Abstract
Background: Systemic adenoviral readministration appears to be limited by immunogenicity.
Aims: We examined the feasibility of repeated adenovirus mediated gene transfer into the liver via the biliary tract.
Methods: Recombinant adenoviruses carrying a reporter lacZ gene were infused retrogradely into the common bile duct of rats. Transduction efficiency of the lacZ gene was estimated histochemically and quantitatively.
Results: Retrograde administration of recombinant adenoviruses into the common bile duct of rats resulted in efficient transgene expression in the liver, specifically in hepatocytes, but not in biliary epithelia. Transduction efficiency induced by intrabiliary adenoviral administration was not substantially different from that induced by intraportal adenoviral infusion. Transgene expression in the liver was however transient, and development of neutralising antibodies against adenovirus was observed in serum but not in bile. When adenoviruses were readministered into the common bile duct, successful re-expression of the transgene in the liver was achieved despite the existence of neutralising antibodies in serum. Interestingly, although proliferation of adenovirus specific T cells in response to adenoviral readministration was suppressed significantly by immunosuppressive FK506 treatment, levels of transgene expression in the liver achieved by intrabiliary adenoviral readministration were not significantly different between animals treated with and without FK506. Furthermore, third adenoviral administration into the common bile duct also induced successful transgene expression in the liver.
Conclusions: These results suggest that adenovirus mediated gene transfer into the liver may be repeatable without immunosuppressive strategies in clinical settings by means of endoscopic retrograde cholangiography.
Keywords: gene therapy, adenovirus, biliary tract, liver, rat
Although adenoviral vectors are a prominent gene transfer vehicle, several factors significantly limit the utility of current early gene region 1 (E1) deleted adenoviral vectors. Transgene expression usually peters out in vivo within 2–3 weeks due, at least in part, to destruction of adenoviral vector transduced cells by host cellular immune responses directed against viral proteins and/or immunogenic transgene products.1–3 Another important limitation of current adenoviral vectors is the difficulty in obtaining successful readministration using a vector of the same adenovirus serotype. Several studies have indicated that neutralising antibodies elicited by input viral particles substantially reduced the efficiency of vector readministration.2–5 Humans are natural hosts of various serotypes of adenoviruses and the majority of humans possess neutralising antibodies against adenovirus serotypes 2 and 5.6–9 Therefore, it should be noted that the first administration of adenoviral vectors to gene therapy patients who have been exposed to wild-type adenoviruses may correspond to readministration of adenoviral vectors into animals. Although successful readministration of adenoviral vectors has been demonstrated in immunocompromised animals, studies using animals lacking a functional immune system do not address key issues pertaining to the use of adenoviral vectors in human clinical trials.
In the present study, using immunocompetent animals, we examined whether adenoviral administration into the liver via the biliary tract resulted in efficient transgene expression. Furthermore, we repeatedly infused adenoviruses retrogradely into the biliary tract with and without immunosuppressive FK506 treatment and examined whether repeated expression of the original gene construct was achievable in the liver.
METHODS
Adenoviral vector
Adex1CAlacZ adenovirus10 was generously provided by Dr Izumu Saito (Institute of Medical Science, University of Tokyo, Tokyo, Japan). This adenoviral vector carries an adenovirus serotype 5 genome lacking the E1A, E1B, and E3 regions, and contains the Escherichia coli β-galactosidase gene, lacZ gene, as a reporter gene. The recombinant adenovirus was propagated and isolated in 293 cells, as described previously.11 A single batch of high titre adenovirus stock (2×109 plaque forming units (pfu)/ml) was used throughout the subsequent experiments.
Adenoviral administration into the biliary tract
Ten week old female Sprague-Dawley rats were anaesthetised with ether and a midline abdominal incision was made. The intestines were displaced to expose the liver and common bile duct. After clamping the distal site of the common bile duct to avoid antegrade outflow of the virus, a 30 gauge needle connected to a 1 ml syringe was inserted directly into the common bile duct. Adenovirus solutions (1×109 pfu/500 μl) were infused retrogradely into the biliary tract over one minute. On completion of the infusion, the needle was removed and pressure was gently applied over the puncture site of the common bile duct for five minutes. After removing the clamp from the common bile duct, the skin and fascia were closed.
Histochemical and quantitative estimations of transgene expression in the liver
LacZ gene expression in rat livers was evaluated histochemically by X-gal staining and quantitatively by a chemiluminescent assay, as described previously.12–15 In all of the experiments performed in the present study, each group consisted of five animals.
