This present review is timely with the increasing use of the molecular adsorbents recirculating system (MARS) for the management of liver failure, with over 3000 patients having been treated with this device worldwide. In the UK, MARS is being used for the treatment of individual patients both in the National Health Service and also in the private sector. In order to investigate the latest position with respect to bioartificial liver devices, a meeting was held at University College London Hospital in September 2003 and this article is based on the most up to date data presented there.
Liver failure, whether of the acute variety with no pre-existing liver disease (acute liver failure (ALF)) or an acute episode of decompensation superimposed on a chronic liver disorder (acute on chronic liver failure (ACLF)), carries a high mortality. In patients with ALF, lack of detoxification, metabolic, and regulatory functions of the liver leads to life threatening complications, including kidney failure, encephalopathy, cerebral oedema, severe hypotension, and susceptibility to infections culminating in multiorgan failure.1 The only established therapy for such patients is liver transplantation (LTx) but currently one third of these patients die while waiting for a transplant and the organ shortage is increasing (fig 1 ▶).2 However, liver failure, whether of the acute or acute on chronic variety, is potentially reversible, and considerable work has been carried out over many years to develop effective liver support devices.
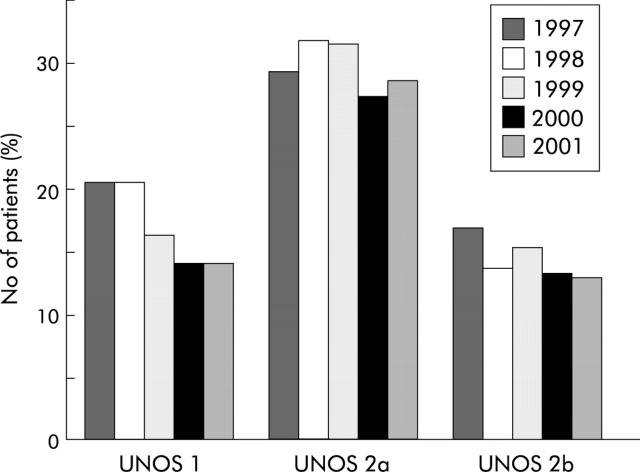
Figure 1.
Annual death rates on the waiting list for liver transplantation between 1997 and 2001 in UNOS categories 1 (acute/fulminant liver failure), 2a (decompensated chronic liver disease urgently requiring transplant), and 2b (decompensated chronic liver disease requiring transplant less urgently). (Source: OPTN/SRTR data, as of 1 August 2002.)
The development of these devices has been approached in two very different ways. The biological devices, which aim to provide all of the functions of the normal liver,3,4 are based on the use of living liver cells with either human hepatic cells as in the extracorporeal liver assist device (ELAD) device5 or porcine hepatocytes as in the BAL device6 and in various other European devices being developed in the Netherlands7 and in Germany.8 The other approach is based on detoxification functions only using membranes and adsorbents which can remove the putative toxins associated with liver failure. Such entirely artificial devices are substantially less costly, by a factor of at least a tenth, than those based on living liver cell lines. The earliest of the artificial systems developed was based on perfusion of the patient’s blood through the adsorbent charcoal.9,10 Although some of the toxins present in liver failure were shown to be adsorbed to the charcoal, other compounds tightly bound to proteins in the plasma were not removed.9,10 Another system known as a Biologic-DT is a combination of flat membrane dialysis against adsorbent solution11 but several studies have shown only limited efficacy in terms of removal of protein bound substances.3,12 MARS is the only available device able to remove free and albumin bound low and middle weight toxins with high selectivity due to use of a polysulfone membrane, and human serum albumin as a selective adsorbent in removal and transport of the toxins.13–15 In addition to the facility for removing protein bound substances, there is an additional dialysis component for removal of water soluble toxins.
In this review, we will define the goals of artificial liver support, discuss the design of the existing liver support systems, and critically analyse the available data from clinical studies to establish their current status in the management of patients with liver failure.
BASIS OF USE OF A LIVER SUPPORT DEVICE
An ideal liver support system would provide many of the normal functions of the liver, be easy to use in clinical practice, have minimal complications, and not be prohibitively expensive. From the pathophysiological perspective, this can be translated to having significant biosynthetic capacity with the ability to detoxify and the capability to biotransform by altering key processes which allows regeneration and healing. Although provision of the biosynthetic function of the liver may be thought to be important, it is possible to provide many of the substances manufactured by the liver, such as glucose, albumin, trace elements and vitamins, and clotting factors either through the oral route or by infusions.4 We believe that the ability of the artificial liver support systems to mimic the detoxification functions of the liver is crucial to their success, as liver failure is associated with accumulation of various toxic substances such as ammonia, mediators of oxidative stress, bile acids, nitric oxide, lactate, products of arachidonic acid metabolism, benzodiazepines, indoles, mercaptans, etc,16,17 which are not only important in the pathogenesis of end organ dysfunction, alteration in vascular function, and acid-base balance but may also impair liver regeneration.18,19 The “biological device” is primarily designed to provide the synthetic functions whereas the “artificial device” is primarily for detoxification function. If all of these functions are to be achieved by a potential liver support system, then it is difficult to imagine that a pure “biological device” would fulfil these roles. On the other hand, removal of toxins alone may allow biotransformation and recovery of biosynthetic and metabolic functions without additional synthetic activity. Although the primary aim of using a liver support device is to improve the transplant free survival of patients with liver failure, the alternative objectives may be to serve as effective “bridge” to liver transplantation, prevent the occurrence of liver failure in those that are predisposed to it, or provide functional capacity to improve end organ dysfunction (see box 1).
Box 1 . Goals of therapy with liver support devices.
Primary goal
In acute liver failure: recovery of patient to normal health
In acute on chronic liver failure: recovery to the state before decompensation
Alternative goals
Bridge to liver transplantation
Prevention of progression to liver failure
Improvement of end organ function in those with established multiorgan failure
TECHNICAL ASPECTS OF THE LIVER SUPPORT DEVICES
Bioartificial devices
In a bioartificial device, isolated cultured hepatocytes are then incorporated into bioreactors.
