calf serum (FCS) and supplemented with 3% inulin (bifidobacteria and ruminococcus) or 3% d-glucose (streptococcus). Bacteria conditioned media (CM) were ultracentrifuged (100 000 g, one hour, 4°C), filtrated on 0.22 μm membranes, and frozen (−80°C) until use. Bacteria CM corresponded to 5×105 and 8×105 CFU/ml for S thermophilus and B breve, and to 2×106, 1.3×106, and 3.3×108 CFU/ml for R gnavus, B bifidum, and the neonatal streptococcus, respectively.
In experiments comparing the anti-TNF-α effect of probiotic or commensal bacteria CM, dilutions were performed in RPMI/FCS to obtain the same final concentration corresponding to 2.5×105 CFU/ml. Commensal bacteria CM were also tested at high concentrations (that is, half diluted).
In some experiments, bacteria CM were treated with pepsin and trypsin (0.15 mg/ml) to hydrolyse proteins. To characterise the size of active bacterial products, we submitted bacteria CM to ultrafiltration through Centriplus YM-3 or YM-10 membranes that retain molecules larger than 3000 or 10 000 Da, respectively. Retained and filtrated fractions were adjusted to the initial volume and frozen until use.
Immune cell culture and experimental protocols
Peripheral blood mononuclear cells (PBMC) of healthy patients were cultured in RPMI 1640 supplemented with 10% human serum AB (SAB), 10 mM HEPES, 1 mM sodium pyruvate, 0.05 mM β-mercapto-ethanol, and 1% penicillin-streptomycin. In some cases, the THP-1 pro-monocytic cell line (ATCC No TIB-202) was cultured in the same culture media containing FCS. PBMC or THP-1 cells were seeded on 24 well (Falcon) plates at 5×105 cells/well. PBMC were allowed to adhere for one hour and were then stimulated with 1–1000 ng/ml lipopolysaccharide (LPS, ref L2654; Sigma, La verpillère, France) for four hours in the presence or absence of dilutions of native or treated (as described above) bacteria CM. After centrifugation, supernatants were collected and assayed for TNF-α. In some cases, IL-10 secretion was also tested in the presence of bacteria CM and LPS. The optimal LPS concentration shown to activate maximal TNF-α secretion was 10 ng/ml (data not shown). The viability of PBMC in the presence of bacteria CM was tested by propidium iodide incorporation using flow cytometry (BDLSR and CELLquest software; Becton Dickinson, Le Pont de Claix, France). In some cases, PBMC were stimulated with lipoteichoic acid (LTA 100 ng/ml; Sigma) for six hours in the presence or absence of bacteria CM.
TNF-α or IL-10 released by PBMC or THP-1 cells was assayed using commercial enzyme linked immunosorbent assays (ELISA kits; Duoset R&D Systems, Lille, France).
Analysis of LPS binding to THP-1 pro-monocytes in the presence of bacteria conditioned media by flow cytometry
LPS has to bind to LPS binding protein (LBP) before LPS-LBP complexes can recognise the CD14 receptor.14 It can also bind to the toll-like receptor 4 (TLR4). In order to test whether lactic acid bacteria CM may interact with the binding of LPS to monocytes, we measured binding of LPS-FITC (Escherichia coli 055:B5; Sigma), used as a fluorescent marker, by flow cytometry. THP-1 cells were incubated for 48 hours at 2×106 cell/ml in the presence of vitamin D3 (12 ng/ml) in order to upregulate CD14 receptor expression.15 Cells were preincubated for 30 minutes at 4°C with the optimal amount of bacteria CM and further incubated at 4°C for one hour in RPMI containing 10% SAB as a source of LBP, and 2 μg/ml LPS-FITC. In some cases, bovine serum albumin 0.2% was used instead of SAB as a negative control to assess CD14 independent LPS binding.
Nuclear translocation of NFκB
Nuclear factor κB (NFκB) activation is characterised by its nuclear translocation. This translocation was demonstrated on nuclear extracts by an electrophoretic migration shift assay.
THP-1 cells (10×106 cells/ml in RPMI/FCS 1%) were stimulated with 10 ng/ml LPS for 30 minutes in the presence or absence of half diluted CM filtrated fractions <3 kDa, and nuclear and cytoplasmic extracts were prepared as previously described.16
Nuclear extracts (5 μg) were placed in binding buffer (10 mM HEPES, pH 7.8, 100 mM NaCl, 1 mM EDTA, and 10% glycerol) containing 1 μg poly(dI-dC) and 0.5 ng of 32P-labelled DNA probe corresponding to the κB site (5′-AGTTGAGGGGACTTTCCCAGG-3′; Promega, Charbonnières, France). Following incubation at room temperature for 30 minutes, samples were run on a 5% polyacrylamide gel in Tris-borate-EDTA buffer. The gel was dried, exposed to a PhosphorImager screen (Molecular Dynamics, Sunnyvale, California, USA), and the ImageQuant software (Molecular Dynamics) was used to perform densitometric analysis.
Intestinal transport of active bacterial metabolites across the filter grown epithelial cell line HT29-19A under control and inflammatory conditions
To analyse whether active bacterial metabolites can cross the intestinal barrier, we used filter grown intestinal cell monolayers. HT29-19A cells were seeded on microporous filters (Falcon) at a concentration of 0.8×106 cells/cm2 in Dulbecco’s modified Eagle’s medium supplemented with 10% FCS and 1% gentamicin. Three weeks later, this resulted in a tight polarised monolayer displaying a transepithelial electrical resistance of ~150 Ω×cm2. Cells were treated (inflammatory conditions) or not (controls) with 100 U/ml interferon (IFN)-γ and 10 ng/ml TNF-α placed in basolateral compartments, and incubated for 48 hours. After two extensive washes with RPMI, bacteria CM (or RPMI as control) were added to the apical compartments. After 24 hours, media in the basolateral compartments were collected and tested for their capacity to inhibit LPS induced TNF-α secretion by PBMC, as described above. Electrical resistances of the intestinal cell monolayers were measured at the end of the experiment (that is, after basolateral treatment with IFN-γ and TNF-α) followed by 24 hour apical incubation with bacteria CM or RPMI. Epithelial cells secrete transforming growth factor β (TGF-β), an anti-inflammatory cytokine known to inhibit TNF-α secretion.17 As this would mask the anti-inflammatory capacity of bacteria CM, the basolateral compartments were coated overnight with an anti-TGF-β antibody (MAB240; R&D) at a concentration of 2 μg/ml before adding bacteria CM to the apical compartment.