Adenoviral readministration into the biliary tract
To examine the transduction efficiency in rat livers by adenoviral readministration, animals were infused with adenoviruses carrying the lacZ gene (1×109 pfu/500 μl) retrogradely into the common bile duct on day 0, as described above. Animals were then separated randomly into two groups. Animals in the former group received reinfusion of adenoviruses carrying the lacZ gene (1×109 pfu/500 μl) into the common bile duct on day 35, in the same way as in the first adenoviral infusion. Animals in the latter group were treated with FK506 around the time of adenoviral readministration. FK506 (5 mg/kg body weight in 100 μl of phosphate buffered saline/day) was injected intramuscularly every day from days 30 to 36. Adenoviruses carrying the lacZ gene (1×109 pfu/500 μl) were reinfused into the common bile duct on day 35, in the same way as in the first adenoviral infusion. Animals were sacrificed on days 37 and 42 (on days 2 and 7 after adenoviral readministration, respectively) and their livers were removed for analysis of lacZ gene expression, as described above.
Statistics
Results are expressed as means (SD). Standard descriptive statistics, Student’s t test, and Welch’s t test were used according to the distribution of experimental values. A p value of <0.05 was considered to indicate a significant difference between groups.
RESULTS
Transgene expression in the liver induced by adenoviral administration into the biliary tract
On day 2 after Adex1CAlacZ adenoviral infusion into the common bile duct, considerable areas in the liver were stained blue with X-gal staining (fig 1A ▶). Although X-gal staining positive cells were seen predominantly at periportal areas, the so-called Rappaport’s zone 1 (fig 1B ▶), a considerable number of cells expressing the lacZ gene were observed in lobular and centrilobular areas, the so-called zones 2 and 3, respectively. Morphometric evaluation of liver sections using the public domain NIH Image program revealed that approximately 30% of cells in the liver expressed the lacZ gene. To identify cells positive for X-gal staining, liver sections after X-gal staining were fixed in 10% buffered formaldehyde, embedded in paraffin, sliced into 4 μm thick sections, and counterstained with haematoxylin-eosin. Interestingly, hepatocytes near the bile duct were positive for X-gal staining while biliary epithelia were negative for the staining (fig 1C ▶). Furthermore, a number of hepatocytes in zones 2 and 3 were also positive for X-gal staining (fig 1D ▶). Infiltration of inflammatory cells was seldom observed in the liver.
Figure 1.
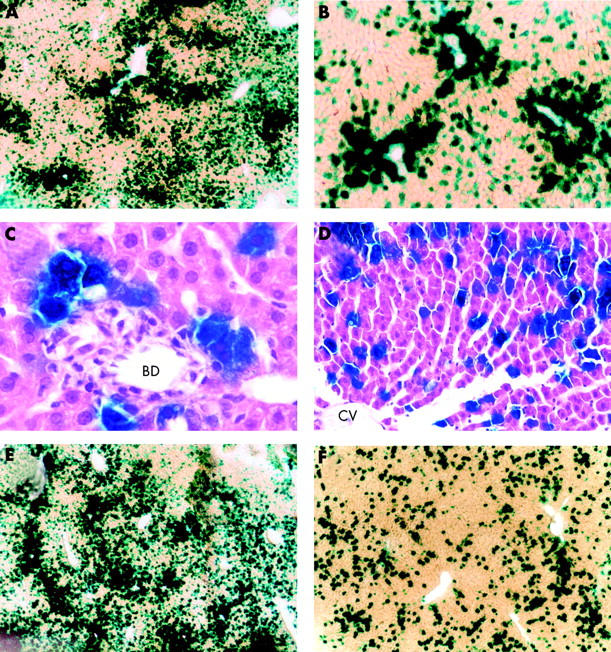
LacZ gene expression in rat livers induced by intrabiliary or intraportal administration of adenoviruses. Recombinant adenoviruses (1×109 pfu/500 μl) carrying the lacZ gene were infused into the common bile duct or into the portal vein of rats. Animals were killed on days 2 (A, B) and 7 (F) after intrabiliary adenoviral infusion, and on day 2 (E) after intraportal adenoviral administration. The livers were removed, sliced into 50 μm thick sections and subjected to X-gal staining. After X-gal staining, liver sections collected two days after intrabiliary adenoviral infusion were fixed in formaldehyde, embedded in paraffin, sliced into 4 μm thick sections, and counterstained with haematoxylin-eosin (C, D). BD, bile duct; CV, central vein. Each group consisted of five animals (a representative image is shown). (Original magnification A, E, F ×40; B ×100; C, D ×200.)