Cellular component
The minimum quantity of cells required to provide enough liver function is not known, but based on experience gathered from patients undergoing hepatic resection,20 approximately 150–450 g of cells (or 1010 hepatocytes), providing the function of 10–30% of the normal liver mass, is required to support the failing liver.3,4,21 The ideal cellular component for use in the devices is the human hepatocyte, which is obviously limited by availability, is difficult to culture, and loses liver specific functions rapidly.3,22 Primary porcine hepatocytes are the most used in the liver support systems that have been evaluated in clinical trials, such as Demetriou’s HepatAssist BAL device6 (as well as others such as Academic Medical Center-BAL (AMC-BAL) developed by Chamuleau et al in Amsterdam7). One of their major advantages, apart from easy availability, is that they can be satisfactorily cryopreserved, thus simplifying the issues of cell storage and transport to the treatment centre.23,24 Concerns remain however regarding immune reactions to foreign antigens,25 as well as xenozoonosis in the form of cross species infection with porcine endogenous retrovirus (which has been demonstrated in vitro26–31 although never in vivo32–34).
An alternative approach is to use genetically engineered human hepatocytes with the required functional and survival characteristics. The C3A hepatocyte line, a subclone of the HepG2 hepatoblastoma cell line, has been used (Sussman’s extracorporeal liver assist device (ELAD)5), while HHY41, another immortalised human hepatocyte cell line which retains many liver specific functions and is particularly resistant to acetaminophen, is currently under investigation.35–37 Concerns regarding functional capacity and escape of tumorigenic cells into patients limit their applicability. Primary human hepatocytes from explanted livers found unsuitable for transplantation have also been used but their supply is limited as only a small number of organs are unacceptable for LTx and both the quality and quantity of hepatocytes recovered from such organs are suboptimal (modular extracorporeal liver support (MELS) developed by Gerlach et al in Berlin).8
Bioreactor component
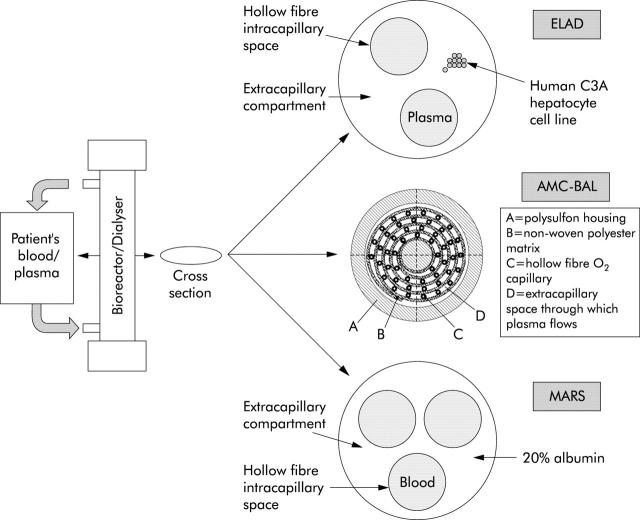
The most basic design of a bioreactor consists of a column containing hollow fibre capillaries through which the patient’s plasma/blood is circulated while hepatocytes are located in the extracapillary space (fig 2 ▶). Plasma can be separated, warmed, and oxygenated in the secondary circuit before being perfused through the bioreactor capillaries. A membrane with a cut off of 50–150 kDa1,3 separates the two compartments, across which exchange of substances can occur between the plasma/blood and hepatocytes. While most toxins and transport proteins (such as albumin) can pass through, larger substances like immunoglobulins, complements, viruses, and cells cannot. This is the basic design used in the HepatAssist BAL6 as well as in the ELAD system.5 The HepatAssist BAL also incorporates two charcoal columns in the circuit prior to the bioreactor for removal of toxins, which could damage or impair the function of the pig hepatocytes.
Figure 2.
Schematic diagram of the structure of a first generation bioreactor (extracorporeal liver assist device (ELAD), with plasma passing through the intracapillary space, and hepatocytes derived from human hepatoblastoma based cell lines in the extracapillary space), a newer generation bioreactor (AMC-BAL, incorporating a spirally wound polyester matrix sheet that includes an integral hollow fibre compartment for oxygenation), and an artificial device dialyser (molecular adsorbents recirculating system (MARS), with blood passing through the intracapillary space, and separated from the extracapillary 20% albumin dialysate by an albumin impregnated membrane).
The MELS system8 uses three sets of capillary tubes—one to provide oxygenation and two to carry inflowing and outflowing plasma. Hepatocytes remain in the extracapillary space. A detoxification module allows single pass albumin dialysis to be performed, and continuous veno-venous haemodiafiltration can be included. The AMC-BAL7 incorporates a spirally wound polyester matrix sheet that includes an integral hollow fibre compartment for oxygenation.
In addition to these hollow fibre based bioreactors, some others have tried designs based on “flate plates and monolayers”, “perfused beds/scaffolds”, and “encapsulation and suspension”.3 A porcine hepatocyte based BAL using a “radial flow” bioreactor is being developed in Italy, and has been tried in three patients with ALF.3,22,38
Artificial devices
These newer systems, based on the use of albumin as transporting medium for toxins and utilising a membrane having a sufficiently small pore size, are substantially more selective with regard to their detoxifying capacity compared with the earlier generation of devices based on charcoal haemoperfusion.9,10 They are thus specific for albumin bound substances which form the majority of the toxins accumulating in liver failure16 while larger molecules (immunoglobulins, growth factors) that might be physiologically important are prevented from crossing over.
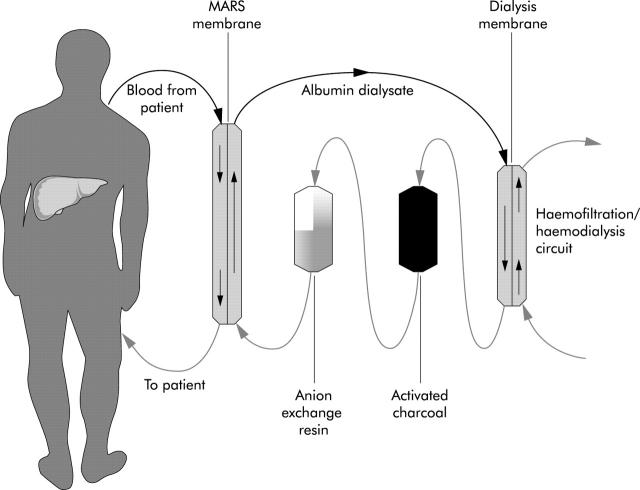
The system that has been developed over the last decade and is currently under extensive clinical investigation is the MARS machine (Teraklin AG, Rostock, Germany)14,39 (fig 3 ▶). This uses a hollow fibre dialysis module where the patient’s blood is dialysed across an albumin impregnated polysulfone membrane (with a cut off of 50 kDa) while maintaining a constant flow of 600 ml of 20% albumin as dialysate in the extracapillary compartment. In vitro studies have demonstrated that toxins bound to albumin in the patient’s blood will detach and bind to the binding sites on the membrane,39 as albumin, when attached to polymers, have a higher affinity for albumin bound toxins.40 These pass on to the albumin in the dialysate which is then cleansed sequentially by a haemodialysis/haemofiltration module (removing water soluble substances) and adsorber columns containing activated charcoal and anion exchange resin (removing most of the albumin bound substances). The dialysate is thus regenerated, and once more capable of taking up more toxins from the blood.