Statistical analysis
Statistical analysis was performed using the SAS package. Results are expressed as mean (SD) and comparisons of different parameters among the groups were performed using analysis of variance and non-parametric tests (Wilcoxon). Differences were considered significant for values of p<0.05.
RESULTS
Effect of lactic acid bacteria and commensal bacteria CM on TNF-α secretion by LPS stimulated PBMC
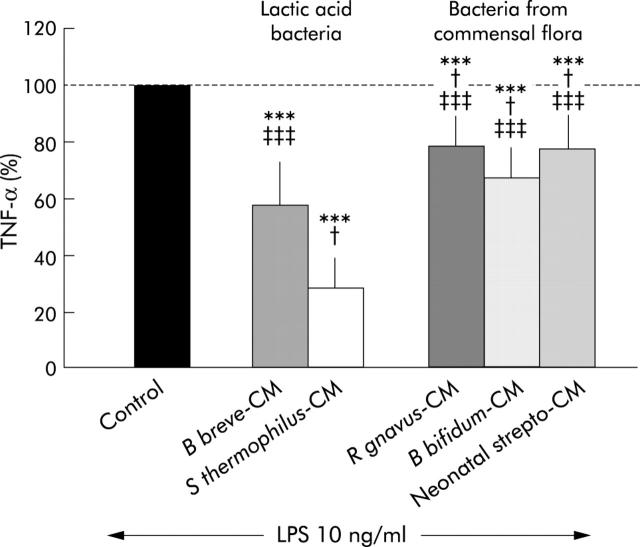
Figure 1 ▶ indicates that both lactic acid bacteria and commensal bacteria CM used at a final concentration corresponding to 2.5×105 CFU/ml exerted an inhibitory effect on LPS induced TNF-α secretion. This inhibitory effect was significantly higher with lactic acid bacteria CM (43% and 71% inhibition for B breve and S thermophilus CM, respectively) than with commensal bacteria CM (21%, 32%, and 22% for R gnavus, B bifidum, and neonatal streptococcus, respectively; p<0.04).
Figure 1.
Comparison of the inhibitory effect of conditioned media (CM) from lactic acid bacteria (Bifidobacterium breve, Streptococcus thermophilus) and Gram positive bacteria from the dominant human microflora (Ruminococcus gnavus, Bifidobacterium bifidum, and neonatal streptococcus). Control bar (100%) represents tumour necrosis factor α (TNF-α) released by peripheral blood mononuclear cells (PBMC) stimulated with lipopolysaccharide (LPS) 10 ng/ml. Addition of bacteria CM (at identical concentration of 2.5×105 CFU/ml) to LPS stimulated PBMC induced a significant decrease in TNF-α secretion. Lactic acid bacteria were more inhibitory than bacteria from the human microflora. n = 8 experiments. ***Significantly different from control (p<0.0001); †significantly different from B breve (p<0.04); ‡‡‡significantly different from S thermophilus (p<0.0001).
We also tested the inhibitory effect of commensal bacteria CM at their highest concentrations (that is, 0.65×106 to 1.65×108 CFU/ml) and found an even more pronounced inhibition, reaching 36%, 77%, and 96% for R gnavus, B bifidum, and neonatal streptococcus CM, respectively. Such high concentrations could not be explored with lactic acid bacteria CM as under identical culture conditions their growth was limited to 5 and 8×105 CFU/ml, respectively.
Dose dependent effect of lactic acid bacteria CM on TNF-α secretion
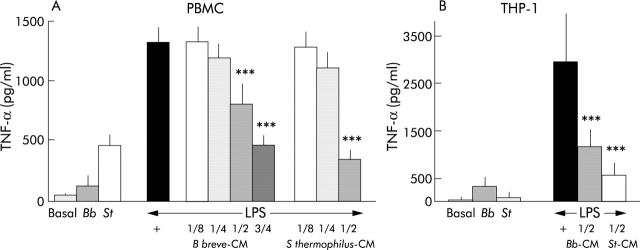
Figure 2A ▶ shows that in the absence of any other stimulation, S thermophilus CM stimulates TNF-α secretion (487 (192) pg/ml; p<0.0003) and B breve CM demonstrates a non-significant increase (150 (96) pg/ml) compared with basal secretion (49 (44) pg/ml). This basal stimulation could be due to the presence in the CM of residual Gram positive membrane components, such as peptigloglycan or LTA, known to stimulate TNF-α secretion.18,19 Both bacteria CM exerted a dose dependent inhibitory effect on LPS induced TNF-α secretion. Compared with LPS induced TNF-α secretion (1315 (413) pg/ml), significant inhibition of TNF-α secretion by PBMC was observed in the presence of B breve CM at 1/2 dilution (804 (400) pg/ml, 39% inhibition; p<0.001) and 3/4 dilution (468 (172) pg/ml, 64% inhibition; p<0.001). S thermophilus CM also inhibited LPS induced TNF-α secretion with a maximal effect at 1/2 dilution (71% inhibition; p<0.001), 3/4 dilution being toxic to immune cells. The culture media used to produce bacteria CM, by themselves, did not inhibit TNF-α secretion (not shown). In THP-1 cells (fig 2B ▶) under basal conditions, B breve CM displayed a non-significant increase (323 (101) pg/ml) in TNF-α secretion compared with basal secretion (46 (34) pg/ml) whereas S thermophilus CM did not stimulate TNF-α secretion (81 (52) pg/ml) in contrast with what was observed with PBMC. Again, in the presence of LPS, TNF-α secretion (2938 (501) ng/ml) was increased, and B breve or S thermophilus CM highly inhibited (1160 (184) and 560 (124) ng/ml, respectively; p<0.001) such secretion.
