Abstract
Background: The role of genetics in the phenotypic manifestations of irritable bowel syndrome (IBS) is unclear. Our aims were: (1) to compare the prevalence of polymorphisms of alpha 2 (α2) adrenoceptors, norepinephrine transporter, and serotonin transporter protein (soluble carrier protein member 4 (SLC6A4)) promoter in patients with lower functional gastrointestinal disorders (FGID) and in healthy controls; and (2) to test associations of these genetic variations with symptoms of IBS and high somatic symptom scores.
Methods: Validated bowel and somatic symptom questionnaires characterised the phenotype: 90 with IBS constipation (IBS-C), 128 IBS diarrhoea, 38 IBS alternating bowel function, and 20 chronic abdominal pain. Logistic regression analyses assessed associations of different polymorphisms for α2 adrenoceptor and SLC6A4 with IBS or chronic abdominal pain phenotypes and high somatic score.
Results: Two distinct polymorphisms independently appeared to be associated with the phenotype IBS-C: α2C Del 322–325 (odds ratio (OR) 2.48 (95% confidence interval (CI) 0.98, 6.28); p = 0.05) and α2A −1291 (C→G) (OR 1.66 (95% CI 0.94, 2.92); p = 0.08) relative to wild-type. Overall, the α2C Del 322–325 polymorphism (alone or combined with other polymorphisms) was also significantly associated with a high somatic symptom score (OR 2.2 (95% CI 1.06, 4.64); p = 0.03). Combinations of polymorphisms were also associated with high somatic scores.
Conclusion: Functionally distinct α2A and α2C adrenoceptor and serotonin transporter polymorphisms are associated with constipation and high somatic symptoms in patients with lower functional gastrointestinal disorders, although the strength of the genetic contribution to the phenotype is unclear.
Keywords: genotype, somatic symptoms, irritable bowel syndrome, irritable bowel syndrome phenotype
Irritable bowel syndrome (IBS) is a biopsychosocial disorder affecting 9–17% of patients of all ethnic groups.1 It is associated with abnormal gastrointestinal motor function, visceral sensitivity, and psychosocial or autonomic dysfunction.2 Psychosocial factors, particularly stress, can alter colonic motility, enhance colonic sensation,2 and influence the timing of patients’ presentation to physicians.3,4
There is evidence of sympathetic adrenergic dysfunction in a subgroup of patients with IBS.5,6 Adrenergic agents alter the motor and sensory function of the human gastrointestinal tract.7–9 The most prominent effects in the human colon were observed with α2 agents.7 Twin studies suggest a genetic component in IBS.10–12 However, the influence of genetic factors that may modulate adrenergic and serotonergic functions in IBS is largely unknown.
Three human α2 adrenoceptor subtypes have been cloned and characterised: 2A, 2B, and 2C subtypes.13–16 Prejunctional α2A and α2C adrenoceptor subtypes regulate the release of norepinephrine from sympathetic nerves through negative feedback at presynaptic nerve endings. The potential of the α2 adrenoceptor subtypes in the motor and sensory dysfunctions of disturbances of gastrointestinal function is shown in the model in fig 1 ▶. It is conceivable that polymorphisms of the genes encoding for these receptors may result in loss of normal synaptic autoinhibitory feedback and enhanced presynaptic release of norepinephrine. Synaptic levels of norepinephrine are modified by the norepinephrine transporter (NET); a mutation of NET in one family has been associated with autonomic dysfunction.17 Genetic disorders of α2 mechanisms could conceivably alter functions of relevance to IBS: gut motility, pain sensation, autonomic imbalance, anxiety, and somatisation.
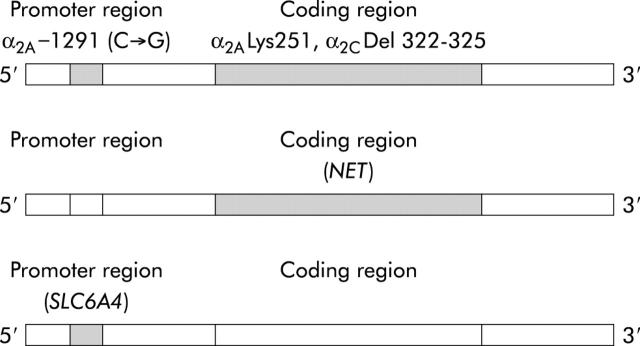
Figure 1.
Candidate polymorphic or mutated genes involved in altered adrenergic and serotonergic functions. Top: α2A and α2C adrenoreceptor polymorphisms. α2A −1291 (C→G), cytosine to guanine transversion in promoter of α2A receptor protein; α2ALys251, a cytosine to guanine transversion at position 753 that changes amino acid 251 of the third intracellular loop of the α2A adrenoceptor from asparagine to lysine; α2C Del 322–325, deletion in third intracellular loop of α2C adrenoceptor protein. Middle: norepinephrine transporter (NET). Bottom: solute carrier family 6 (neurotransmitter transporter, serotonin), member 4 (SLC6A4) (serotonin transporter promoter (SERT-P)).
Serotonin (5-hydroxytryptamine (5-HT)) modulates sensorimotor functions in the digestive tract. There are seven subclasses of serotonergic receptors, differentiated on the basis of structure, molecular mechanism, and function.18 The actions of enteric 5-HT are terminated by reuptake by the 5-HT transporter (SERT). SERT in the gut is similar to that in the brain of the same species19; gastrointestinal motility is abnormal in SERT knockout mice.20 The approved gene symbol for SERT is SLC6A4 (solute carrier family 6 (neurotransmitter transporter, serotonin), member 4); this abbreviation will be used in the remainder of this manuscript. Adaptive changes occur in the subunit composition of enteric 5-HT3 receptors in SLC6A4 knockout mice. Such changes are reflected in altered 5-HT3 receptor affinity and desensitisation and hence in function in response of the receptor to 5-HT released from enteroendocrine cells.21 Serotonin type 3 (5-HT3) receptors are involved in the colonic motor response to feeding in health and IBS.18 The colonic transit response to the 5-HT3 antagonist, alosetron, in IBS patients with diarrhoea is influenced by the genotype controlling the promoter for SLC6A4.22
There is also evidence that the two biogenic amines, serotonin and norepinephrine, interact in modulating gastrointestinal functions. For example, norepinephrine causes 5-HT release from enterochromaffin cells in mouse ileal tissues via α2 adrenoceptor subtypes coupled to a pertussis toxin sensitive G protein23 or via β2 adrenoceptors in rat duodenal mucosa.24 Thus we were interested in exploring the potential combined effects of genetic mechanisms that influence serotonin and norepinephrine. Similarly, underactivation of serotonergic function and overactivation of noradrenergic function modulate the brain circuitry involved in euthymic and abnormal mood and anxiety states.25 Thus altered control of noradrenergic and serotonergic systems may result in symptoms of depression and anxiety, which are frequently associated with IBS at the time of presentation to physicians.
Selection of candidate genes for association studies
α2A and α2C adrenoceptor polymorphisms can act synergistically to alter the feedback regulation of norepinephrine release through their effect on the prejunctional α2 adrenoceptor.16 Moreover, most of the presynaptic receptors inhibiting acetylcholine release are of the α2A subtype.26 α2A Adrenoceptors are located on somatic and visceral afferents and may be associated with reporting of chronic abdominal pain and somatic symptoms.27 α2B Adrenoceptor gene polymorphisms were not included in our candidate gene approach as the biological action of α2B adrenoceptors on vascular function (for example, hypertension) appears dependent on a sodium retention state, and renal medullary actions (for example, release of nitric oxide) also counteract the hypertensive effect of norepinephrine27,28 mediated through the α2B adrenoceptor.