Figure 3.
Schematic diagram of the molecular adsorbents recirculating system (MARS) circuit showing direction of flow of the blood and the dialysate (20% albumin). Albumin bound toxins from the patient’s blood pass on to the albumin in the dialysate which is then cleansed sequentially by a haemodialysis/haemofiltration module (removing water soluble substances) and adsorber columns containing activated charcoal and anion exchange resin (removing most of the albumin bound substances). The dialysate is thus regenerated, and once more capable of taking up more toxins from the blood.
Another type of albumin dialysis that has been introduced recently (1999) is the fractionated plasma separation and adsorption (FPSA),41 using an albumin permeable membrane with a cut off of 250 kDa. Albumin and albumin bound toxins cross the membrane and pass through special adsorbers (one or two columns in series in the secondary circuit, containing a neutral resin adsorber and an anion exchanger) that remove the toxins. The cleansed albumin is returned to plasma. In the newly introduced Prometheus system (Fresenius Medical Care AG, Bad Homburg, Germany)42 the FPSA method is combined with high flux haemodialysis (of the blood directly, as opposed to the MARS system where haemodialysis/filtration of the albumin dialysate is performed).
RESULTS FROM CLINICAL STUDIES USING DEVICES
Bioartificial devices
The first clinical use of a BAL device, using rabbit hepatocytes, was in 1987 to treat a single patient with ALF.43 Of the many trials with different devices since then, only those relevant to currently available or recently used systems are discussed below. Table 1 ▶ summarises some of the important studies evaluating bioartificial devices.
Table 1.
Summary of important studies evaluating the bioartificial devices (BAL and extracorporeal liver assist device (ELAD))
| Study | Patient population | System used | Study design | End point | Outcome | Comments |
| Chen et al (1996)44 | ALF (n = 12), ACLF (n = 8) | BAL | Prospective case series | Inhospital mortality or LTx | ALF: all bridged to LTx; ACLF: 6 died, 2 bridged to LTx | |
| Samuel et al (2002)45 | ALF (n = 10) | BAL | Prospective case series | Neurological improvement, LTx | Improvement in Glasgow coma score, 6; all bridged to LTx (8 alive at 18 mths) | Bleeding complications in 5; haemodynamic instability in 6 |
| Stevens et al (2001)46 | ALF (n = 147), primary graft non-function (n = 24) | Hepat-Assist (BAL) | Multicentre, randomised, controlled trial | 30 day mortality | 30 day survival. All patients: BAL 71%, controls 62%. Subgroups: All ALF: BAL 73%, controls 59% (p = 0.1). ALF due to paracetamol: BAL 70%, controls 37% (p<0.05) | Substantial impact of LTx (54% of all patients) on outcome |
| Ellis et al (1996)47 | ALF (n = 24). Grp I: not fulfilling LTx criteria* (n = 17); Grp II: fulfilling LTx criteria (n = 7) | ELAD | Single centre, randomised, controlled trial | Inhospital mortality or LTx | Survival. Grp I: ELAD 78%, controls 75%; Grp II: ELAD 1/3, controls 1/4 | Survival among controls in Grp I was much higher than anticipated |
| Millis et al (2001)48 | ALF (n = 24) (19 listed for LTx, 5 not listed) | ELAD | Randomised, controlled, phase I trial | 30 day mortality | In those listed for LTx. 30 day survival: ELAD 83%, controls 43%; LTx received: ELAD 92%, controls 43% | Not adequately powered to look at outcome |
*Kings College criteria.
Porcine hepatocyte based BAL
Most of the available clinical data relate to treatment of patients with ALF in whom neurological improvement,44,45 with minimal reduction of serum bilirubin44,45 and arterial ammonia (in some studies44), have been observed. Adverse events in the form of bleeding complications and haemodynamic instability have also been noted.45
The device has been evaluated in a large multicentre randomised controlled trial in 171 patients (ALF 147, primary graft non-function 24), conducted in the USA and Europe. The preliminary results of this trial were reported in 1991 but the final data are still not published fully.46 While some improvement in intracranial pressure and consciousness level were seen, there was little evidence of improved synthetic function. Most disappointingly, a survival advantage was evident only in the subgroup with acetaminophen aetiology (n = 39) (BAL 70% v controls 37%). Thirty day survival in the entire study population was 62% for controls versus 71% for BAL treated patients, while among fulminant liver failure patients alone it was 59% versus 73%, respectively (p = 0.1). However, this primary end point was confounded by the major impact of LTx; 54% of the entire study population were transplanted. Thirty day survival with LTx (n = 90) was 84% (BAL 89% v controls 80%) while that without LTx (n = 81) was 46% (BAL 51% v controls 40%). After accounting for the impact of LTx and other factors predictive of survival (including aetiology, stage of encephalopathy), a 47% reduction in mortality favouring BAL treatment (p = 0.03) in the ALF group (n = 147) was found. However, as we understand it, a further phase III trial has been requested in the USA by the Food and Drug Administration.
Hepatoblastoma based extracorporeal liver assist device (ELAD)
The ELAD device developed by Sussman et al was tried in an early randomised controlled study in London.47 Twenty four ALF patients were enrolled, comprising 17 patients not fulfilling criteria for transplantation (predicted survival 50%; group I) and seven patients fulfilling criteria (predicted survival <10%; group II). Arterial ammonia decreased marginally in the ELAD group, and the rise in serum bilirubin was more pronounced in the controls. Worsening of encephalopathy was less in ELAD treated patients. However, a clear survival advantage was not shown. Survival in group I was 7/9 (78%) with ELAD and 6/8 (75%) for controls, the much higher value than anticipated making it difficult to show a survival advantage. There were only a small number of patients in group II, and one each of three treated and four control patients survived.