Figure 2.
Effect of Streptococcus thermophilus (St) and Bifidobacterium breve (Bb) conditioned media (CM) on basal and lipopolysaccharide (LPS) induced tumour necrosis factor α (TNF-α) secretion by peripheral blood mononuclear cells (PBMC) (A) and the pro-monocytic cell line THP-1 (B). Dose dependent inhibition of TNF-α secretion was observed in the presence of the conditioned media obtained from both bacterial strains. ***Significantly different from LPS induced TNF-α secretion (p<0.0001). n = 8 (THP-1 experiments); n = 9–18 (PBMC experiments).
Viability studies using flow cytometry showed that B breve CM did not induce cell death even at a high dose (3/4 dilution) as PBMC apoptosis was approximately 11% under control conditions and 13% in the presence of B breve CM. However, incubation of S thermophilus CM with PBMC led to a death rate of 20% at 1/2 dilution but of 90% at the highest concentration (3/4 dilution).
Effect of bacteria CM on TNF-α secretion by LTA induced PBMC
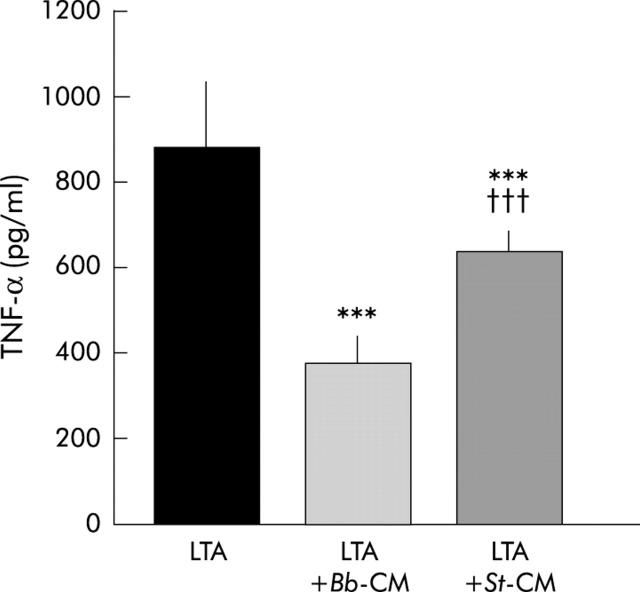
Figure 3 ▶ shows that LTA, a major compound of the Gram positive bacterial cell wall, stimulates TNF-α secretion (880 (147) pg/ml) and that both lactic acid bacteria CM inhibit LTA stimulated TNF-α secretion (383 (50) and 642 (39); p<0.0001 for Bb and St CM, respectively).
Figure 3.
Effect of Streptococcus thermophilus (St) and Bifidobacterium breve (Bb) conditioned media (CM) on lipoteichoic acid (LTA) induced tumour necrosis factor α (TNF-α) secretion by peripheral blood mononuclear cells. Inhibition of TNF-α secretion was observed in the presence of Bb-CM (3/4 dilution) and St-CM (1/2 dilution). ***Significantly different from LTA induced TNF-α secretion (p<0.0001); †††significantly different from LTA+Bb-CM (p<0.0001); n = 4–7 separate experiments.
Effect of bacteria CM on IL-10 secretion by PBMC
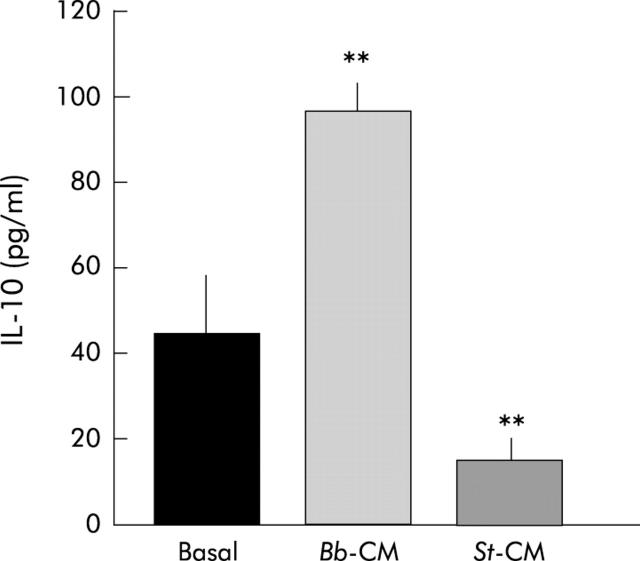
IL-10 secretion by PBMC was identical in the presence or absence of LPS (not shown). Figure 4 ▶ shows that basal secretion of IL-10 (52 (27) pg/ml) was significantly increased in the presence of B breve CM (3/4 dilution) (98 (24) pg/ml; p<0.0005). In the presence of S thermophilus CM (1/2 dilution), IL-10 secretion was significantly decreased (13 (5) pg/ml; p<0.0006) compared with basal secretion.
Figure 4.
Effect of Bifidobacterium breve (Bb) and Streptococcus thermophilus (St) conditioned media (CM) on interleukin 10 (IL-10) secretion by peripheral blood mononuclear cells. CM were used at their maximal concentration (1/2 and 3/4 for S thermophilus and B breve, respectively). **Significantly different from lipopolysaccharide (p<0.0005); n = 4.