To compare the transduction efficiency in the liver induced by intrabiliary adenoviral administration with that induced by intraportal adenoviral infusion, Adex1CAlacZ adenoviruses were infused directly into the portal vein. On day 2 after adenoviral infusion, a considerable number of cells in the liver were stained blue with X-gal staining (fig 1E ▶). Similar to the results for adenoviral administration into the common bile duct, X-gal staining positive cells were seen predominantly in zone 1. Morphometric evaluation of liver sections revealed that approximately 40% of cells in the liver expressed the lacZ gene. Although transduction efficiency induced by intraportal adenoviral infusion was slightly higher than that induced by intrabiliary adenoviral administration, the difference was not substantial.
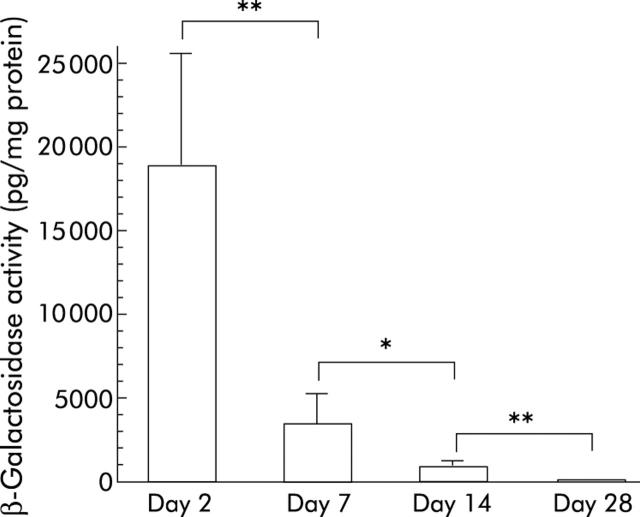
We then examined duration of transgene expression in the liver caused by retrograde adenoviral administration into the biliary tract. X-gal staining positive areas were decreased significantly on day 7 after adenoviral infusion and approximately 5% of cells in the liver were stained blue with X-gal staining (fig 1F ▶). X-gal staining positive cells in the liver were decreased markedly thereafter and there were few cells expressing the lacZ gene by day 28 (data not shown). As shown in fig 2 ▶, considerable levels of lacZ gene expression were observed in the liver on day 2 after adenoviral infusion, with mean level of β-galactosidase activity being approximately 19 000 pg/mg protein. Expression of the lacZ gene in the liver was decreased rapidly thereafter and mean levels of β-galactosidase activity on days 7, 14, and 28 were approximately 3500, 910, and 47 pg/mg protein, respectively. On day 28, β-galactosidase activity in the liver was not significantly different from that of naive untreated control rats.
Figure 2.
Quantitative estimation of lacZ gene expression in rat livers induced by retrograde adenoviral administration into the biliary tract. Recombinant adenoviruses (1×109 pfu/500 μl) carrying the lacZ gene were infused retrogradely into the common bile duct of rats. Animals were killed on days 2, 7, 14, and 28 after adenoviral infusion. Their livers were homogenised and β-galactosidase activity in the liver was estimated by the chemiluminescent reporter gene assay. Livers of rats that did not receive adenoviral administration were also subjected to the chemiluminescent assay to estimate background values of β-galactosidase activity in rat livers. Each bar represents the mean (SD) of five animals. *0.01<p<0.05; **0.001<p<0.01.
Development of neutralising antibodies against adenovirus
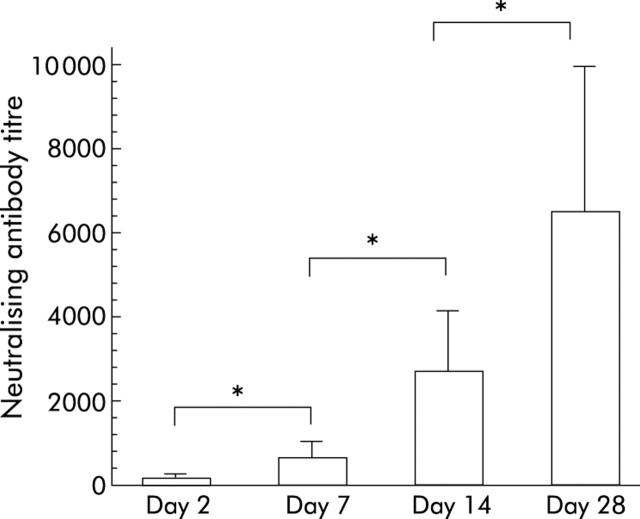
Sera were collected from rats administered with adenoviruses retrogradely into the biliary tract and neutralising antibodies against adenovirus serotype 5 were examined, as previously described.16 As rats are not natural hosts of adenovirus serotype 5, there were no detectable levels of neutralising antibodies against adenovirus in naive control rats. However, as shown in fig 3 ▶, development of neutralising antibodies against adenovirus was observed as early as day 2 after intrabiliary adenoviral infusion, with the mean titre of neutralising antibodies being ×120. Titres of neutralising antibodies against adenovirus were increased profoundly with the passage of time and mean titres were ×704, ×2816, and ×6656 on days 7, 14, and 28, respectively.