In a more recent randomised controlled phase I trial in patients with fulminant hepatic failure, patients were stratified into those listed for LTx (n = 19) and those not listed (n = 5).48 Of the 19 patients listed for LTx, 12 received ELAD therapy. Eleven of 12 (92%) ELAD patients went on to receive LTx while only 3/7 (43%) controls were transplanted (p<0.05). Ten of 12 (83%) ELAD patients also achieved the primary end point of 30 day survival compared with 3/7 (43%) controls (p = 0.12). The device appeared to be safe, and although the study was not powered to look at outcome, there was a significant advantage for patients receiving LTx in the ELAD group, including those listed for LTx. On this basis, funding for a phase II randomised controlled trial is being sought.
Other devices
A phase I clinical trial with the AMC-BAL (Amsterdam) has been carried out in Italy.49 Seven ALF patients with grade 3–4 encephalopathy, listed for LTx, were treated. Neurological improvement was observed in all patients. Serum bilirubin and arterial ammonia decreased. The only adverse effect observed was transient hypotension in two patients immediately after starting the device. One of the patients improved sufficiently not to require LTx while the remaining six were transplanted.
A phase I trial with MELS (Berlin) in eight patients with ALF (all fulfilling criteria for high urgency LTx) has recently been reported.50 The treatments were safely performed and well tolerated, with thrombocytopenia being the only adverse event encountered. All patients were successfully transplanted and were alive after a follow up of three years. No patient showed any evidence of porcine endogenous retrovirus infection.
Artificial devices
MARS
Compared with the biological devices, MARS is easy to use and relatively inexpensive, with a cost of approximately £4000–£7000 for a full treatment, as purchased by individual hospital centres in the UK. This is a much more manageable expense than treatments such as bioartificial devices that may cost £50 000–£60 000 for a full treatment. Table 2 ▶ summarises some of the important studies evaluating MARS. In contrast with trials of the biological devices which have been tested in the context of ALF, most of the clinical studies with MARS have been in patients with ACLF51–53 with a survival benefit shown in two small randomised controlled trials.54,55 This is an effective detoxification device which can remove substances bound to a wide variety of plasma proteins, and which also have the potential to bind to albumin.56 MARS therapy in patients with ACLF has a major beneficial effect on circulating neurohormones, nitric oxide, free radicals production, and reduction in the markers of oxidative stress.57 The clinical effects of these changes are reflected in individual organ function with temporal improvement in cholestasis and liver function, renal function, encephalopathy, and in some patients in mean arterial pressure.58 The improvement in liver function may result from reduced hepatocyte cell death and improved environment for regeneration. Alternatively, the improvement in liver function may be the result of improved hepatic haemodynamics. The most marked and consistent effect of MARS is on the severity of hepatic encephalopathy without affecting circulating ammonia levels,57,59 suggesting that MARS may modify the blood-brain barrier characteristics which determine the brain effects of hyperammonaemia. Alternatively, it may exert this effect through reduction in oxidative stress and/or remove unknown protein bound factors that either act alone or in concert with ammonia to produce encephalopathy (see box 2). These data are supported by studies in animal model of ALF where MARS treated animals were observed to have significantly lower intracranial pressure without any differences in arterial ammonia.
Table 2.
Summary of important studies evaluating the molecular adsorbents recirculating system (MARS) device
| Study | Patient population | Study design | End-point | Outcome |
| Stange et al (2000)62 | ACLF with intrahepatic cholestasis (bilirubin>20 mg/dl) (n = 26) | Prospective case series | Inhospital mortality | UNOS 2a status: 7/16 survived. UNOS 2b status: 10/10 survived |
| Mitzner et al (2000)54 | Type I hepatorenal syndrome (n = 13) | Two centre, randomised, controlled | 30 day mortality | Mortality: controls 100% (day 7); MARS 62.5% (day 7) and 75% (day 30) (p<0.01) |
| Heemann et al (2002)55 | ACLF (n = 24) | Two centre, randomised, controlled | Primary: reduction of serum bilirubin. Secondary: inhospital mortality | Improvement in bilirubin and 30 day survival with MARS (11/12 v 6/11 controls; p<0.05) |
| Jalan et al (2003)63 | ACLF due to acute alcoholic hepatitis (Maddrey’s discriminant function >32) (n = 8) | Prospective case series | Inhospital mortality | Improvement in 3 month predicted mortality (pre-MARS 76%, post-MARS 27%). 3 month survival: 4/8 |
Box 2 . Indications and contraindications of MARS therapy.
Indications
Acute on chronic liver failure
Severe alcoholic hepatitis
Severe pruritus due to cholestasis
Intoxication from protein bound substances
Relative contraindications
Progressive coagulopathy indicative of DIC
Uncontrolled sepsis
Uncontrolled bleeding
Monitoring during therapy
Electrolytes including phosphates, magnesium, and calcium
Coagulation (look for DIC)
Drug levels (protein bound drugs may be removed)
In the first randomised trial of MARS, 13 ACLF patients with type I hepatorenal syndrome were allocated to treatment with MARS or standard medical therapy, including haemodiafiltration.54 The mortality rates was 100% in the group receiving haemodiafiltration (n = 5) on day 7 compared with 62.5% in the MARS group (n = 8) on day 7 and 75% on day 30, respectively (p<0.01). Mean survival was 25.2 (34.6) days in the MARS group and 4.6 (1.8) days in the control group (p<0.05). In addition, a significant decrease in serum bilirubin and creatinine, and increase in serum sodium and prothrombin activity were observed in the MARS group but not in the control group. Mean arterial pressure at the end of treatment was significantly greater in the MARS group. Although there was no significant increase in urine volume in the MARS group, four of eight patients showed an increase in this group compared with none of the controls.