Norepinephrine reuptake requires a transporter protein (NET). A mutation of the gene encoding NET17 results in a non-functional NET, increased norepinephrine, and functional overstimulation of the sympathetic nervous system in response to physiological stimuli, such as orthostatic hypotension syndrome, neurocirculatory asthenia, and chronic fatigue syndrome.
The actions of enteric 5-HT are terminated by 5-HT transporter mediated uptake. We elected to study a polymorphism in the functional promoter region. This has been associated with gastrointestinal and neurobiological dysfunctions,20,21,29 neuropsychiatric disorders,29 and with altered response of IBS patients to a 5-HT3 antagonist.22
Homozygous wild-type or long alleles reflect normal function. For the association studies performed, we assumed that the gene confers a functional disadvantage if there was a homozygous (short) or heterozygous polymorphism. This was based on the literature that shows that the heterozygote state in knockout mice confers a change in biological function that mirrors that of the homozygous state. Thus, for example, marked increases in the stress hormone adrenocorticotropin were found in the plasma of homozygous −/− and heterozygous +/− knockout mice compared with their control littermates. These data suggest that homozygous short and heterozygous SLC6A4 genotypes in mice are both associated with an increased stress-responsive phenotype.30 Moreover, the SLC6A4 heterozygous state was associated with reduced colonic transit response to alosetron compared with wild-type.22 There is also evidence that heterozygous variation in α2 receptors may impart a change in some α2 mediated functions or responses to medications. α2 Agonist treatment of mice heterozygous for the α2A adrenoceptor (α2A adrenoceptor +/−) lowers blood pressure without inducing sedation.31 Thus there is compelling evidence to hypothesise that the heterozygous polymorphisms may be biologically relevant.
Study hypothesis and aims
Our hypothesis is that single or combined polymorphisms of α2 adrenoceptors and serotonergic receptors are associated with IBS phenotypes and somatic symptom scores. To assess the potential role of genetic determinants in IBS, we considered five polymorphisms in four candidate genes or their promoters. The polymorphisms selected for study modify adrenergic or serotonergic functions, and have been previously demonstrated to affect smooth muscle function, visceral sensation, bioamine metabolism, sympathetic function (for example, cardiac or vascular tone), or psychological state.32–35
The aims of this study were: (1) to compare the distributions of polymorphisms of α2A and α2C adrenoceptors, norepinephrine transporter, and serotonin transporter protein promoter in patients with lower functional gastrointestinal disorders (FGID—that is, IBS or chronic functional abdominal pain (CAP)) and in healthy controls; and (2) to assess the association of these polymorphisms with phenotypes of IBS and CAP, and with high somatic symptom scores.
METHODS
Asymptomatic healthy controls and patients with IBS
All participants (18–75 years) completed a validated bowel disease questionnaire (including questions that corresponded to Rome II criteria2) and a somatic symptom checklist.36 The latter has been used extensively in the literature to identify patients with a propensity to report somatic symptoms.36 The somatic symptom score was summarised as a mean of the frequency and severity scores over the 16 items, each recorded on a scale of 0–4.
Symptoms surveyed were: headache, backache, wheezing, trouble breathing, difficulty sleeping, fatigue (tiredness), depression (feeling sad or blue), general stiffness, palpitations, joint pains, eye pain associated with reading, dizziness, weakness, nervousness (or shakiness), hot or cold spells, and high blood pressure. Subjects were classified as having a high somatic score when a subject’s mean score across the 16 domains was >0.75, which was the 90th percentile of mean scores in the healthy participants in this study. We have used these two questionnaires extensively in epidemiological studies (for example, in patients with diabetes).37
IBS participants were selected from an administrative database of 752 patients with IBS residing within a 150 mile radius of Rochester, Minnesota, USA, and were recruited by mailing. All IBS patients had already been evaluated by a staff gastroenterologist using clinically indicated tests, including endoscopy, biopsies, and tests of rectal evacuation. Healthy volunteers were recruited by public advertisement in Rochester, Minnesota. All participants gave informed consent for the study which was approved by the Mayo Foundation Institutional Review Board.
There is evidence of a significant likelihood of category transitions38 between constipation predominant IBS (IBS-C) and functional constipation (FC), and similar transitions in the categories of diarrhoea predominant IBS (IBS-D) and functional diarrhoea (FD). Hence we have grouped patients whose symptoms at the time of the questionnaire suggested IBS-C and FC into one group (designated IBS-C) and those with IBS-D and FD into a second group (designated IBS-D).
DNA analysis by polymerase chain reaction amplification, identification, and sequencing
Venous blood drawn from a forearm vein was stored as de-identified samples. We isolated DNA from whole blood from 394 participants by the alkaline lysis method using the QIAamp DNA Blood Maxi Kit, (Qiagen Inc., Valencia, California, USA). Molecular assays were adapted from previously published papers to detect the candidate mutations or polymorphisms of interest.
We used the polymerase chain reaction (PCR) based fragment length assays to identify polymorphisms for the α2C and α2A adrenoceptor coding regions and in the α2A adrenoceptor promoter. We confirmed polymorphisms by direct sequencing. Where there was no restriction site available, as for NET and the SLC6A4, polymorphisms were identified by direct sequence alone.
Sequences used in these studies were obtained from GenBank/EBI Data Bank, and accession numbers are given below. Briefly, we performed PCR amplification using TaKaRa LA Taq with GC buffers (TaKaRa Shuzo Co., Ltd, Japan) in a total volume of 50 μl solution containing 750 ng template DNA, 0.4 μM primers, 2.5 units TaKaRa LA Taq, 20 mM dNTPs, and GC buffer I containing 2.5 mM MgCl2. After denaturing DNA samples at 94°C for one minute, we set up cycling conditions at 30 cycles of 94°C for 30 seconds, 60°C for 30 seconds, 72°C for two minutes followed by an extension at 72°C for five minutes. The polymorphisms were amplified using a Perkin Elmer Gene Amp 9700 PCR thermal cycler.
α2C and α2A PCR products were digested for one hour and visualised by electrophoresis using 3% metaphor agarose (BioWhittaker Molecular Applications Inc., Rockland, Maine, USA). Polymorphisms were confirmed by direct sequencing performed at the Mayo Molecular Biology Core Facility using an ABI PRISM 377 DNA sequencer with XL Upgrade and 96 well Upgrade (Perkin-Elmer Applied Biosystems, Foster City, California, USA).
Detection of polymorphisms in the α2 adrenoceptor subtypes
(A) Within the promoter region for the α2A adrenoceptor, a C to G transversion results in a Msp1 restriction fragment length polymorphism located at −1291 base pairs upstream of the origin of transcription.39 This polymorphism is designated by the abbreviation α2A −1291 (C→G). Genotype GG has been detected as an apolymorphic band of 174 bp, and genotype CC as two polymorphic bands of 121 and 53 bp. In a Japanese control group, 36% had C allele and 64% G allele, with GG homozygosity in 16%, CG heterozygosity in 40%, and CC homozygosity in 44%.39 In contrast, a study of White Swedish men revealed allele frequencies of 23% allele C and 77% allele G, with no CC, 46% CG, and 54% GG genotypes.40 Data from European American healthy controls have not been reported previously. Altered expression of the gene causes loss of receptor function.