Figure 3.
Development of neutralising antibodies against adenovirus elicited by retrograde adenoviral administration into the biliary tract. Serum samples were collected from rats infused with adenoviruses (1×109 pfu/500 μl) into the common bile duct when they were killed on days 2, 7, 14, and 28 after adenoviral infusion. Serum samples were decomplemented, serially diluted, and then analysed for neutralising antibodies against adenovirus serotype 5. The titre of neutralising antibody for each sample is expressed as the reciprocal dilution of serum that inhibited adenoviral infection by 50%. Each bar represents the mean (SD) of five animals. *0.01<p<0.05.
Adenoviral readministration into the biliary tract
We have already shown that adenoviral readministration into the portal vein or into the tail vein of rats without immunosuppressive strategies failed to induce significant transgene expression in the liver.13,14 Furthermore, we have also shown that a second adenoviral infusion into the tail vein of rats performed six or 12 months after the initial administration could not induce detectable levels of transgene expression in the liver.17 Therefore, in the present study, we did not perform intravenous adenoviral readministration. Instead, to examine the feasibility of adenoviral readministration into the biliary tract, Adex1CAlacZ adenoviruses (1×109 pfu/500 μl) were readministered retrogradely into the common bile duct on day 35 after the first administration. Furthermore, to examine the effect of an immunosuppressive strategy on intrabiliary adenoviral readministration, animals were separated randomly into two groups. Animals in the former group did not receive any immunosuppressive treatment and those in the latter group received intramuscular injections of FK506 daily from days 30 to 36 after the first adenoviral administration. Animals were killed on days 37 and 42 (on days 2 and 7, respectively, after adenoviral readministration) for analysis of transgene expression in the liver. As shown in fig 4A ▶, X-gal staining of liver sections of animals without FK506 treatment revealed that there were a considerable number of cells expressing the lacZ gene in the liver. Although X-gal staining positive areas were seen predominantly in zone 1, a number of cells expressing the lacZ gene were observed in zones 2 and 3. As shown in fig 4B ▶, X-gal staining positive cells were also observed in the liver of animals treated with FK506. The number of cells expressing the lacZ gene in the liver appeared to be similar between animals treated with and without FK506. Subsequent morphometric evaluation revealed that approximately 5% of cells in the liver expressed the lacZ gene, irrespective of FK506 treatment.