The most recent (and largest completed) randomised controlled trial, performed in two centres (Rostock and Essen), included 24 patients with ACLF with marked hyperbilirubinaemia (serum bilirubin >20 mg/dl (340 μmol/l)) who were randomised to receive standard medical therapy alone (controls, n = 12) or MARS in addition (n = 12).55 The primary end point of bilirubin less than 15 mg/dl for three consecutive days was reached in five of 12 patients in the MARS group and in two of 12 patients in the control group. Compared with controls, bilirubin, bile acids, and creatinine decreased and mean arterial pressure and encephalopathy improved in the MARS group. Most importantly, albumin dialysis was associated with a significant improvement in 30 day survival (11/12 v 6/11 in controls). At present, a multicentre randomised controlled trial with ACLF patients is being conducted in the UK and Europe, which is designed to look at mortality, while another on short term benefit in hepatic encephalopathy is nearing completion in the USA.
The safety of this device has been evaluated through its use in over 3000 patients worldwide. The MARS registry, which is maintained by the University of Rostock, contains data on approximately 500 patients treated with this device.59,60 In general, treatment is well tolerated and the only consistent adverse finding with the use of MARS is thrombocytopenia. Critical analysis of the data from the registry in patients with ACLF suggests that its use should be contraindicated in those with established disseminated intravascular coagulation (DIC) or in those patients with “incipient” DIC characterised by progressive thrombocytopenia and coagulopathy.
Prometheus
The results of Prometheus treatment in 11 patients with ACLF and accompanying renal failure have been published recently.42 Improvement in serum levels of conjugated bilirubin, bile acids, ammonia, cholinesterase, creatinine, urea, and blood pH occurred. A drop in blood pressure in two patients and uncontrolled bleeding in one patient were the adverse events noted. Prospective controlled trials are planned for the future.
PERSPECTIVES FROM CLINICAL TRIALS OF “LIVER SUPPORT DEVICES”
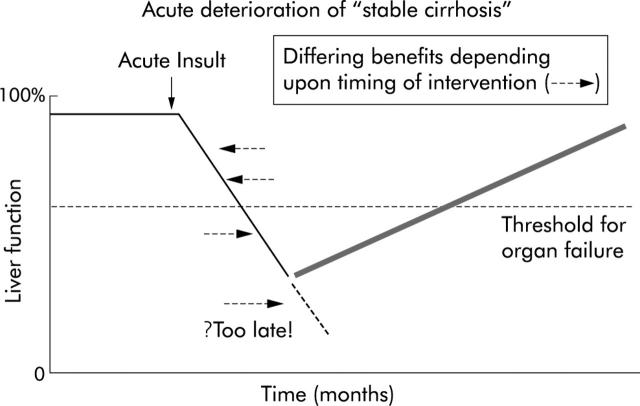
The results of randomised clinical trials evaluating the various liver support systems are summarised in fig 4 ▶. The results of trials using the biological liver support devices in ALF have been disappointing, with little evidence of significant benefits in terms of synthetic functions. This may be either because they are ineffective or the trials that have been performed with these devices have not allowed the effect of these devices to be explored fully. Stevens’ study using the bioartificial liver device illustrates this impeccably. Although their trial was in patients with ALF,46 for some strange reason patients with primary graft dysfunction were also included in the study. Although in the overall analysis they observed no significant differences in survival, a post hoc analysis revealed a near survival benefit in the ALF group. In addition, the group of patients that they chose to assess the efficacy of their liver support device was on a group of patients in whom recovery of native liver function, even with auxiliary liver transplant, can take up to a year. On the other hand, the use of MARS has been shown to improve systemic haemodynamics, severity of hepatic encephalopathy, and renal function, which has translated into improved survival in two small studies in patients with ACLF.17,54,55,60 These two randomised clinical trials illustrate that if treatment is applied before organ failure is manifest, progression to full blown ACLF may be prevented. In the study by Heemann et al,55 mortality in the control group was approximately 45% but in the study by Mitzner and colleagues54 mortality of the control group was 100%. Intervention with MARS resulted in an increase in survival in the Heemann study to over 90% but survival in MARS treated patients in the Mitzner study was approximately 20%. In our opinion, the timing of intervention with liver support is of critical importance in determining whether a device will improve outcome (fig 5 ▶). By the time multiorgan failure is manifest, the benefits of intervention with these devices is not likely to be fully realised.
Figure 4.
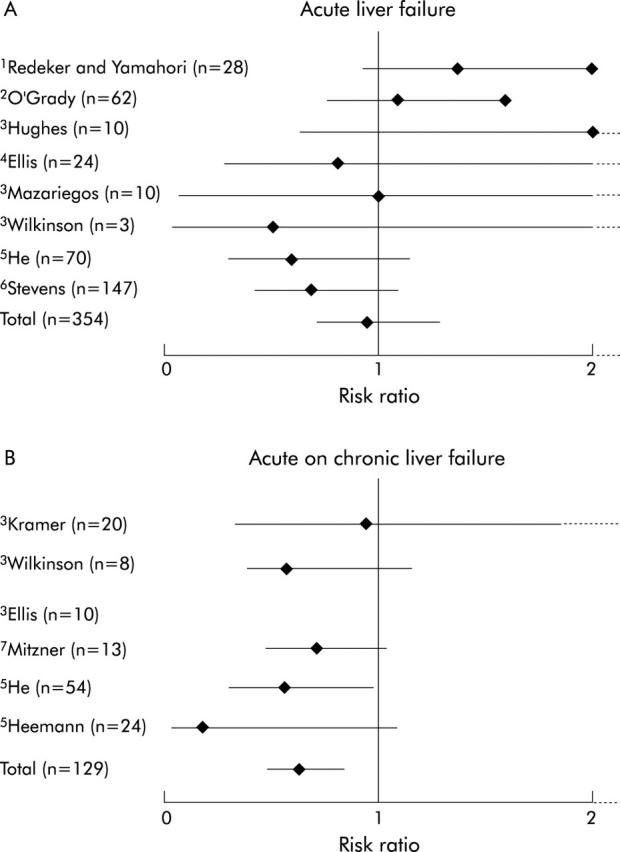
Randomised controlled trials with artificial and bioartificial liver support devices, showing effects on mortality in acute (A) and acute on chronic (B) liver failure.61 1, Whole blood exchange; 2, charcoal haemoperfusion; 3, Biologic-DT; 4, extracorporeal liver assist device (ELAD); 5, haemoperfusion; 6, BAL; and 7, molecular adsorbents recirculating system (MARS). The only device to show significant effects on mortality was the MARS device in the context of acute on chronic liver failure.