Characterisation of active bacterial metabolites
Pepsin-trypsin hydrolysis
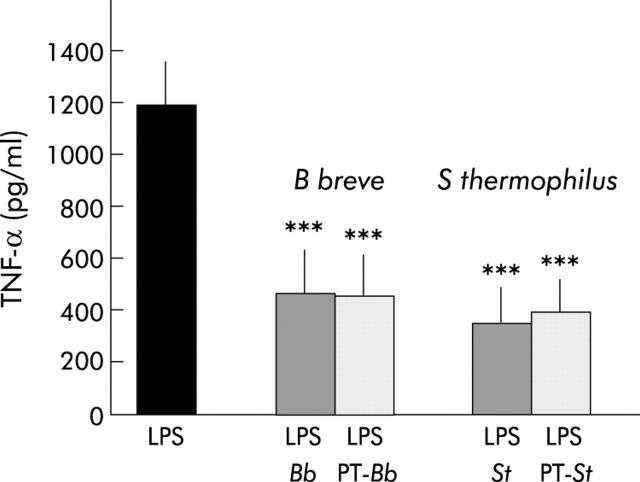
Pepsin/trypsin treated B breve (462 (153) pg/ml) and S thermophilus CM (394 (139) pg/ml) were found to have an inhibitory effect compared with control LPS stimulation (1185 (165) pg/ml; p<0.0001) (fig 5 ▶). Native and hydrolysed bacteria CM had the same inhibitory effect, indicating that digestive enzymes do not alter their inhibitory capacity and that active bacterial metabolites are probably not proteins.
Figure 5.
Effect of Bifidobacterium breve (Bb) and Streptococcus thermophilus (St) conditioned media (CM), after treatment with pepsin/trypsin (PT), on lipopolysaccharide (LPS) induced tumour necrosis factor α (TNF-α) secretion. Native and PT hydrolysed bacteria CM had the same inhibitory effect on LPS induced TNF-α secretion. ***Significantly different from LPS (p<0.0001); n = 8–13.
Selective ultrafiltration
Figure 6 ▶ shows that filtrates containing metabolites <10 kDa still decreased TNF-α secretion (330 (46) pg/ml and 680 (96) pg/ml for Bb and St, respectively; p<0.0001) compared with LPS induced secretion (1189 (66) pg/ml). Filtrates containing metabolites <3 kDa also significantly decreased TNF-α secretion (690 (74) pg/ml and 759 (325) pg/ml for Bb and St, respectively; p<0.0001) whereas retained fractions did not, indicating that most of the active bacterial metabolites have a molecular mass lower than 3 kDa.
Figure 6.
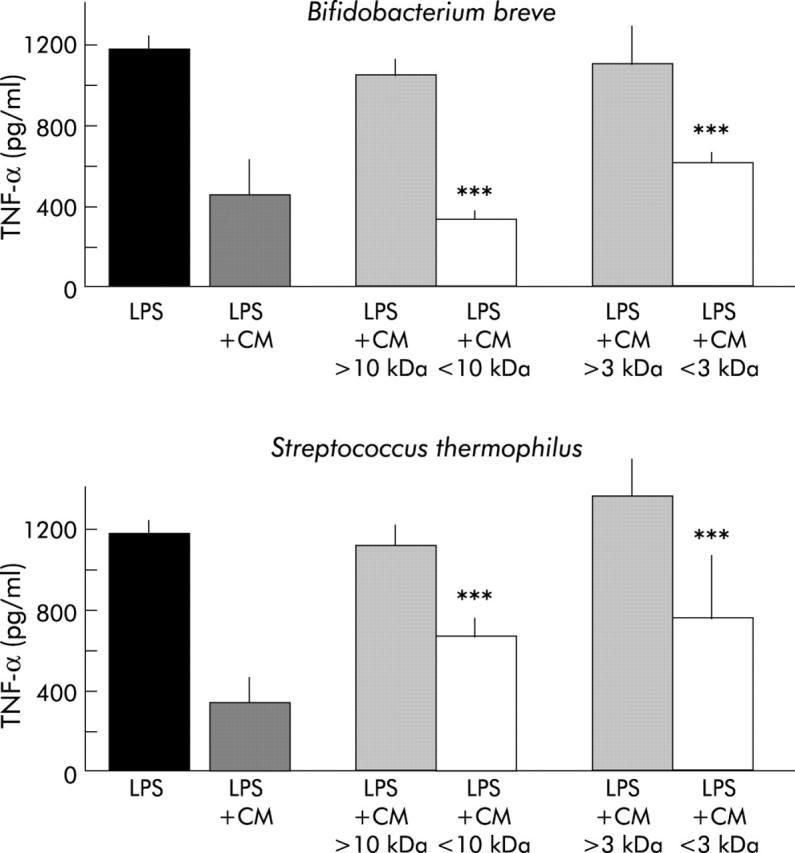
Assessment of the molecular weight of active bacterial metabolites. Ultrafiltration of bacteria conditioned media (CM) was performed on selective membranes (molecular weight cut off of 3 and 10 kDa). Retained and filtrated fractions were tested for their capacity to inhibit tumour necrosis factor α (TNF-α) secretion. Ultrafiltrated CM were used at their maximal inhibitory concentration. Only filtrated but not retained fractions displayed inhibitory capacities, indicating the low molecular weight (<3 kDa) of active bacterial metabolites. ***Significantly lower than lipopolysaccharide (LPS) and retained fraction (p<0.0001); n = 4–10 separate experiments.
Active bacterial metabolites inhibit LPS binding to THP-1 cells
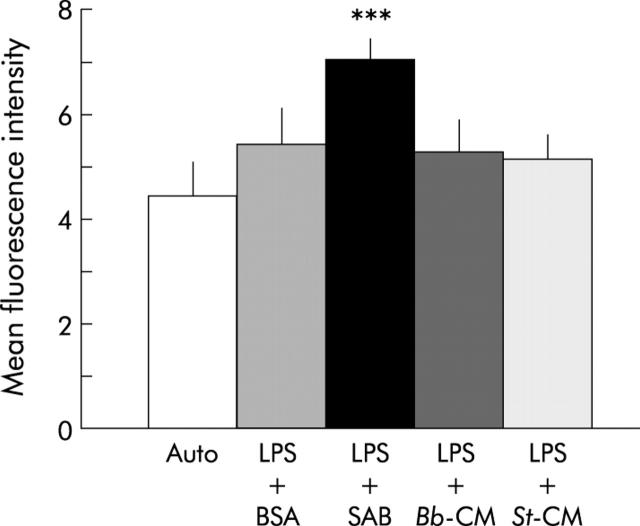
As shown in fig 7 ▶, in the presence of LBP (contained in serum AB), LPS-FITC binding to CD14 receptors expressed on mature THP-1 cells was small but significant (mean fluorescence intensity 7.0 (0.4)). There was also a small amount (non-significant) of binding of LPS-FITC in the absence of LBP (LPS+BSA). However, B breve and S thermophilus CM completely inhibited LBP dependent LPS binding to THP-1 cells (mean fluorescence 5.2 (0.6) and 5.1 (0.5); p<0.0001). The culture media used to grow both lactic acid bacteria did not, by themselves, inhibit this binding.