GenBank accession number was #M23533. A 523 bp region containing the Msp1 polymorphic site was amplified by PCR using the following primers: sense position 661: 5′-TCACACCGGA GGTTACTTCCCTCG 3′ and antisense position 1165: 5′-TCCGACGACA GCGCGAGTT 3′. The Msp1 restriction enzyme (New England Biolabs, Beverly, Massachusetts, USA) was used at 100 units per reaction.
(B) A single nucleotide polymorphism at position 753 consists of a C to G transversion that changes amino acid 251 of the third intracellular loop of the α2A adrenoceptor from asparagine to lysine (α2ALys251). This polymorphism results in enhanced Gi protein coupling and increased agonist promoted receptor function. The polymorphism is identified by the restriction enzyme Sty1, and it shows a higher prevalence in African-Americans (0.04) than in European Americans (0.004).41 GenBank accession number was #AF281308. A 310 bp region containing the polymorphic site was amplified by PCR using the following primers: sense position 581: 5′-AGTGGTACGTCATCTCGTCG 3′ and antisense position 871: 5′-GAGCTCTCCTCCAGGTCCAG 3′. The Sty1 restriction enzyme (Invitrogen, Carlsbad, California, USA) was used at 10 units per reaction.
(C) There is a 12 bp deletion beginning with nucleotide 964 in the region coding for the third intracellular loop of the α2C adrenoceptor.42 It results in loss of four amino acids (Gly-Ala-Gly-Pro) at positions 322–325 (denoted α2C Del 322–325), and loss of an Nci1 restriction site. The ethnic distribution of this polymorphism in controls in a US population showed an allele frequency of 41.1% in African-Americans and 3.8% in European Americans. The genotype distribution for the two groups were, respectively: wild-type 34.5% and 94.3%; heterozygous 48.8% and 3.8%, and homozygous deleted 16.6% and 1.9%. GenBank accession number was #J03853. A 723 bp region containing the polymorphic site was amplified by PCR using the following primers: sense position 547: 5′-CCACCATCGT CGCCGTGTGG CTCATCT 3′ and antisense position 1246: 5′-AGGCCTCGCG GCAGATGCCG TACA 3′. The Nci1 restriction enzyme (New England Biolabs) was used at 20 units per reaction.
Detection of norepinephrine transporter (NET) gene mutation
A guanine (G) to cytosine(C) missense mutation in nucleotide 237 of the coding region of NET is associated with Ala457Pro mutation.17 For examination of the NET gene, upstream and downstream primers were synthesised as follows: sense 5′ CCGGAAACTCTTCACATTTG 3′ and antisense 5′ CGCTGAATTGAGGATGCTGG 3′.16 GenBank accession number was #X76753. PCR products were visualised on a 1% agarose gel and confirmed by direct sequencing.
Detection of polymorphism in the serotonin transporter protein promoter
A polymorphism in the 5′ promoter region (5′-HTTLPR) of SLC6A4 (42) consists of a repetitive sequence of 22 bp located 1 kb upstream of the SLC6A4 transcription start site.29 This polymorphism is biallelic in most populations. The allele with the smallest number of repeats, commonly called the short (S) allele, has lower transcriptional activity, leading to marked reductions in messenger RNA levels, 5-HT binding, and 5-HT uptake in both platelets and lymphoblasts compared with the long (L) allele.29 In European-Americans, the S and L alleles have generally been observed to occur at frequencies of approximately 0.43 and 0.57 with wild-type (LL) 32%, heterozygous 49%, and homozygous short 19%.29
Identification of the polymorphisms in the promoter for SLC6A4, the serotonin transporter protein, was by PCR based fragment length polymorphisms.22 GenBank accession number was #X76753. We synthesised oligonucleotide primers flanking the long polymorphic region corresponding to the nucleotide positions 1671 sense 5′-GCCGCTCTGAATGCCAGCAC 3′ and position 2219 antisense 5′-GGAGGAACTGACC-CCTGAAAACTG 3′ to generate 572 bp PCR amplified fragments.
Data and statistical analysis
Logistic regression models were used to estimate the associations (odds ratios (OR)) for specific phenotypes of IBS and high somatic symptom scores with the different adrenergic and serotonergic polymorphisms.
A high somatic symptom score was defined based on the subject’s mean score greater than the 90th percentile of healthy participants. The odds ratios (95% confidence interval CI)) for a specific phenotype were computed from the estimated logistic regression model coefficients (and their standard errors) examining individual polymorphisms or their combinations relative to the homozygous type. Race and sex were included as covariates in each of the logistic regression models. The statistical software used for all analyses was SAS.
After the study was completed, we estimated the effect size detectable given the number of subjects in different subgroups included in this study.
RESULTS
Lower FGID symptoms and high somatic symptom scores
The symptom phenotypes of patients with lower FGID were: 90 IBS-C, 128 IBS-D, 38 IBS alternating bowel function, and 20 CAP. The demographics of patients in the different subgroups are shown in table 1 ▶. Female participants predominated in the patient (82%) and control (79%) groups. Caucasian patients were 89% of controls and 97% of patients; there were 6% Asian and 3% Hispanic healthy participants. Other racial groups were <1% in the two groups.
Table 1.
Patient characteristics, prevalence of SLC6A4, α2C Del 322–325, and α2A −1291 (C→G) polymorphisms, and mean somatic score (SoSC) in 276 patients with lower functional gastrointestinal disorders (FGID) and 120 healthy controls
| Controls | Literature controls* (%) | All Lower FGID Patients | IBS-C | IBS-D | IBS-Alt | CAP | |
| N | 120 | 276 | 90 | 128 | 38 | 20 | |
| Age (mean, range) | 36 (18–72) | 49 (18–82) | 52 (23–82) | 47 (18–77) | 47 (18–69) | 47 (26–78) | |
| Sex (n (% female)) | 95 (79) | 226 (82) | 83 (92) | 98 (77) | 30 (79) | 15 (75) | |
| SoSC (mean (SD)) | 0.31 (0.31) | 0.9 (0.6) | 0.89 (0.57) | 0.83 (0.55) | 0.93 (0.65) | 0.84 (0.62) | |
| Caucasian (n (%)) | 107 (89) | 267 (97) | 86 (96) | 124 (97) | 37 (97) | 20 (100) | |
| SLC6A4 | |||||||
| Wild-type (%) | 31 | 36 | 36 | 40 | 32 | 42 | 35 |
| Heterozygous (%) | 49 | 47 | 46 | 43 | 48 | 45 | 45 |
| Homozygous polymorphism (%) | 20 | 16 | 18 | 17 | 20 | 13 | 20 |
| Any polymorphism (%) | 69 | 63 | 64 | 60 | 68 | 58 | 65 |
| α2C Del 322–325 | |||||||
| Wild-type (%) | 92 | 94 | 89 | 85 | 91 | 86 | 95 |
| Heterozygous (%) | 6 | 4 | 9 | 11 | 7 | 11 | 5 |
| Homozygous polymorphism (%) | 2 | 2 | 2 | 4 | 2 | 3 | 0 |
| Any polymorphism (%) | 8 | 6 | 11 | 15 | 9 | 14 | 5 |
| α2A −1291 (C→G) | |||||||
| Wild-type(%) | 57 | 48 | 52 | 45 | 55 | 53 | 55 |
| Heterozygous (%) | 36 | 43 | 43 | 47 | 39 | 47 | 45 |
| Homozygous polymorphism(%) | 7 | 8 | 5 | 8 | 6 | 0 | 0 |
| Any polymorphism (%) | 43 | 51 | 48 | 55 | 45 | 47 | 45 |
SoSC, mean somatic symptom score (mean often and mean bothersome scores for 16 symptom “items”). Each “item” is on based on a scale of 0–4.