Figure 5.
Possibility of different clinical outcomes related to timing of therapeutic intervention in the course of acute deterioration of “stable cirrhosis”.
CURRENT STATUS OF LIVER SUPPORT DEVICES
The difficulties with the use of porcine hepatocytes for application in humans due to the perceived dangers of transmission of known and unknown retroviruses coupled with the lack of demonstrable efficacy of these devices in the human setting has led to the folding of the two companies that were supporting the development of Demetriou’s BAL and the ELAD device. Trials with AMC-BAL are currently stopped because of logistical difficulties introduced by the use of porcine cells in this system. MELS continues to be tested but its widespread application and trials is likely to be limited by the availability of human hepatocytes. Clearly, the factor that is likely to revive the bioartificial liver support systems is discovery of a new hepatocyte cell line which retains good functional capacity and can be scaled up to adequate quantities.
The best data are for MARS, and its use in clinical practice is supported by studies showing improvement in organ function with its use and early data showing improvement in survival in patients with ACLF. But an important question with the MARS device is whether the clinical course of these patients is altered and survival of the patient improved? Based on critical evaluation of the results presented above, guidelines have been drawn up as to the use and contraindications, and these are being followed in the present multicentre trial in the UK and are strongly recommended to all those who use it for such therapy for individual cases. Of particular importance is the clear appreciation that the use of this device should be contraindicated in cases of severe thrombocytopenia, progressive coagulopathy, and sepsis which together may be indicative of incipient DIC.
Not surprisingly, MARS is also being used to treat ALF. The largest single centre experiences from Helsinki suggest possible benefits in maintaining patients alive until the transplant can be performed and in some instances with spontaneous recovery. However, controlled clinical trials will be needed before any firm recommendations can be made. Such a trial is being planned in France but given the experience with trials of bioartificial devices in ALF, the timing of randomisation may well determine the outcome of the study. Further difficulties in the design and analysis of such trials are introduced by the effects of different timing of LTx. The device, as well as having uses in liver failure, would appear to be of clinical benefit in poisoning with protein bound drugs/toxins. This is related to the ability of MARS to remove protein bound drugs. In addition, early data show that MARS is effective in the treatment of pruritus in the context of cholestasis.
Clearly, the place of MARS in liver failure will only become proven when the results of current trials are completed. However, all this interest in MARS has resulted in the development of a great deal of interest in the pathophysiology of ACLF. There is a growing realisation that there are large numbers of patients occupying hospital beds who have a very high mortality and require considerable resources. With better understanding of the basis of acute decompensation, the availability of emerging medical therapies, as well as extracorporeal therapies such as MARS, one could hope to see a considerable improvement both in survival and in the reduction of hospital inpatient stay.
Summary.
Mortality in patients with liver failure remains unacceptably high. The major cause of liver failure in the UK is acute decompensation of cirrhosis and the incidence of acute liver failure in the UK is decreasing.
Acute decompensation of cirrhosis carries as poor a prognosis as ALF, and improvement in the outcome of these patients may be improved with earlier referral and emerging therapies.
The bioartificial liver systems have failed to live up to their initial promise and currently cannot be recommended for the treatment of patients outside of carefully controlled clinical trials.
-
MARS therapy has been shown to
- – alter some of the pathophysiological mechanisms thought to be important in the development of liver failure.
- – meta-analysis of the available data suggest that MARS may reduce mortality in patients with acute on chronic liver failure.
- – progressive thrombocytopenia, coagulopathy, and uncontrolled sepsis are relative contraindications to the use of MARS.
The first meeting attempting to define the pathophysiological basis, reversibility, prognostic factors, and the potential role of liver support in ACLF took place at the annual meeting of the American Association for the Study of Liver Diseases, 2003, Boston. The International Working Party emerging from this meeting in Boston will produce data in relation to ACLF and the role of emerging therapies by next years meeting in November.
Acknowledgments
We thank all the speakers of the Pre-BASL meeting “Prospects for Liver Support in the UK” held at UCL, and Dr D Shawcross for help with data collection. Part of this work was funded by the Sir Siegmund Warburg Settlement Fund.
REFERENCES
- 1.Stockmann HB, JN IJ. Prospects for the temporary treatment of acute liver failure. Eur J Gastroenterol Hepatol 2002;14:195–203. [DOI] [PubMed] [Google Scholar]
- 2.OPTN/SRTR data. 1August2002. www.optn.org.