Figure 7.
Effect of Streptococcus thermophilus (St) and Bifidobacterium breve (Bb) conditioned media (CM) on lipopolysaccharide (LPS)-FITC binding to differentiated THP-1 cells. Mean fluorescence intensity was determined by flow cytometry. Compared with autofluorescence, there was a small amount of binding of LPS to THP-1 cells in the presence of bovine serum albumin (BSA) (CD14 independent binding). This binding was significantly increased in the presence of LPS binding protein (contained in human serum AB (SAB)) (CD14 dependent binding). After pretreatment at 4°C with St-CM or Bb-CM in the presence of SAB, CD14 dependent LPS-FITC binding to THP-1 cells was significantly inhibited. ***Significantly different from the other conditions (p<0.0001); n = 6 separate experiments.
Active metabolites from B breve and S thermophilus decrease NFκB nuclear translocation in THP-1 cells
In this study, active metabolites of molecular mass <3 kDa were used. As shown in fig 8 ▶, LPS stimulated nuclear translocation of NFκB and the density of this band was arbitrarily taken as 100% activation. In the presence of LPS, B breve and S thermophilus CM (filtrated fraction <3 kDa) inhibited LPS induced NFkB nuclear translocation by 34% and 35%, respectively.
Figure 8.
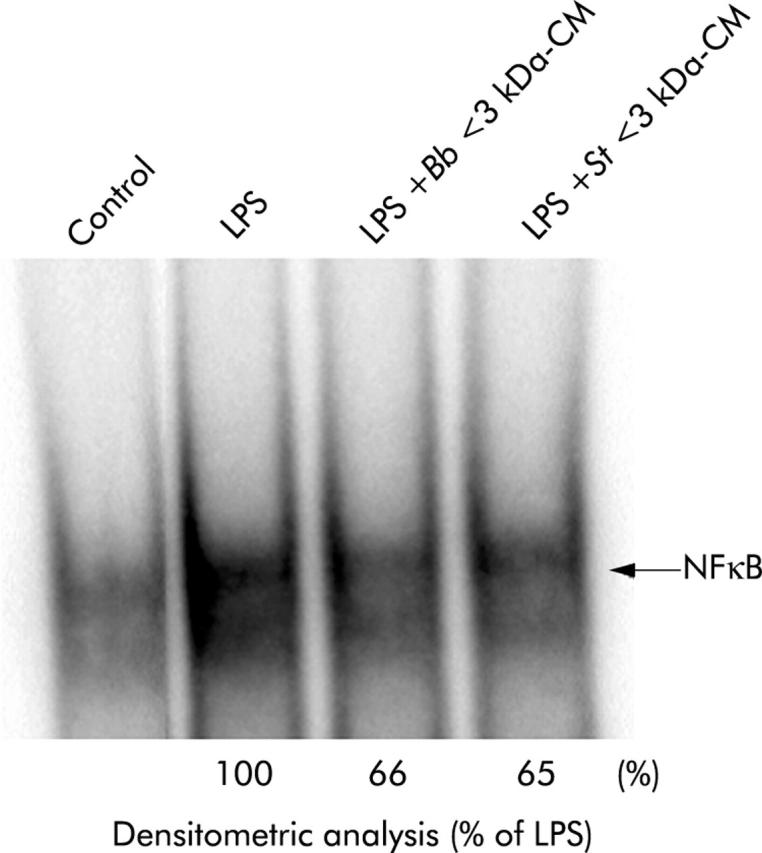
Effect of Streptococcus thermophilus (St) and Bifidobacterium breve (Bb) conditioned media (CM) on nuclear translocation of nuclear factor κB (NFκB) in THP-1 cells. Electrophoretic migration shift assays were performed following extraction of nuclear proteins, and the presence of NFκB assessed using hybridisation with a 32P labelled DNA probe. Results are representative of two separate experiments. LPS, lipopolysaccharide.