Lower FGID group comprises the four groups: irritable bowel syndrome with predominant constipation (IBS-C), irritable bowel syndrome with predominant diarrhoea (IBS-D), irritable bowel syndrome with alternating bowel function (IBS-Alt), and chronic abdominal pain (CAP).
*Genotype frequencies for controls are from: Serretti and colleagues,49 Small and colleagues,46 and Tsai and colleagues.50
Lower FGID was associated with significantly higher somatic symptom scores relative to healthy controls (p<0.05).
Prevalence of polymorphisms in lower FGID
α2ALys251 and NET polymorphisms were not identified in the first 100 patients and 20 controls, and were not measured in the remaining participants.
The distributions of polymorphisms for SLC6A4, α2C Del 322–325, and α2A −1291 (C→G) were not significantly different between patients with lower FGID compared with controls (table 1 ▶). The distribution of these polymorphisms in the controls of the present study was similar to the distribution in control populations from previous studies.43–45 In tables 2 ▶ and 3 ▶, we provide the distribution of lower FGID phenotypes and controls in each genotype category or combinations of genotypes.
Table 2.
Distributions (%) of irritable bowel syndrome and chronic abdominal pain phenotypes, and controls, in each genotype category
| n* | Controls (%) | IBS-C (%) | IBS-D (%) | IBS-Alt (%) | CAP (%) | |
| SLC6A4 wild-type | 137 | 27 | 26 | 30 | 12 | 5 |
| Heterozygous | 185 | 32 | 21 | 33 | 9 | 5 |
| Homozygous polymorphism | 74 | 33 | 20 | 35 | 7 | 5 |
| Any polymorphism | 259 | 32 | 21 | 34 | 8 | 5 |
| α2C Del 322–325 wild-type | 348 | 32 | 21 | 33 | 9 | 5 |
| Heterozygous | 31 | 23 | 32 | 29 | 13 | 3 |
| Homozygous polymorphism | 8 | 25 | 38 | 25 | 12 | 0 |
| Any polymorphism | 39 | 23 | 33 | 28 | 13 | 3 |
| α2A −1291 (C→G) wild-type | 210 | 33 | 19 | 34 | 9 | 5 |
| Heterozygous | 160 | 27 | 26 | 31 | 10 | 6 |
| Homozygous polymorphism | 22 | 36 | 32 | 32 | 0 | 0 |
| Any polymorphism | 182 | 28 | 26 | 31 | 10 | 5 |
*A total of 396 (276 patients and 120 controls) had SLC6A4, 387 had α2C Del 322−325, and 392 had α2A −1291(C→G) genotypes assayed.
IBS-C, irritable bowel syndrome with predominant constipation; IBS-D, irritable bowel syndrome with predominant diarrhoea; IBS-Alt, irritable bowel syndrome with alternating bowel function; CAP, chronic abdominal pain.
Table 3.
Distributions (%) of different phenotypes by combinations of different serotonergic and adrenergic genotype. n refers to number of people (patients and controls) with a specified genotype or genotype combination
| n | SLC 6A4 | α2C Del 322–325 | α2A −1291 (C→G) | Controls (%) | IBS-C (%) | IBS-D (%) | IBS-Alt (%) | CAP (%) |
| 58 | Wt | Wt | Wt | 31 | 24 | 28 | 12 | 5 |
| 59 | Wt | Wt | Het/hom | 24 | 27 | 32 | 10 | 7 |
| 9 | Wt | Het/hom | Wt | 22 | 44 | 33 | 0 | 0 |
| 7 | Wt | Het/hom | Het/hom | 29 | 0 | 42 | 29 | 0 |
| 126 | Het/hom | Wt | Wt | 36 | 13 | 37 | 9 | 5 |
| 103 | Het/hom | Wt | Het/hom | 31 | 25 | 32 | 7 | 5 |
| 12 | Het/hom | Het/hom | Wt | 25 | 34 | 25 | 8 | 8 |
| 11 | Het/hom | Het/hom | Het/hom | 18 | 46 | 18 | 18 | 0 |
Wt, wild-type; Het, heterozygous; hom, homozygous polymorphism.
IBS-C, irritable bowel syndrome with predominant constipation; IBS-D, irritable bowel syndrome with predominant diarrhoea; IBS-Alt, irritable bowel syndrome with alternating bowel function; CAP, chronic abdominal pain.
Odds ratios for the associations between the different polymorphisms and FGID phenotypes are shown in table 4 ▶ and fig 2 ▶. The presence of either α2C Del 322–325 or α2A −1291 (C→G) polymorphism appeared to be associated with an increased OR (95% CI) for the phenotype of IBS-C versus controls: α2C Del 322–325, OR = 2.48 (0.98, 6.28), p = 0.05 and α2A −1291 (C→G) OR = 1.66 (0.94, 2.92), p = 0.08. There were no significant associations between the genetic polymorphisms tested and other phenotypes of IBS or CAP.
Table 4.
Odds ratio (95% CI) for functional gastrointestinal disorder phenotype versus controls in polymorphic (heterozygous or homozygous polymorphism) relative to respective wild-type genotypes
| IBS-C | IBS-D | IBS-Alt | CAP | |
| SLC6A4 | 0.7 (0.4, 1.2) | 1.0 (0.6, 1.7) | 0.7 (0.3, 1.4) | 0.9 (0.3, 2.5) |
| α2C Del 322–325 | 2.5 (1.0, 6.3) | 1.3 (0.5, 3.4) | 2.2 (0.7, 7.1) | 0.8 (0.1, 6.8) |
| α2A −1291 (C→G) | 1.7 (0.9, 2.9) | 1.2 (0.7, 2.0) | 1.3 (0.6, 2.8) | 1.2 (0.5, 3.2) |
| α2A −1291 (C→G) and SLC6A4 | 1.1 (0.5, 2.7) | 1.4 (0.6, 3.2) | 0.7 (0.2, 2.2) | 1.2 (0.3, 5.6) |
| α2C Del 322–325 and SLC6A4 | 2.2 (0.4, 12.1) | 1.3 (0.2, 7.5) | 1.0 (0.1, 11.7) | 2.4 (0.2, 32.4) |
| α2A −1291 (C→G) and α2C Del 322–325 | NE | 2.3 (0.3, 16.0) | 3.3 (0.4, 30.4) | NE |
| α2A −1291 (C→G) and SLC6A4 and α2C Del 322–325 | 4.1 (0.6, 25.8) | 1.5 (0.2, 12.7) | 3.5 (0.4, 31.8) | NE |
SLC6A4 (heterozygous or homozygous polymorphism).
α2C Del 322–325 (heterozygous or homozygous polymorphism).
α2A −1291 (C→G) (heterozygous or homozygous polymorphism).