Figure 4.
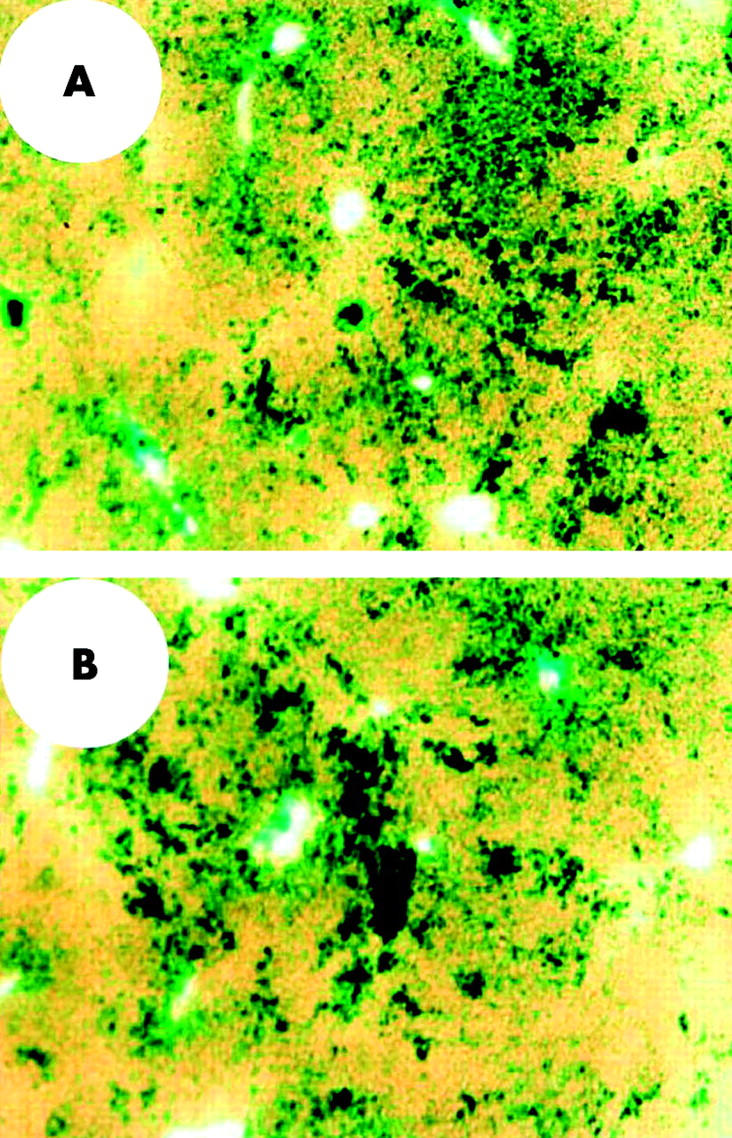
LacZ gene expression in rat livers induced by adenoviral readministration into the biliary tract. Recombinant adenoviruses (1×109 pfu/500 μl) carrying the lacZ gene were infused retrogradely into the common bile duct of rats. Animals were then separated randomly into two groups and treated with and without FK506. Animals in the FK506 treatment group were given intramuscular injections of FK506 (5 mg/kg body weight) from days 30 to 36. Animals in both groups were reinfused with adenoviruses (1×109 pfu/500 μl) retrogradely into the common bile duct on day 35. Animals were killed on day 37 and lacZ gene expression in the liver was examined by X-gal staining. Cells stained blue were observed in the liver of animals treated with (B) and without (A) FK506. A representative image is shown. (Original magnification ×40.)
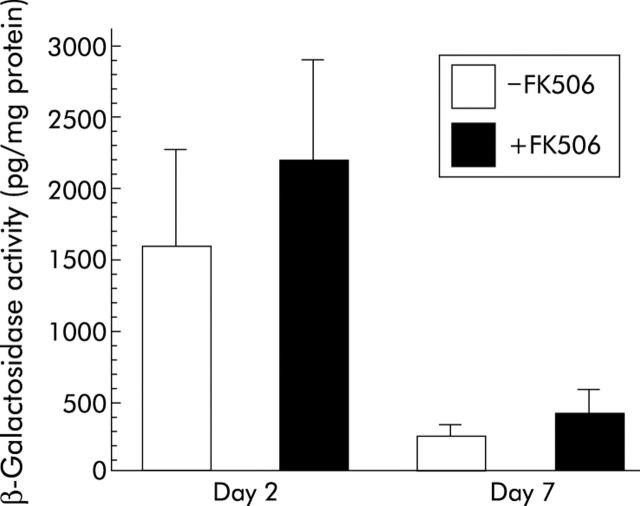
The results were estimated quantitatively by the subsequent chemiluminescent reporter gene assay. As shown in fig 5 ▶, levels of β-galactosidase activity in the liver of animals readministered with adenoviruses into the common bile duct with and without FK506 treatment were approximately 2200 and 1600 pg/mg protein, respectively, on day 2 after adenoviral readministration. Differences were not statistically significant between the groups. Levels of β-galactosidase activity in the liver induced by adenoviral readministration into the biliary tract were decreased significantly not only in animals without FK506 treatment but also in those with FK506 treatment, with levels of β-galactosidase activity being approximately 260 and 430 pg/mg protein, respectively, on day 7 after adenoviral readministration. Levels of transgene expression in the liver on day 7 after adenoviral readministration were not significantly different between the groups.
Figure 5.
Quantitative estimation of lacZ gene expression in rat livers induced by adenoviral readministration into the biliary tract. Recombinant adenoviruses carrying the lacZ gene were infused retrogradely into the common bile duct of rats, as described in the legend of fig 4 ▶. Animals treated with and without FK506 around the time of intrabiliary adenoviral readministration were killed on days 2 and 7 after readministration. LacZ gene expression in the liver induced by adenoviral readministration was quantitatively estimated by the chemiluminescent assay. Each bar represents the mean (SD) of five animals. There were no significant differences in β-galactosidase activity between animals treated with and without FK506 not only on day 2 but also on day 7 after adenoviral readministration.