Active bacterial products retain their anti-TNF-α properties after intestinal transport
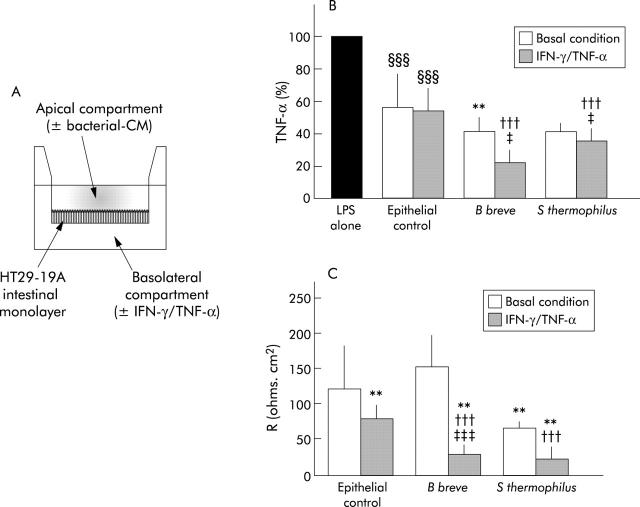
We tested the capacity of bacterial metabolites to cross the epithelial barrier as their anti-inflammatory effect should normally take place in the lamina propria where immune cells are located rather than in the intestinal lumen. As presented in fig 9A ▶, basolateral compartments, potentially containing inhibitory factors after transepithelial transport of bacterial CM, were tested for their capacity to inhibit LPS induced TNF-α secretion by PBMC, under basal and inflammatory conditions. As shown in fig 9B ▶, basolateral compartments obtained under basal conditions (epithelial control) had an inhibitory effect on TNF-α secretion (44% and 45% inhibition under basal and inflammatory conditions, respectively; p<0.0001). This suggests that epithelial cells by themselves release anti-TNF-α factors that may be distinct from TGF-β as the culture plates are coated with anti-TGF-β. Under basal conditions, B breve CM were still able to inhibit TNF-α secretion (p<0.0012) following intestinal transport (S thermophilus CM also inhibited secretion although this was not significant (p = 0.07)) compared with epithelial controls. After treatment of intestinal monolayers with the inflammatory cytokines TNF-α and IFN-γ, B breve and S thermophilus CM further decreased TNF-α secretion to 23 (7)%, and 37 (8)% of LPS activated secretion, respectively (p<0.0001). Under inflammatory conditions, the inhibitory capacity of bacteria CM was higher than that observed under basal conditions (p<0.02). As a control of the integrity of the epithelial barrier, the electrical resistance, R, of HT29-19A monolayers was measured in Ussing chambers following treatment with TNF-α and IFN-γ for 48 hours and then incubation with bacteria CM for 24 hours in the apical compartments (fig 9C ▶). Under basal conditions, HT29-19A monolayers had an electrical resistance R of 122 (59) Ω×cm2. B breve CM did not significantly modify R (153 (44) Ω×cm2) compared with control monolayers whereas S thermophilus CM decreased R to 67 (7) Ω×cm2 (p<0.002). Under inflammatory conditions (IFN-γ and TNF-α), in epithelial controls, R significantly decreased (78 (19) Ω×cm2; p<0.0001) compared with basal conditions (122 (59) Ω×cm2). This decrease was even more pronounced in the presence of bacteria CM (R = 29 (14) and 24 (16) Ω×cm2, respectively, for B breve and S thermophilus CM; p<0.0001) indicating that bacterial metabolites do not display a curative effect on the inflamed epithelial barrier.
Figure 9.
Inhibitory capacity of bacteria conditioned media (CM) after crossing the intestinal epithelial monolayer. (A) HT29-19A intestinal cells were grown as tight monolayers on microporous filters separating apical and basolateral compartments. After 48 hours of treatment with (or without) tumour necrosis factor α (TNF-α)/interferon γ (IFN-γ), cells were washed and bacteria CM were added to the apical compartment for 24 hours. Basolateral compartments were then tested for their inhibitory capacity on TNF-α secretion. (B) Basolateral epithelial cell CM, by themselves (epithelial control), already inhibited lipopolysaccharide (LPS) induced TNF-α secretion. The inhibitory capacities of bacteria CM were retained after crossing the intestinal epithelial layer of HT29-19A cells, especially under inflammatory conditions. The inhibitory capacity of Bifidobacterium breve CM was retained after transepithelial transport, under basal and more extensively under inflammatory conditions. The inhibitory capacity of Streptococcus thermophilus CM was statistically significant under inflammatory conditions. **Significantly different from epithelial control under basal conditions (p<0.0012); †††significantly different from epithelial control under inflammatory condition (p<0.0001); ‡significantly different from bacteria CM under basal conditions (p<0.02); §§§significantly different from LPS alone (p<0.0001); n = 4–15 separate experiments. (C) Electrical resistance of epithelial layers 48 hours after treatment with inflammatory cytokines, followed by incubation for 24 hours with S thermophilus and B breve CM or RPMI in the apical compartment. **Significantly different from epithelial control under basal conditions (p<0.002); †††significantly different from epithelial control under inflammatory conditions (p<0.0001); ‡‡‡significantly different from B breve CM under basal conditions (p<0.0001); n = 10 monolayers per condition.
DISCUSSION
Beneficial effects have been observed with probiotic bacteria used as adjuvants in the treatment of various digestive diseases. The most convincing effect in humans has been observed in the treatment of pouchitis.6 However, the mechanisms operating in the improvement in inflammatory conditions associated with digestive diseases are not completely understood and are probably complex. The beneficial activity of probiotics may be exerted through the immunomodulation of gut associated lymphoid tissue. The suggestion that commensal bacteria may have a role in induction of anti-inflammatory signals, as tested in intestinal epithelial cell lines, was reported in a study showing that non-pathogenic Salmonella were capable of inhibiting the NFκB activation pathway by preventing ubiquitination of IκB and therefore the release of proinflammatory cytokines.20 These results suggested that commensal bacteria, and perhaps probiotic bacteria, might induce downregulation of proinflammatory signals mediated through NFκB, a transcription factor involved in the production of many proinflammatory cytokines or chemokines.
In the present study, secretion metabolites of two lactic acid bacteria (B breve and S thermophilus) and to a lesser extent three commensal Gram positive bacteria (R gnavus, B bifidum, and neonatal streptococcus) were shown to be capable, in vitro, of inhibiting LPS induced TNF-α secretion by immune cells.
The present results suggest the possibility of self-limiting properties of commensal bacteria with respect to their own proinflammatory capacities as commensal bacteria, which are part of the dominant microflora, also displayed a dose dependent anti-inflammatory effect. Therefore, the question arises as to the benefit of using probiotic bacteria when the commensal microflora has the same beneficial effects on inflammation. One of the advantages of probiotic bacteria could reside in maintaining a higher (although transient) bacterial density in the upper gastrointestinal tract, a location normally hosting very few bacteria. This “transient colonisation” may favour downregulation of inflammatory processes developing in intestinal areas which are normally not in contact with commensal microflora or in areas where the composition of the resident microflora is altered. It is possible that probiotics represent an “exogenous” microflora exerting a local beneficial effect during intestinal transit, and that continuous intake is needed to exert such an effect. By contrast, the resident microflora would exert such an effect permanently, at least in the distal part of the small intestine and colon.