IBS-C, irritable bowel syndrome with predominant constipation; IBS-D, irritable bowel syndrome with predominant diarrhoea; IBS-Alt, irritable bowel syndrome with alternating bowel function; CAP, chronic abdominal pain; NE, non-estimable.
Figure 2.
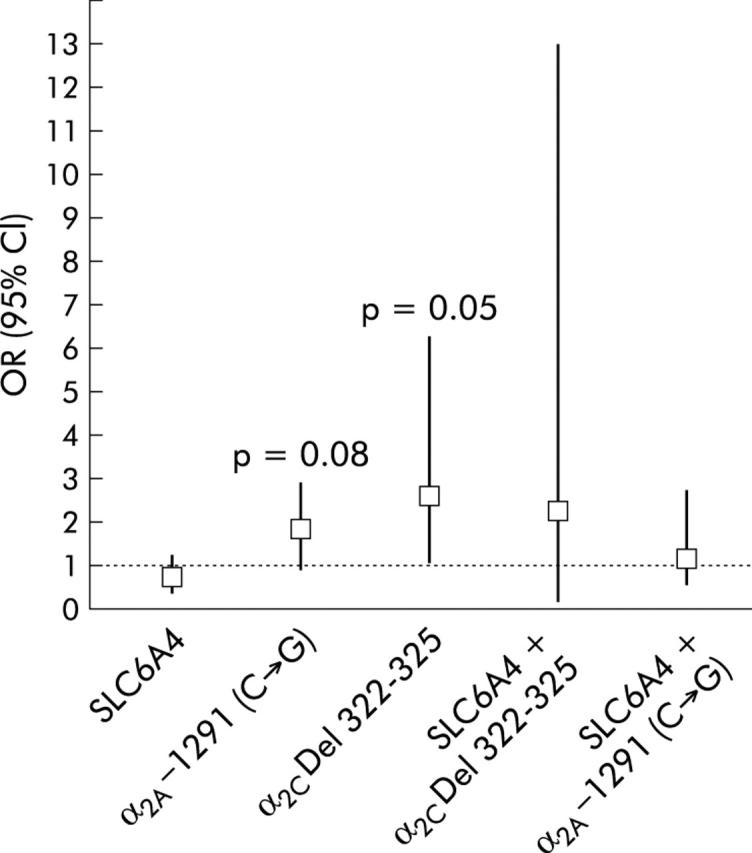
Odds ratio (95% confidence interval (CI)) for irritable bowel syndrome (IBS) constipation phenotype versus controls in polymorphic relative to respective wild-type genotypes. Note the significant association between the constipation predominant phenotype of IBS and the α2C Del 322–325 deletion, which alters the coding region of the gene.
Association of polymorphisms with somatic symptoms in lower FGID
The mean somatic symptom scores, percentage of high somatic scores, and the associations with different polymorphisms are shown in table 5 ▶. Overall, the α2C Del 322–325 polymorphism (alone or combined with other polymorphism) was significantly associated with a high somatic symptom score (OR = 2.2 (1.06, 4.64); p = 0.03). Combinations of polymorphisms were also associated with high somatic symptom scores: α2C Del 322–325 with SLC6A4 (OR = 5.0 (1.11, 22.22); p = 0.04) and α2C Del 322–325 with α2A −1291 (C→G) (OR = 11.1 (1.15, 108.1); p = 0.04) relative to respective wild-type genotype(s). The significant OR for combined α2C Del 322–325 with α2A −1291 (C→G) polymorphisms had a wide confidence interval due to the small sample size.
Table 5.
Mean somatic scores (SoSc) and percentage of abnormal SoSc for each genotype combination in patients with lower functional gastrointestinal disorders and healthy controls
| N | SLC6A4 | α2C Del 322–325 | α2A −1291 (C→G) | SoSC score (mean) (SD) | % High score | OR (95% CI)* |
| 51 | Wt | Wt | Wt | 0.67 (0.57) | 33% | 1.0 (ref) |
| 51 | Wt | Wt | Het/hom | 0.74 (0.49) | 41% | 1.4 (0.6,3.1) |
| 9 | Wt | Het/hom | Wt | 0.77 (0.65) | 44% | 1.7 (0.4,7.3) |
| 6 | Wt | Het/hom | Het/hom | 0.94 (0.24) | 83% | 11.1 (1.1, 100.1) |
| 108 | Het/hom | Wt | Wt | 0.59 (0.57) | 30% | 0.8 (0.4, 1.7) |
| 90 | Het/hom | Wt | Het/hom | 0.76 (0.58) | 40% | 1.3 (0.6, 7.8) |
| 10 | Het/hom | Het/hom | Wt | 1.23 (0.79) | 70% | 5.0 (1.1, 22.2) |
| 9 | Het/hom | Het/hom | Het/hom | 0.53 (0.43) | 22% | 0.6 (0.1, 3.4) |
The high somatic symptom score refers to >0.75, the 90th percentile for healthy controls.
*Estimated odds ratio (95% confidence interval) from logistic regression model adjusting for race and sex.
Wt, wild-type; Het, Heterozygous; hom, homozygous polymorphism.
Statistical power to detect associations
Table 6 ▶ summarises the “degree of association” between the presence/absence of specific polymorphisms in α2C Del 322–325, α2A −1291 (C→G), and SLC6A4 genes versus specific symptom subgroups (including overall FGID). This assessment was based on a comparison of the observed proportion of patients in the group without a specific polymorphism (number of patients divided by the sum of the number of patients plus number of controls) and the proportion that could have been detected among those with the corresponding polymorphism.
Table 6.
Statistical power to detect genotype differences with this study sample
| No with genotype SLC6A4 (Wt) | % with phenotype | No with genotype SLC6A4 (het/hom) | ⩾80% power† to detect this proportion (%) with phenotype |
| 137 | Any FGID: 73% | 259 | Any FGID: ⩾85% (or⩽61%) |
| 73 | IBS-C: 49% | 137 | IBS-C: ⩾69% (or ⩽29%) |
| 78 | IBS-D: 53% | 170 | IBS-D: ⩾72% (or ⩽32%) |
| No with genotype α2C Del 322–325 (Wt) | % with phenotype | No with genotype α2C Del 322–325 (het/hom) | ⩾80% power† to detect this proportion (%) with phenotype |
| 348 | Any FGID: 68% | 39 | Any FGID: ⩾88% (or ⩽48%) |
| 183 | IBS-C: 40% | 22 | IBS-C: ⩾71% (or ⩽9%) |
| 224 | IBS-D: 51% | 20 | IBS-D: ⩾82% (or ⩽20%) |
| No with genotype α2A −1291 (C→G) (Wt) | % with phenotype | No with genotype α2A −1291 (C→G) (het/hom) | ⩾80% power† to detect this proportion (%) with phenotype |
| 210 | Any FGID: 67% | 182 | Any FGID: ⩾80% (or ⩽54%) |
| 109 | IBS-C: 37% | 99 | IBS-C: ⩾57% (or ⩽17%) |
| 140 | IBS-D: 51% | 108 | IBS-D: ⩾69% (or ⩽33%) |
| No with genotype α2A −1291 (C→G) (Wt) and SLC6A4 (Wt) | % with phenotype | No with genotype α2A −1291 (C→G) (het/hom) and SLC6A4 (het/hom) | ⩾80% power† to detect this proportion (%) with phenotype |
| †Based on two sample test for proportions using a two sided alpha level of 0.05. | |||
| IBS-C, irritable bowel syndrome with predominant constipation; IBS-D, irritable bowel syndrome with predominant diarrhoea; FGID, functional gastrointestinal disorders. | |||
| 68 | Any FGID: 71% | 115 | Any FGID: >88% (or <52%) |
| 39 | IBS-C: 49% | 66 | IBS-C: >76% (or <22%) |
| 39 | IBS-D: 49% | 69 | IBS-D: >76% (or <22%) |
The data indicate that clinically meaningful associations of these three candidate genotypes could have been detected with at least 80% power—for example, a difference in prevalence of wild-type versus polymorphic genotypes of 12% for all lower FGID, and 19–20% for IBS-C and IBS-D.