- 3.Allen JW, Hassanein T, Bhatia SN. Advances in bioartificial liver devices. Hepatology 2001;34:447–55. [DOI] [PubMed] [Google Scholar]
- 4.Strain AJ, Neuberger JM. A bioartificial liver—state of the art. Science 2002;295:1005–9. [DOI] [PubMed] [Google Scholar]
- 5.Sussman NL, Chong MG, Koussayer T, et al. Reversal of fulminant hepatic failure using an extracorporeal liver assist device. Hepatology 1992;16:60–5. [DOI] [PubMed] [Google Scholar]
- 6.Rozga J, Williams F, Ro MS, et al. Development of a bioartificial liver: properties and function of a hollow-fiber module inoculated with liver cells. Hepatology 1993;17:258–65. [PubMed] [Google Scholar]
- 7.Flendrig LM, la Soe JW, Jorning GG, et al. In vitro evaluation of a novel bioreactor based on an integral oxygenator and a spirally wound nonwoven polyester matrix for hepatocyte culture as small aggregates. J Hepatol 1997;26:1379–92. [DOI] [PubMed] [Google Scholar]
- 8.Gerlach JC, Schnoy N, Encke J, et al. Improved hepatocyte in vitro maintenance in a culture model with woven multicompartment capillary systems: electron microscopy studies. Hepatology 1995;22:546–52. [PubMed] [Google Scholar]
- 9.O’Grady JG, Gimson AE, O’Brien CJ, et al. Controlled trials of charcoal hemoperfusion and prognostic factors in fulminant hepatic failure. Gastroenterology 1988;94:1186–92. [DOI] [PubMed] [Google Scholar]
- 10.McGuire BM, Sielaff TD, Nyberg SL, et al. Review of support systems used in the management of fulminant hepatic failure. Dig Dis 1995;13:379–88. [DOI] [PubMed] [Google Scholar]
- 11.Ash SR, Blake DE, Carr DJ, et al. Clinical effects of a sorbent suspension dialysis system in treatment of hepatic coma (the BioLogic-DT). Int J Artif Organs 1992;15:151–61. [PubMed] [Google Scholar]
- 12.Hughes RD, Pucknell A, Routley D, et al. Evaluation of the BioLogic-DT sorbent-suspension dialyser in patients with fulminant hepatic failure. Int J Artif Organs 1994;17:657–62. [PubMed] [Google Scholar]
- 13.Mitzner S, Stange J, Freytag J, et al. Role of transport proteins in bioartificial liver assist systems. Int J Artif Organs 1996;19:49–52. [PubMed] [Google Scholar]
- 14.Stange J, Mitzner S. A carrier-mediated transport of toxins in a hybrid membrane. Safety barrier between a patients blood and a bioartificial liver. Int J Artif Organs 1996;19:677–91. [PubMed] [Google Scholar]
- 15.Stange J, Mitzner S, Ramlow W, et al. A new procedure for the removal of protein bound drugs and toxins. Asaio J 1993;39:M621–5. [PubMed] [Google Scholar]
- 16.Mitzner SR, Stange J, Klammt S, et al. Extracorporeal detoxification using the molecular adsorbent recirculating system for critically ill patients with liver failure. J Am Soc Nephrol 2001;12(suppl 17):S75–82. [PubMed] [Google Scholar]
- 17.Sen S, Jalan R, Williams R. Extracorporeal albumin dialysis in acute-on-chronic liver failure: will it stand the test of time? Hepatology 2002;36:1014–16. [DOI] [PubMed] [Google Scholar]
- 18.Sen S, Williams R, Jalan R. The pathophysiological basis of acute-on-chronic liver failure. Liver 2002;22(Suppl 2):5–13. [DOI] [PubMed] [Google Scholar]
- 19.Hughes RD, Cochrane AM, Thomson AD, et al. The cytotoxicity of plasma from patients with acute hepatic failure to isolated rabbit hepatocytes. Br J Exp Pathol 1976;57:348–53. [PMC free article] [PubMed] [Google Scholar]
- 20.Nagasue N, Yukaya H, Ogawa Y, et al. Human liver regeneration after major hepatic resection. A study of normal liver and livers with chronic hepatitis and cirrhosis. Ann Surg 1987;206:30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arkadopoulos N, Lilja H, Suh KS, et al. Intrasplenic transplantation of allogeneic hepatocytes prolongs survival in anhepatic rats. Hepatology 1998;28:1365–70. [DOI] [PubMed] [Google Scholar]
- 22.Riordan SM, Williams R. Acute liver failure: Targeted artificial and hepatocyte-based support of liver regeneration and reversal of multiorgan failure. J Hepatol 2000;32(suppl 1):63–76. [DOI] [PubMed] [Google Scholar]
- 23.Wu L, Sun J, Wang L, et al. Cryopreservation of primary porcine hepatocytes for use in bioartificial liver support systems. Transplant Proc 2000;32:2271–2. [DOI] [PubMed] [Google Scholar]
- 24.Yagi T, Hardin JA, Valenzuela YM, et al. Caspase inhibition reduces apoptotic death of cryopreserved porcine hepatocytes. Hepatology 2001;33:1432–40. [DOI] [PubMed] [Google Scholar]
- 25.Baquerizo A, Mhoyan A, Shirwan H, et al. Xenoantibody response of patients with severe acute liver failure exposed to porcine antigens following treatment with a bioartificial liver. Transplant Proc 1997;29:964–5. [DOI] [PubMed] [Google Scholar]
- 26.Nyberg SL, Hibbs JR, Hardin JA, et al. Influence of human fulminant hepatic failure sera on endogenous retroviral expression in pig hepatocytes. Liver Transpl 2000;6:76–84. [DOI] [PubMed] [Google Scholar]
- 27.Patience C, Takeuchi Y, Weiss RA. Infection of human cells by an endogenous retrovirus of pigs. Nat Med 1997;3:282–6. [DOI] [PubMed] [Google Scholar]
- 28.Martin U, Kiessig V, Blusch JH, et al. Expression of pig endogenous retrovirus by primary porcine endothelial cells and infection of human cells. Lancet 1998;352:692–4. [DOI] [PubMed] [Google Scholar]
- 29.Wilson CA, Wong S, Muller J, et al. Type C retrovirus released from porcine primary peripheral blood mononuclear cells infects human cells. J Virol 1998;72:3082–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyberg SL, Hibbs JR, Hardin JA, et al. Transfer of porcine endogenous retrovirus across hollow fiber membranes: significance to a bioartificial liver. Transplantation 1999;67:1251–5. [DOI] [PubMed] [Google Scholar]
- 31.Martin U, Winkler ME, Id M, et al. Productive infection of primary human endothelial cells by pig endogenous retrovirus (PERV). Xenotransplantation 2000;7:138–42. [DOI] [PubMed] [Google Scholar]
- 32.Heneine W, Tibell A, Switzer WM, et al. No evidence of infection with porcine endogenous retrovirus in recipients of porcine islet-cell xenografts. Lancet 1998;352:695–9. [DOI] [PubMed] [Google Scholar]
- 33.Paradis K, Langford G, Long Z, et al. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. The XEN 111 Study Group. Science 1999;285:1236–41. [DOI] [PubMed] [Google Scholar]