Humoral and cellular immune responses to adenoviral readministration into the biliary tract
To examine humoral responses to adenoviral readministration into the biliary tract, development of neutralising antibodies against adenovirus was examined. Adenoviruses were reinfused retrogradely into the common bile duct on day 35 after the first administration, and animals treated with and without FK506 around the time of adenoviral readministration were killed on days 37 and 42 (on days 2 and 7, respectively, after adenoviral readministration). The mean titre of neutralising antibodies against adenovirus reached approximately ×6656 on day 28 after the first adenoviral infusion into the common bile duct (see fig 3 ▶). Irrespective of FK506 treatment, neutralising antibodies against adenovirus were further increased in response to adenoviral readministration and titres were >×40 960 (the highest tested) not only on day 2 but also on day 7 after adenoviral readministration. In marked contrast, irrespective of FK506 treatment, titres of neutralising antibodies against adenovirus in bile were <×10 (the lowest tested) in all animals that were given adenoviral readministration into the common bile duct.
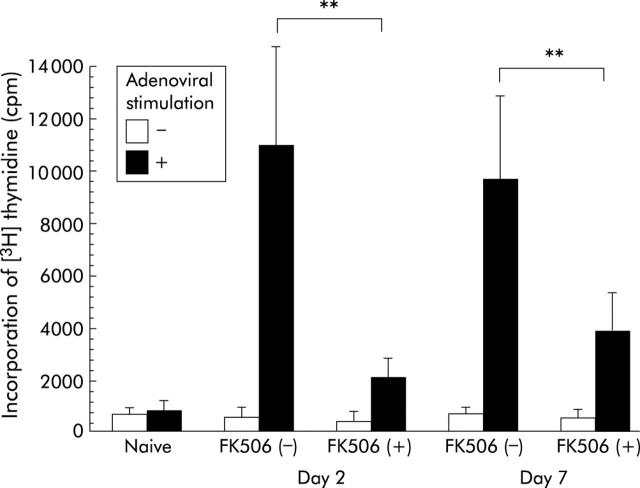
To examine cellular immune responses to adenoviral readministration into the biliary tract, activation of adenovirus specific splenic T cell proliferation was estimated, as previously described.18 As shown in fig 6 ▶, splenic cells collected from naive control animals did not proliferate significantly with stimulation of heat inactivated adenoviruses. Splenic cells collected from animals on days 2 and 7 after adenoviral readministration also did not proliferate when they were not stimulated with heat inactivated adenoviruses. Conversely, when splenic cells collected from animals readministered with adenoviruses into the biliary tract were stimulated with heat inactivated adenoviruses, they exhibited marked proliferation. Levels of incorporation of [3H] thymidine into splenic cells stimulated with heat inactivated adenoviruses were significantly higher in animals without FK506 treatment than in those with FK506 treatment not only on day 2 but also on day 7 after adenoviral readministration.
Figure 6.
Cellular immune responses to adenoviral readministration into the biliary tract. To examine cellular immune responses to adenoviral readministration into the common bile duct, splenic cells were collected from rats and incubated with (+) or without (−) heat inactivated adenoviruses for five days and pulsed with [3H] thymidine for the last 18 hours of incubation. Values are means (SD) of five animals. **0.001<p<0.01.
Third administration of adenovirus into the biliary tract
To confirm the feasibility of repeated adenovirus mediated gene transfer into the liver by way of the biliary tract, we performed the third adenoviral administration into the common bile duct. Adex1CAlacZ adenoviruses (1×109 pfu/500 μl) were infused retrogradely into the common bile duct of rats on days 0, 35, and 70 without FK506 treatment. Animals were killed on days 72 and 77 (on days 2 and 7, respectively, after the third administration) for analysis of transgene expression in the liver. As shown in fig 7 ▶, X-gal staining of liver sections of animals that were given the third adenoviral administration revealed that approximately 5% of cells in the liver, predominantly in zone 1, were positive for X-gal staining. Similar to the results of the first and the second adenoviral administrations into the biliary tract, X-gal staining positive cells were decreased significantly on day 7 after the third adenoviral administration (data not shown). The subsequent chemiluminescent assay showed that mean levels of β-galactosidase activity in the liver were approximately 1500 and 310 pg/mg protein on days 2 and 7, respectively, after the third adenoviral administration into the common bile duct. Duration of β-galactosidase expression in the liver after the third adenoviral administration was not substantially different from that after the first and second adenoviral administrations. Ratios of β-galactosidase activity on day 7 to those on day 2 after the first, second, and third adenoviral administrations were approximately 18%, 12%, and 21%, respectively.
Figure 7.