DISCUSSION
We have observed associations between functionally distinct genetic polymorphisms (α2A and α2C adrenoceptor and SLC6A4) and specific phenotypes in patients with IBS. Our data suggest that the α2C Del 322–325 adrenoceptor polymorphism is associated with increased odds for constipation (p = 0.05) and, separately, increased odds for higher frequency and severity of somatic symptoms in participants with lower functional gastrointestinal disorders. These studies are quite challenging in a functional disorder such as the irritable bowel syndrome, given the fluctuation in symptoms that characterise the phenotype; in future studies, we also perceive that attention needs to be given to the stability of symptoms over time as the phenotype is characterised.
Combinations of α2A and α2C polymorphisms or α2C Del 322–325 adrenoceptor and SLC6A4 polymorphisms also showed significant associations with high somatic scores. Combinations of polymorphisms may be functionally important. For example, the combined β1 adrenoceptor and α2C adrenoceptor polymorphisms were reported to predispose to heart failure.46 Norepinephrine causes 5-HT release from enterochromaffin cells in mouse ileal tissues via α2 adrenoceptors.23 Functional alteration of both serotonin and noradrenergic control by the concurrence of α2C Del 322–325 adrenoceptor and SLC6A4 polymorphisms may also influence gastrointestinal functions.
The α2 adrenoceptor polymorphisms tested alter the third intracellular loop (which binds to G proteins) or synthesis of the receptor; association of distinct α2 adrenoceptor genotypes with the IBS constipation phenotype supports a role for genetic predisposition in IBS in twin studies and epidemiological reports.10–12 These genetic variations may alter manifestations of the disorder. α2C Del 322–325 and α2A −1291 (C→G) polymorphisms result in reduced prejunctional α2 adrenoceptor function, and norepinephrine released from the prejunctional site is not effectively inactivated by reuptake and subsequent monoamine oxidation. Increased synaptic norepinephrine may inhibit cholinergic enteric motor neurones,47 reducing gastrointestinal motility (for example, constipation). Moreover, α2A mechanisms directly alter gut motor function.26
Genetic variation in α2 adrenoceptor functions may influence visceral sensation and behaviour in IBS. α2A Adrenoceptors on primary visceral afferents facilitate the transmission of pain to the dorsal horn neurone in the spinal cord, and those in high density in locus coeruleus neurones influence the sedative effects of α2 agonists,27 which may relieve psychological or somatisation disorders in IBS. α2C Adrenoreceptors are involved in control of behaviour and those on spinal interneurones modify descending inhibitory pathways from the brainstem27 that downregulate the dorsal horn neurones and peripheral sensation.48 Thus loss of normal α2C adrenoceptor function may reduce descending modulation, thereby increasing the “sensitivity” of the dorsal horn neurone.27 Higher somatic scores with the α2C adrenoceptor polymorphisms may reflect a somatisation disorder, or altered peripheral pathways or central sensation (for example, spinal pathway sensitivity or induction of affective disturbance). Loss of function α2C adrenoreceptor function may also result in reduced feedback through presynaptic receptors, thereby allowing continued release of norepinephrine which results in inhibition of excitatory (cholinergic) neurones in the gut and hence colonic transit delay.
The allele frequencies and genotype distributions observed in the healthy controls in this study illustrate the importance of having ethnic controls in these studies. Thus distributions for the α2A −1291 (C→G) polymorphism is very different in Japanese, Swedish men, and the predominantly female European American populations in our study. On the other hand, distributions for α2C Del 322–325, α2ALys251, and SLC6A4 were very similar to those for Caucasians in the published literature.
The association (p = 0.04) of the combination of α2C Del 322–325 and SLC6A4 polymorphisms with IBS is consistent with evidence that modulation of α2 adrenoceptor function may alter the biological effects of serotonin.43 An increased availability of 5-HT at the synapse resulting from less effective reuptake due to the SLC6A4 polymorphism may result in greater stimulation of afferent pathways. Decreased α2C function by the α2C Del 322–325 polymorphism might reduce 5-HT release from enterochromaffin cells.23,43 The lack of a significant association observed between IBS phenotype and SLC6A4 polymorphism does not negate a potential role of this genetic variation in responses to stress (as recently demonstrated in depression, see below) or to responses to serotonergic medications.22SLC6A4 polymorphisms have been associated with a variety of psychological disorders and their response to therapy.29,43
Further studies are needed to assess the biological effects of the combined α2C and SLC6A4 polymorphisms. Specific hypotheses that are plausible and should be addressed in future studies are: firstly, that α2C Del 322–325 is associated with a greater prevalence and colonic transit delay in IBS than in IBS patients without the deletion; and secondly, that there is greater severity and frequency of pain and lower thresholds for sensation of rectal or colonic distension in patients with functional lower gastrointestinal disorders with the combined α2C and SLC6A4 polymorphisms compared with patients without the genetic polymorphisms.
Our study followed the standards recommended for appraising genotype prevalence and gene-disease association45: analytical validity by DNA sequencing to confirm results by PCR and restriction; blinding of investigators with laboratory personnel assessing genotype and clinicians categorising the phenotype; confirmation that genotype frequencies conform to those reported in controls46,49,50 from the same source population; and presentation of genotype frequencies. There were no patients with the mutation tested for NET or for the α2ALys251 polymorphism in the first 100 patients and 20 controls. However, the investigated NET mutation had been identified in a specific family with orthostatic intolerance syndrome.17 This mutation was not confirmed in a recent study of 14 patients with orthostatic intolerance syndrome.51 Therefore, we cannot exclude the possibility that alternative genetic alterations in the NET gene may be relevant to disorders of gastrointestinal function.
We assessed the power to detect associations between phenotypes and candidate genes. We detected no NET mutation or α2ALys251 polymorphisms in 120 predominantly Caucasian patients and controls. Absence of any genetic variation in these two candidate genes suggests that they are unlikely to play significant roles in determining IBS or somatic symptom phenotype. However, given the low prevalence of the α2ALys251 polymorphism in European Americans, further studies in African-Americans are required. Table 6 ▶ summarises the “degree of association” between the presence/absence of specific polymorphisms in α2C Del 322–325, α2A −1291 (C→G), and SLC6A4 genes or combinations versus specific symptom subgroups (including overall FGID). It indicates that clinically meaningful associations of these three candidate genotypes could have been detected with at least 80% power.