- 34.Patience C, Patton GS, Takeuchi Y, et al. No evidence of pig DNA or retroviral infection in patients with short-term extracorporeal connection to pig kidneys. Lancet 1998;352:699–701. [DOI] [PubMed] [Google Scholar]
- 35.Kono Y, Yang S, Letarte M, et al. Establishment of a human hepatocyte line derived from primary culture in a collagen gel sandwich culture system. Exp Cell Res 1995;221:478–85. [DOI] [PubMed] [Google Scholar]
- 36.McCloskey P, Edwards RJ, Tootle R, et al. Resistance of three immortalized human hepatocyte cell lines to acetaminophen and N-acetyl-p-benzoquinoneimine toxicity. J Hepatol 1999;31:841–51. [DOI] [PubMed] [Google Scholar]
- 37.McCloskey P, Tootle R, Selden C, et al. Modulation of hepatocyte function in an immortalized human hepatocyte cell line following exposure to liver-failure plasma. Artif Organs 2002;26:340–8. [DOI] [PubMed] [Google Scholar]
- 38.Morsiani E, Pazzi P, Puviani AC, et al. Early experiences with a porcine hepatocyte-based bioartificial liver in acute hepatic failure patients. Int J Artif Organs 2002;25:192–202. [DOI] [PubMed] [Google Scholar]
- 39.Stange J, Ramlow W, Mitzner S, et al. Dialysis against a recycled albumin solution enables the removal of albumin-bound toxins. Artif Organs 1993;17:809–13. [DOI] [PubMed] [Google Scholar]
- 40.Hughes R, Ton HY, Langley P, et al. Albumin-coated Amberlite XAD-7 resin for hemoperfusion in acute liver failure. Part II: in vivo evaluation. Artif Organs 1979;3:23–6. [DOI] [PubMed] [Google Scholar]
- 41.Falkenhagen D, Strobl W, Vogt G, et al. Fractionated plasma separation and adsorption system: a novel system for blood purification to remove albumin bound substances. Artif Organs 1999;23:81–6. [DOI] [PubMed] [Google Scholar]
- 42.Rifai K, Ernst T, Kretschmer U, et al. Prometheus—a new extracorporeal system for the treatment of liver failure. J Hepatol 2003;39:984–90. [DOI] [PubMed] [Google Scholar]
- 43.Matsumura KN, Guevara GR, Huston H, et al. Hybrid bioartificial liver in hepatic failure: preliminary clinical report. Surgery 1987;101:99–103. [PubMed] [Google Scholar]
- 44.Chen SC, Hewitt WR, Watanabe FD, et al. Clinical experience with a porcine hepatocyte-based liver support system. Int J Artif Organs 1996;19:664–9. [PubMed] [Google Scholar]
- 45.Samuel D, Ichai P, Feray C, et al. Neurological improvement during bioartificial liver sessions in patients with acute liver failure awaiting transplantation. Transplantation 2002;73:257–64. [DOI] [PubMed] [Google Scholar]
- 46.Stevens AC, Busuttil R, Han S, et al. An interim analysis of a phase II/III prospective randomized, multicenter, controlled trial of the Hepatassist(R) bioartificial liver support system for the treatment of fulminant hepatic failure. Hepatology 2001;34:299A. [Google Scholar]
- 47.Ellis AJ, Hughes RD, Wendon JA, et al. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology 1996;24:1446–51. [DOI] [PubMed] [Google Scholar]
- 48.Millis JM, Kramer DJ, O’Grady J, et al. Results of phase I trial of the extracorporeal liver assist device for patients with fulminant hepatic failure. Am J Transplantation 2001;1(suppl 1):391. [Google Scholar]
- 49.van de Kerkhove MP, Di Florio E, Scuderi V, et al. Phase I clinical trial with the AMC-bioartificial liver. Academic Medical Center. Int J Artif Organs 2002;25:950–9. [DOI] [PubMed] [Google Scholar]
- 50.Sauer IM, Kardassis D, Zeillinger K, et al. Clinical extracorporeal hybrid liver support--phase I study with primary porcine liver cells. Xenotransplantation 2003;10:460–9. [DOI] [PubMed] [Google Scholar]
- 51.Mitzner SR, Klammt S, Peszynski P, et al. Improvement of multiple organ functions in hepatorenal syndrome during albumin dialysis with the molecular adsorbent recirculating system. Ther Apher 2001;5:417–22. [DOI] [PubMed] [Google Scholar]
- 52.Sorkine P, Ben Abraham R, Szold O, et al. Role of the molecular adsorbent recycling system (MARS) in the treatment of patients with acute exacerbation of chronic liver failure. Crit Care Med 2001;29:1332–6. [DOI] [PubMed] [Google Scholar]
- 53.Stange J, Mitzner SR, Risler T, et al. Molecular adsorbent recycling system (MARS): clinical results of a new membrane-based blood purification system for bioartificial liver support. Artif Organs 1999;23:319–30. [DOI] [PubMed] [Google Scholar]
- 54.Mitzner SR, Stange J, Klammt S, et al. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transpl 2000;6:277–86. [DOI] [PubMed] [Google Scholar]
- 55.Heemann U, Treichel U, Loock J, et al. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatology 2002;36:949–58. [DOI] [PubMed] [Google Scholar]
- 56.Sen S, Rose C, Ytrebo LM, et al. Molecular adsorbents recirculating systems (MARS): A potential model for removing midazolam, fentanyl and possibly other protein-bound drugs. Hepatology 2003;38:238A.12830007 [Google Scholar]
- 57.Sen S, Davies NA, Mookerjee RP, et al. Pathophysiological basis of extracorporeal albumin dialysis with MARS in acute-on-chronic liver failure due to alcohol. Hepatology 2003;38:239A. [Google Scholar]
- 58.Sen S, Jalan R, Williams R. Liver failure: basis of benefit of therapy with the molecular adsorbents recirculating system. Int J Biochem Cell Biol 2003;35:1306–11. [DOI] [PubMed] [Google Scholar]
- 59.Steiner C, Zinggrebe A, Viertler A. Experiences with MARS therapy in liver disease: analysis of 385 patients of the International MARS Registry. Hepatology 2003;38:239A. [Google Scholar]
- 60.Sen S, Steiner C, Williams R, et al. Artificial liver support: overview of registry and controlled clinical trials. In: Arroyo V, Forns X, Garcia-Pagan JC, eds. Progress in the treatment of liver diseases. Barcelona: Ars Medica, 2003:429–35.
- 61.Kjaergard LL, Liu J, Als-Nielsen B, et al. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure: a systematic review. JAMA 2003;289:217–22. [DOI] [PubMed] [Google Scholar]
- 62.Stange J, Mitzner SR, Klammt S, et al. Liver support by extracorporeal blood purification: a clinical observation. Liver Transpl 2000;6:603–13. [DOI] [PubMed] [Google Scholar]
- 63.Jalan R, Sen S, Steiner C, et al. Extracorporeal liver support with molecular adsorbents recirculating system in patients with severe acute alcoholic hepatitis. J Hepatol 2003;38:24–31. [DOI] [PubMed] [Google Scholar]