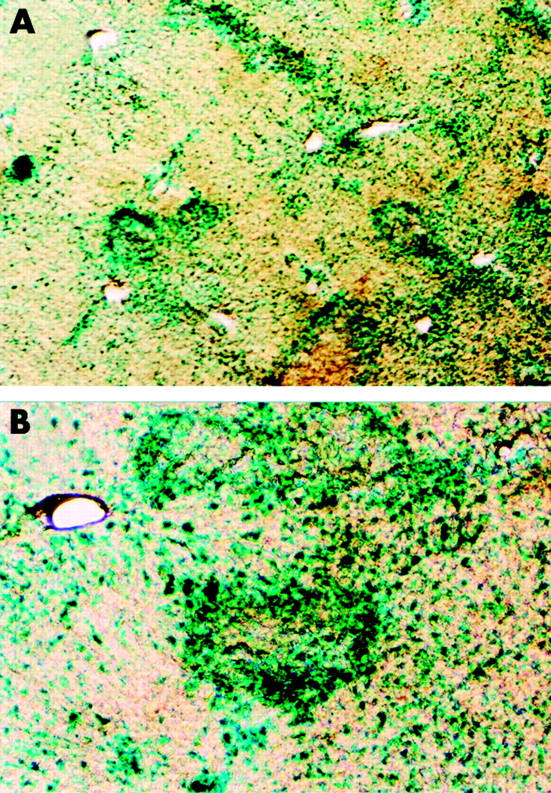
LacZ gene expression in rat livers induced by third adenoviral administration into the biliary tract. Recombinant adenoviruses (1×109 pfu/500 μl) carrying the lacZ gene were infused retrogradely into the common bile of rats on days 0, 35, and 70. Animals were killed on day 72 and lacZ gene expression in the liver was examined by X-gal staining. A representative image is shown. (Original magnification A ×40; B ×100.)
Serum alanine aminotransferase (ALT) levels in animals administered intrabiliary with adenoviruses
To examine the adverse reaction caused by intrabiliary adenoviral administration, serum samples were collected from rats two days after the first, second, and third adenoviral infusions into the common bile duct. Serum samples were also collected from rats two days after intraportal adenoviral administration, as well as from naive control animals. Serum ALT levels of naive animals were 42 (9) U/l/37°C and those of animals that received intraportal adenoviral administration were 66 (18) U/l/37°C. Serum ALT levels of rats that received intrabiliary adenoviral administration once, twice, and thrice were 51 (23), 64 (27), and 59 (15) U/l/37°C, respectively. Although values were slightly higher in animals that were given intraportal or intrabiliary adenoviral infusion than in naive controls, the differences were not statistically significant.
DISCUSSION
We have demonstrated here that retrograde administration of adenoviruses into the common bile duct could induce efficient transgene expression in rat livers. Although cells expressing the transgene were observed predominantly in zone 1, a considerable number of cells expressing the transgene were also observed in zones 2 and 3. Furthermore, it was shown that intrabiliary adenoviral administration resulted in transgene expression in hepatocytes but not in biliary epithelia. Transduction efficiency in the liver induced by adenoviral administration into the common bile duct was not substantially different from that induced by adenoviral administration into the portal vein.
One of the major problems with systemic adenoviral administration is that the animal becomes resistant to a second therapeutic administration of adenoviruses.2,4,19,20 As systemic readministration of adenoviruses appears to be limited, several studies have been performed to examine whether local direct readministration of adenoviruses might be possible without immune suppression in immunocompetent animals. Bennett and colleagues21 have shown that subretinal administration of an adenoviral vector resulted in minimal antiadenovirus antibody production relative to subcutaneous administration, and successful repeated administration was observed. Effective repeated dosing in this organ is probably a reflection of the immune privileged status of the anterior chamber of the eye.22 Chen and colleagues23 have also shown that successful readministration of an adenoviral vector to skeletal muscle was possible without using any immunosuppressive drugs. They reasoned that the concentration of neutralising antibodies against adenovirus in the muscle might be considerably lower than the concentration in serum and thus permitted effective readministration to the muscle under conditions that did not allow readministration to the liver via the intravenous route. Conversely, McClane and colleagues24 have demonstrated that local delivery of an adenoviral vector into the pancreas induced systemic responses that prevented local direct readministration of the vector. However, the feasibility of adenoviral readministration into the biliary tract has not been examined extensively to date. In the present study, we demonstrated that although adenoviral readministration into the biliary tract induced both humoral and cellular immune responses in rats, it could induce re-expression of the original gene construct in rat livers without immunosuppressive strategies. We showed here that even after adenoviral readministration into the common bile duct, titres of neutralising antibodies against adenovirus in bile were less than ×10 (the lowest tested) while those in serum were more than ×40 960 (the highest tested). Therefore, the plausible explanation for this outcome is that because concentrations of neutralising antibodies against adenovirus in bile were not elevated substantially after adenoviral administration into the common bile duct, readministration directly into the biliary tract allowed adenoviruses to infect hepatocytes through the biliary system without encountering neutralising antibodies, resulting in re-expression of the same transgene in the liver. Furthermore, we showed that the third adenoviral administration into the biliary tract could induce similar transduction efficiency in the liver compared with the second adenoviral administration. Although adenoviral administration into the common bile duct resulted in mild elevation of serum ALT levels, the values were not significantly different from those of naive control animals. Furthermore, substantial histological damage was not caused in the liver by intrabiliary adenoviral administration. These results suggest that repeated gene transduction into the liver can be achieved safely by adenoviral administration into the biliary tract.