B breve CM was more efficient than S thermophilus CM in exerting anti-inflammatory properties, especially following intestinal transport, with no side effects on epithelial or immune cell viability. The inhibitory capacity of B breve and S thermophilus CM may not involve the same active metabolites as some discordance has been observed in experiments designed to better understand the mechanisms of the inhibitory effect (lower IL-10 secretion by S thermophilus CM and lower inhibition of LTA induced TNF-α secretion).
Active metabolites seem to be non-protein molecules (<3000 Da) but their exact nature remains to be investigated. Some bacterial metabolites, particularly short chain fatty acids such as butyrate, produced by bacterial fermentation of dietary fibres, have been shown to exert inhibitory effects on NFκB activation, especially in the presence of TNF-α both, in an intestinal epithelial cell line21 and in macrophages.22 In the present study, this possibility was excluded as bacteria CM did not contain significant amounts of butyrate. Indeed, butyrate concentration was very low (15 and 20 μM for B breve and S thermophilus CM, respectively) suggesting that butyrate was not involved as active concentrations have been reported in the micromolar range.22 Substantial amounts of lactic acid were found (4.5 and 9 mM for B breve and S thermophilus CM, respectively) but at these concentrations, lactic acid did not inhibit LPS induced TNF-α secretion (data not shown).
It is interesting to note that even though lactic acid bacteria CM are obtained from a relatively small bacterial density (~2.5×105 CFU/ml), the inhibitory effect is significant, underlining the efficiency of the active bacterial metabolites. Indeed, in vivo, the density of probiotic bacteria orally administered is generally high (~1010 CFU/day) and our results suggest that despite large amounts of bacteria being lost due to the hostile intestinal environment (gastric acidity, digestive enzymes, defensins), surviving bacteria may play a role in downregulating inflammation. The mechanisms by which bacterial secretion products inhibit LPS activation of immune cells are probably multifactorial. In the present study, bacterial products seem to limit access of LPS to CD14 receptors on monocytes/macrophages. This is associated with lowering of NFκB signalling in immune cells and downregulation of TNF-α secretion. Although intestinal macrophages do not express CD14 under basal conditions, their expression is upregulated under inflammatory conditions,23 underlining the potential beneficial effect of probiotic bacteria under these conditions. However, the mechanism involved in inhibition of TNF-α secretion may not rely solely on inhibition of the LPS receptor as bacteria CM also inhibited LTA induced TNF-α secretion, a process dependent on TLR-2. Thus the possibility that bacteria CM also act by affecting the intracellular signalling cascade leading to NFκB activation has not been excluded.
B breve CM also stimulated IL-10 secretion, a cytokine with anti-inflammatory properties.24 Indeed, IL-10 downregulates TNF-α secretion by macrophages25 and decreases MHC class II expression on antigen presenting cells.26 In atopic children, administration of Lactobacillus rhamnosus GG (2×1010 CFU daily) for a period of four weeks leads to increased levels of serum IL-10 and to increased production of mitogen induced IL-10 secretion by PBMC.27 In the same way, intragastric administration of Lactococcus lactis engineered to secrete IL-10 leads to a 50% decrease in dextran sulphate induced colitis in mice.28
It is interesting that after crossing the epithelial barrier, bacteria CM still exerted an inhibitory effect, especially under inflammatory conditions. It was difficult to dissociate the inhibitory capacity of bacteria CM from that of intestinal epithelial cells (IEC) themselves as the latter are known to release suppressive factors, including prostaglandins and TGF-β.29,30 Although we tried to eliminate this problem by capturing TGF-β in the basolateral compartment of filter grown epithelial cells using anti-TGF-β antibodies, an inhibitory effect was still caused by epithelial cell CM, suggesting that either TGF-β is not totally blocked by anti-TGF-β antibodies or that suppressive factors other than TGF-β are released by IEC. However, addition of bacteria CM (especially from B breve) significantly added to the epithelial inhibitory effect, suggesting that bacterial active metabolites by themselves help in downregulating inflammation. Two possibilities may explain such an inhibitory activity in the intestinal environment: (1) an indirect inhibitory effect linked to modulation of epithelial cell derived anti-inflammatory compounds by bacterial metabolites or (2) most likely, transepithelial passage of active bacterial metabolites across the epithelial barrier. The transepithelial pathway, along which bacterial metabolites may cross the epithelial barrier, could be paracellular as well as transcellular. Indeed, although it is well recognised that proinflammatory cytokines facilitate paracellular leakage to small molecules, increased transport of molecules along a transcellular pathway by transcytosis is also observed in the presence of IFN-γ.31 Alternatively, receptor mediated transport of active metabolites at the apical membrane of enterocytes could be involved.
Taken together, the present results indicate that both commensal Gram positive bacteria and the probiotic bacteria Bifidobacterium breve and Streptococcus thermophilus exert an anti-TNF-α effect, at least in vitro. In addition, the findings suggest that active metabolites released by probiotic bacteria during intestinal transit may cross the intestinal layer to exert anti-inflammatory effects. This would add to the suppressive tone of the intestinal microenvironment, under basal conditions, and help downregulation of inflammation under pathological conditions. Thus immunomodulation of the gut associated lymphoid tissue may explain, at least in part, the beneficial effects of probiotic bacteria as adjuvants in the treatment of digestive inflammation.
Acknowledgments
This work was partly supported by Blédina-SA and by financial assistance from the Princesse de Monaco foundation. We also acknowledge Dr Butel for her help in performing short chain fatty acids assays and Dr G Corthier for providing us with the commensal bacterial strains.