In summary, we have shown that two functionally distinct α2 adrenoceptor polymorphisms, alone or in combination with a SLC6A4 polymorphism, are associated with constipation and high somatic symptom scores in patients with lower functional gastrointestinal disorders. These data suggest that genetic factors may interact with environmental factors and contribute to the manifestations of IBS; however, the strength of the genetic contribution to the phenotype is unclear. A gene-by-environment interaction was recently demonstrated in depression as SLC6A4 polymorphism influences the effect of life event stress on depression.52 Similar gene-environment interaction studies are needed in IBS in view of the evidence that life event stress is associated with IBS,2–4 models of visceral hypersensitivity,53 and depression.54 Moreover, in view of the reduced global IBS symptoms and bowel dysfunction in IBS patients treated with an α2 agonist55 and modulation of μ opioid responses to pain by genetic variation in activation of norepinephrine by catechol-o-methyl transferase,56 pharmacogenomic studies would be of significant interest and potential clinical relevance.
Acknowledgments
This study was supported in part by General Clinical Research Center grant RR00585, by grants R01 DK54681 and K24 DK02638 (to MC), and grants RO1 DK52913 and DK56220 (to RU) from National Institutes of Health. This work was also supported by the COSAT Foundation and the American Gastroenterological Association Miles and Shirley Fiterman Foundation (to MC) and by the American College of Gastroenterology and the Miles and Shirley Fiterman Digestive Diseases Research Grant to Mayo Foundation (supporting HJK). We thank Brenda Luther, RN, and Elena Atanasova, PhD, for support in performing these studies and Mrs Cindy Stanislav for excellent secretarial assistance.
Abbreviations
IBS, irritable bowel syndrome
IBS-C, irritable bowel syndrome with predominant constipation
IBS-D, irritable bowel syndrome with predominant diarrhoea
FGID, functional gastrointestinal disorders
FC, functional constipation
FD, functional diarrhoea
CAP, chronic abdominal pain
SoSc, somatic symptom score
NET, norepinephrine transporter
5-HT, 5-hydroxytryptamine (serotonin)
SERT, serotonin transporter
SERT-P, serotonin transporter promoter
SLC6A4, solute carrier family 6 (neurotransmitter transporter, serotonin), member 4
α2C Del 322–325, deletion in third intracellular loop of α2C adrenoceptor protein
α2ALys251, a cytosine to guanine transversion at position 753 that changes amino acid 251 of the third intracellular loop of the α2A adrenoceptor from asparagine to lysine
α2A −1291 (C→G), cytosine to guanine transversion in promoter of α2A receptor protein
OR, odds ratio
PCR, polymerase chain reaction
REFERENCES
- 1.Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci 1993;38:1569–80. [DOI] [PubMed] [Google Scholar]
- 2.Drossman DA, Camilleri M, Mayer EA, et al. AGA technical review on irritable bowel syndrome. Gastroenterology 2003;123:2108–31. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA, McKee DC, Sandler RS, et al. Psychosocial factors in the irritable bowel syndrome. A multivariate study of patients and nonpatients with irritable bowel syndrome. Gastroenterology 1988;95:701–8. [DOI] [PubMed] [Google Scholar]
- 4.Whitehead WE, Bosmajian L, Zonderman AB, et al. Symptoms of psychologic distress associated with irritable bowel syndrome. Comparison of community and medical clinic samples. Gastroenterology 1988;95:709–14. [DOI] [PubMed] [Google Scholar]
- 5.Bharucha AE, Camilleri M, Low PA, et al. Autonomic dysfunction in gastrointestinal motility disorders. Gut 1993;34:397–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal A, Cutts TF, Abell TL, et al. Predominant symptoms in irritable bowel syndrome correlate with specific autonomic nervous system abnormalities. Gastroenterology 1994;106:945–50. [DOI] [PubMed] [Google Scholar]
- 7.Bharucha AE, Camilleri M, Zinsmeister AR, et al. Adrenergic modulation of human colonic motor and sensory function. Am J Physiol 1997;273:G997–1006. [DOI] [PubMed] [Google Scholar]
- 8.Thumshirn M, Camilleri M, Choi M-G, et al. Modulation of gastric sensory and motor functions by nitrergic and alpha2-adrenergic agents in humans. Gastroenterology 1999;116:573–85. [DOI] [PubMed] [Google Scholar]
- 9.Viramontes BE, Malcolm A, Camilleri M, et al. Effects of α2-adrenergic agonist on gastrointestinal transit, colonic motility and sensation in humans. Am J Physiol 2001;281:G1468–76. [DOI] [PubMed] [Google Scholar]
- 10.Morris-Yates A, Talley NJ, Boyce PM, et al. Evidence of a genetic contribution to functional bowel disorder. Am J Gastroenterol 1998;93:1311–17. [DOI] [PubMed] [Google Scholar]
- 11.Locke GR III, Zinsmeister AR, Talley NJ, et al. Familial association in adults with functional gastrointestinal disorders. Mayo Clinic Proceedings 2000;75:907–12. [DOI] [PubMed] [Google Scholar]
- 12.Levy RL, Jones KR, Whitehead WE, et al. Irritable bowel syndrome in twins: heredity and social learning both contribute to etiology. Gastroenterology 2001;121:799–804. [DOI] [PubMed] [Google Scholar]
- 13.Kobilka BK, Matsui H, Kobilka TS, et al. Cloning, sequencing, and expression of the gene coding for the human platelet alpha 2-adrenergic receptor. Science 1987;238:650–6. [DOI] [PubMed] [Google Scholar]
- 14.Lomasney JW, Lorenz W, Allen LF, et al. Expansion of the alpha 2-adrenergic receptor family: cloning and characterization of a human alpha 2-adrenergic receptor subtype, the gene for which is located on chromosome 2. Proc Natl Acad Sci U S A 1990;87:5094–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regan JW, Kobilka TS, Yang-Feng TL, et al. Cloning and expression of a human kidney cDNA for an alpha 2-adrenergic receptor subtype. Proc Natl Acad Sci U S A 1988;85:6301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Small KM, McGraw DW, Liggett SB. Pharmacology and physiology of human adrenergic receptor polymorphisms. Ann Rev Pharmacol Toxicol 2003;43:381–411. [DOI] [PubMed] [Google Scholar]
- 17.Shannon JR, Flattem NL, Jordan J, et al. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med 2000;342:541–9. [DOI] [PubMed] [Google Scholar]
- 18.Kim D-Y, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol 2000;95:2698–709. [DOI] [PubMed] [Google Scholar]
- 19.Chen J-X, Pan H, Rothman TP, et al. Guinea pig 5-HT transporter: cloning, expression, distribution, and function in intestinal sensory reception. Am J Physiol 1998;275:G433–8. [DOI] [PubMed] [Google Scholar]
- 20.Chen JJ, Li Z, Pan H, et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci 2001;21:6348–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu MT, Rayport S, Jiang Y, et al. Expression and function of 5-HT3 receptors in the enteric neurons of mice lacking the serotonin transporter. Am J Physiol 2002;283:G1398–411. [DOI] [PubMed] [Google Scholar]
- 22.Camilleri M, Atanasova E, Carlson PJ, et al. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology 2002;123:425–32. [DOI] [PubMed] [Google Scholar]