Abbreviations
FCS, fetal calf serum
IFN, interferon
IL, interleukin
LBP, LPS binding protein
LPS, lipopolysaccharide
LTA, lipoteichoic acid
NFκB, nuclear factor κB
PBMC, peripheral blood mononuclear cells
SAB, human serum AB
Bb CM, Bifidobacterium breve conditioned media
St CM, Streptococcus thermophilus conditioned media
TGF-β, transforming growth factor β
TNF-α, tumour necrosis factor α
TLR, toll-like receptor
IEC, intestinal epithelial cells
REFERENCES
- 1.Heyman M, Menard S. Probiotic microorganisms: how they affect intestinal pathophysiology. Cell Mol Life Sci 2002;59:1151–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majamaa H, Isolauri E. Probiotics: a novel approach in the management of food allergy. J Allergy Clin Immunol 1997;99:179–85. [DOI] [PubMed] [Google Scholar]
- 3.Canducci F, Armuzzi A, Cremonini F, et al. A lyophilized and inactivated culture of Lactobacillus acidophilus increases Helicobacter pylori eradication rates. Aliment Pharmacol Ther 2000;14:1625–9. [DOI] [PubMed] [Google Scholar]
- 4.Madsen K, Cornish A, Soper P, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001;121:580–91. [DOI] [PubMed] [Google Scholar]
- 5.Madsen KL, Doyle JS, Jewell LD, et al. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology 1999;116:1107–14. [DOI] [PubMed] [Google Scholar]
- 6.Gionchetti P, Rizzello F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology 2000;119:305–9. [DOI] [PubMed] [Google Scholar]
- 7.Borruel N, Carol M, Casellas F, et al. Increased mucosal tumour necrosis factor alpha production in Crohn’s disease can be downregulated ex vivo by probiotic bacteria. Gut 2002;51:659–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta P, Andrew H, Kirschner BS, et al. Is lactobacillus GG helpful in children with Crohn’s disease? Results of a preliminary, open-label study. J Pediatr Gastroenterol Nutr 2000;31:453–7. [DOI] [PubMed] [Google Scholar]
- 9.Caplan MS, Miller-Catchpole R, Kaup S, et al. Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Gastroenterology 1999;117:577–83. [DOI] [PubMed] [Google Scholar]
- 10.Pena JA, Versalovic J. Lactobacillus rhamnosus GG decreases TNF-alpha production in lipopolysaccharide-activated murine macrophages by a contact-independent mechanism. Cell Microbiol 2003;5:277–85. [DOI] [PubMed] [Google Scholar]
- 11.Prantera C, Scribano ML, Falasco G, et al. Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn’s disease: a randomised controlled trial with Lactobacillus GG. Gut 2002;51:405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maassen CB, Holten-Neelen C, Balk F, et al. Strain-dependent induction of cytokine profiles in the gut by orally administered Lactobacillus strains. Vaccine 2000;18:2613–23. [DOI] [PubMed] [Google Scholar]
- 13.Dabard J, Bridonneau C, Phillipe C, et al. Ruminococcin A, a new lantibiotic produced by a Ruminococcus gnavus strain isolated from human feces. Appl Environ Microbiol 2001;67:4111–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamping N, Dettmer R, Schroder NW, et al. LPS-binding protein protects mice from septic shock caused by LPS or gram-negative bacteria. J Clin Invest 1998;101:2065–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi T, Nakamura K, Iho S. Differentiation of myeloid cells and 1,25-dihydroxyvitamin D3. Leuk Lymphoma 1997;27:25–33. [DOI] [PubMed] [Google Scholar]
- 16.Courtois G, Whiteside ST, Sibley CH, et al. Characterization of a mutant cell line that does not activate NF-kappaB in response to multiple stimuli. Mol Cell Biol 1997;17:1441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai K, Takeshita A, Hanazawa S. Transforming growth factor-beta inhibits lipopolysaccharide-stimulated expression of inflammatory cytokines in mouse macrophages through downregulation of activation protein 1 and CD14 receptor expression. Infect Immun 2000;68:2418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhakdi S, Klonisch T, Nuber P, et al. Stimulation of monokine production by lipoteichoic acids. Infect Immun 1991;59:4614–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Timmerman CP, Mattsson E, Martinez-Martinez L, et al. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect Immun 1993;61:4167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neish AS, Gewirtz AT, Zeng H, et al. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science 2000;289:1560–3. [DOI] [PubMed] [Google Scholar]
- 21.Inan MS, Rasoulpour RJ, Yin L, et al. The luminal short-chain fatty acid butyrate modulates NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology 2000;118:724–34. [DOI] [PubMed] [Google Scholar]
- 22.Segain JP, Raingeard DLB, Bourreille A, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut 2000;47:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimm MC, Pavli P, Van de PE, et al. Evidence for a CD14+ population of monocytes in inflammatory bowel disease mucosa—implications for pathogenesis. Clin Exp Immunol 1995;100:291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asseman C, Mauze S, Leach MW, et al. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 1999;190:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiorentino DF, Zlotnik A, Mosmann TR, et al. IL-10 inhibits cytokine production by activated macrophages. J Immunol 1991;147:3815–22. [PubMed] [Google Scholar]
- 26.McBride JM, Jung T, de Vries JE, et al. IL-10 alters DC function via modulation of cell surface molecules resulting in impaired T-cell responses. Cell Immunol 2002;215:162–72. [DOI] [PubMed] [Google Scholar]
- 27.Pessi T, Sutas Y, Hurme M, et al. Interleukin-10 generation in atopic children following oral Lactobacillus rhamnosus GG. Clin Exp Allergy 2000;30:1804–8. [DOI] [PubMed] [Google Scholar]
- 28.Steidler L, Hans W, Schotte L, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 2000;289:1352–5. [DOI] [PubMed] [Google Scholar]
- 29.Koyama SY, Podolsky DK. Differential expression of transforming growth factors alpha and beta in rat intestinal epithelial cells. J Clin Invest 1989;83:1768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nathens AB, Rotstein OD, Dackiw AP, et al. Intestinal epithelial cells down-regulate macrophage tumor necrosis factor-alpha secretion: a mechanism for immune homeostasis in the gut-associated lymphoid tissue. Surgery 1995;118:343–50. [DOI] [PubMed] [Google Scholar]
- 31.Terpend K, Blaton MA, Candalh C, et al. Intestinal barrier function and cow’s milk sensitization in guinea-pigs fed milk or fermented milk. J Pediatr Gastroenterol Nutr 1998;28:191–8. [DOI] [PubMed] [Google Scholar]