- 23.Hirafuji M, Ogawa T, Kato K, et al. Norepinephrine stimulates 5-hydroxytryptamine release from mouse ileal tissues via alpha(2)-adrenoceptors. Eur J Pharmacol 2001;432:149–52. [DOI] [PubMed] [Google Scholar]
- 24.Pettersson G, Dahlstrom A, Larsson I, et al. The release of serotonin from rat duodenal enterochromaffin cells by adrenoceptor agonists studied in vitro. Acta Physiol Scand 1978;103:219–24. [DOI] [PubMed] [Google Scholar]
- 25.Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depression Anxiety 2000;12:2–19. [DOI] [PubMed] [Google Scholar]
- 26.Scheibner J, Trendelenburg AU, Hein L, et al. Alpha 2-adrenoceptors in the enteric nervous system: a study in alpha 2A-adrenoceptor-deficient mice. Br J Pharmacol 2002;135:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Philipp M, Brede M, Hein L. Physiological significance of alpha(2)-adrenergic receptor subtype diversity: one receptor is not enough. Am J Physiol 2002;283:R287–95. [DOI] [PubMed] [Google Scholar]
- 28.Zou A-P, Cowley AW Jr. α2-Adrenergic receptor-mediated increase in NO production buffers renal medullary vasoconstriction. Am J Physiol 2000;279:R769–77. [DOI] [PubMed] [Google Scholar]
- 29.Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996;274:1527–31. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Wichems C, Heils A, et al. Reduction of 5-HT1A-mediated temperature and neuroendocrine responses and 5-HT1A binding sites in 5-HT transporter knockout mice. J Pharmacol Exp Ther 1999;291:999–1007. [PubMed] [Google Scholar]
- 31.Tan CM, Wilson MH, MacMillan LB, et al. Heterozygous alpha 2A-adrenergic receptor mice unveil unique therapeutic benefits of partial agonists. Proc Natl Acad Sci USA 2002;99:12471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sallinen J, Link RE, Haapalinna A, et al. Genetic alteration of alpha 2C-adrenoceptor expression in mice: influence on locomotor, hypothermic, and neurochemical effects of dexmedetomidine, a sub-type-nonselective alpha 2-adrenoceptor agonist. Mol Pharmacol 1997;51:36–46. [DOI] [PubMed] [Google Scholar]
- 33.Hein L, Altman JD, Kobilka BK. Two functionally distinct alpha2-adrenergic receptors regulate sympathetic neurotransmission. Nature 1999;402:181–4. [DOI] [PubMed] [Google Scholar]
- 34.Gavin KT, Colgan MP, Moore D, et al. Alpha 2C adrenoceptors mediate contractile responses to norepinephrine in the human saphenous vein. Naunyn Schmiedebergs Arch Pharmacol 1997;355:406–11. [DOI] [PubMed] [Google Scholar]
- 35.Sallinen J, Haapalinna A, Macdonald E, et al. Genetic alteration of the alpha2 adrenoceptor subtype c in mice affects the development of behavioral despair and stress-induced increases in plasma corticosterone levels. Mol Psychiatr 1999;4:443–52. [DOI] [PubMed] [Google Scholar]
- 36.Talley NJ, Phillips SF, Wiltgen CM, et al. Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc 1990;65:1456–79. [DOI] [PubMed] [Google Scholar]
- 37.Maleki D, Locke GR III, Camilleri M, et al. Gastrointestinal symptoms among persons with diabetes in the community. Arch Intern Med 2000;160:2808–16. [DOI] [PubMed] [Google Scholar]
- 38.Locke GR III, Fett SL, Zinsmeister AR. Transition probabilities between functional gastrointestinal disorders in the community. Gastroenterology 2001;120:A76. [Google Scholar]
- 39.Ohara K, Nagai M, Tani K, et al. Polymorphism in the promoter region of the alpha 2A adrenergic receptor gene and mood disorders. Clin Neurosci 1998;9:1291–4. [DOI] [PubMed] [Google Scholar]
- 40.Rosmond R, Bouchard C, Bjorntorp P. A C-1291G polymorphism in the alpha2A-adrenergic receptor gene (ADRA2A) promoter is associated with cortisol escape from dexamethasone and elevated glucose levels. J Intern Med 2002;251:252–7. [DOI] [PubMed] [Google Scholar]
- 41.Small KM, Forbes SL, Brown KM, et al. An asn to lys polymorphism in the third intracellular loop of the human alpha 2A-adrenergic receptor imparts enhanced agonist-promoted Gi coupling. J Biol Chem 2000;275:38518–23. [DOI] [PubMed] [Google Scholar]
- 42.Small KM, Forbes SL, Rahman FF, et al. A four amino acid deletion polymorphism in the third intracellular loop of the human 2C-adrenergic receptor confers impaired coupling to multiple effectors. J Biol Chem 2000;275:23059–64. [DOI] [PubMed] [Google Scholar]
- 43.Scheibner J, Trendelenburg AU, Hein L, et al. Alpha2-adrenoceptors modulating neuronal serotonin release: a study in alpha2-adrenoceptor subtype-deficient mice. Br J Pharmacol 2001;132:925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weizman A, Weizman R. Serotonin transporter polymorphism and response to SSRIs in major depression and relevance to anxiety disorders and substance abuse. Pharmacogenomics 2000;1:335–41. [DOI] [PubMed] [Google Scholar]
- 45.Little J, Bradley L, Bray MS, et al. Reporting, appraising, and integrating data on genotype prevalence and gene-disease associations. Am J Epidemiol 2002;156:300–10. [DOI] [PubMed] [Google Scholar]
- 46.Small KM, Wagoner LE, Levin AM, et al. Synergistic polymorphisms of beta 1- and alpha 2c-adrenergic receptors and the risk of congestive heart failure. N Engl J Med 2002;374:1135–42. [DOI] [PubMed] [Google Scholar]
- 47.Tack JF, Wood JD. Actions of norepinephrine on myenteric neurons in the guinea pig gastric antrum. J Auton Nerv Syst 1992;41:67–77. [DOI] [PubMed] [Google Scholar]
- 48.Coote JH, Macleod VH. Evidence for the involvement in the baroreceptor reflex of a descending inhibitory pathway. J Physiol 1974;241:477–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serretti A, Lilli R, Lorenzi C, et al. Serotonin transporter gene (5-HTTLPR) and major psychoses. Mol Psychiatr 2002;7:95–9. [DOI] [PubMed] [Google Scholar]
- 50.Tsai SJ, Wang YC, Younger WYY, et al. Association analysis of polymorphisms in the promoter region of the alpha2a-adrenoceptor gene with schizophrenia and clozapine response. Schizophr Res 2001;49:53–8. [DOI] [PubMed] [Google Scholar]
- 51.Ivancsits S, Heider A, Rudiger HW, et al. Orthostatic intolerance is not necessarily related to a specific mutation (Ala457Pro) in the human norepinephrine transporter gene. Am J Med Sci 2003;325:63–5. [DOI] [PubMed] [Google Scholar]
- 52.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003;301:386–9. [DOI] [PubMed] [Google Scholar]
- 53.Coutinho SV, Plotsky PM, Sablad M, et al. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol 2002;282:G307–16. [DOI] [PubMed] [Google Scholar]
- 54.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psych 1999;156:837–41. [DOI] [PubMed] [Google Scholar]
- 55.Camilleri M, Kim D-Y, McKinzie S, et al. A randomized, controlled exploratory study of clonidine in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol 2003;1:111–21. [DOI] [PubMed] [Google Scholar]
- 56.Zubieta J-K, Heitzeg MM, Smith YR, et al. COMT val158met genotype affects µ-opioid neurotransmitter responses to a pain stressor. Science 2003;299:1240–3. [DOI] [PubMed] [Google Scholar]