To inhibit the production of neutralising antibodies against adenovirus that prevent further readministration of vectors, immunosuppressive strategies have been undertaken. It has been demonstrated that treatment regimens with immunosuppressive drugs, such as cyclophosphamide, FK506, cyclosporin A, and deoxyspergualin, around the time of initial exposure to adenoviruses, permitted prolonged transgene expression, reduced inflammation, prevented the formation of neutralising antibodies, and permitted successful readministration of adenoviral vectors.25–28 The practicality of these approaches is however questionable because the majority of prospective gene therapy patients have already been infected with adenovirus serotypes 2 and 5. Therefore, the first adenoviral administration to humans may correspond to readministration of adenoviruses to animals. We have shown that human sera with a relatively high titre (>×128) of antiadenovirus antibody completely inhibited adenovirus mediated gene transfer not only in vitro but also in vivo. Furthermore, human sera with the lowest positive titre (×4) of antiadenovirus antibody also substantially inhibited adenovirus mediated gene transduction.29 It is therefore expected that humans may not be susceptible to immune downregulation because activation requirements tend to be reduced in primed lymphocyte populations.30 Furthermore, when these immunosuppressive strategies are applied in clinical settings, there is a risk associated with systemic immunosuppression.
In the present study, we demonstrated that adenoviral readministration into the biliary tract induced significant humoral and cellular immune responses to adenoviruses. Nevertheless, when adenoviruses were readministered into the biliary tract without any immunosuppressive agents, successful re-expression of the original gene construct was achieved in the liver. We also examined the effect of an immunosuppressive strategy on adenoviral readministration into the biliary tract. To provide a closer approximation of the expected clinical setting, we gave the immunosuppressive agent FK506 around the time of the secondary adenoviral administration but not around the time of the first adenoviral administration. FK506 treatment around the time of adenoviral readministration into the common bile duct significantly suppressed cellular immune responses to adenoviral readministration. However, there were no significant differences in transgene expression in the liver induced by adenoviral readministration between animals treated with and without FK506 treatment. Our results support the feasibility of administering recombinant adenoviruses to the human biliary tract by a relatively non-invasive approach, namely endoscopic retrograde cholangiography. This widely practised procedure is relatively safe with rare complications. In this procedure, the common bile duct is cannulated during endoscopic visualisation of the papilla of Vater of the duodenum. In clinical practice, endoscopically placed biliary cannulas are used to safely deliver radio-opaque contrast agents to the biliary tract, and this approach should be effective for infusion of recombinant adenoviruses. It allows for retrograde infusion of adenoviruses while antegrade outflow can be limited by balloon catheterisation of the distal common bile duct. Furthermore, after termination of adenoviral infusion, excessive adenoviruses are delivered immediately into the duodenum and excreted in stool.
In conclusion, retrograde adenoviral administration into the biliary tract may be a clinically practical modality for inducing repeated expression of therapeutic genes in the liver. Although more investigations should be performed to establish useful gene therapy with adenoviruses, our results support the feasibility of adenovirus mediated gene transfer into the liver in the clinic.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (B-14370185) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
Abbreviations
E1, early gene region 1
pfu, plaque forming units
ALT, alanine aminotransferase
REFERENCES
- 1.Tripathy SK, Black HB, Goldwasser E, et al. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med 1996;2:545–50. [DOI] [PubMed] [Google Scholar]
- 2.Yang Y , Li Q, Ertl HCJ, et al. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol 1995;69:2004–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan JM, St George JA, Pennington SE, et al. Humoral and cellular immune responses of nonhuman primates to long-term repeated lung exposure to Ad2/CFTR-2. Gene Ther 1996;3:117–27. [PubMed] [Google Scholar]
- 4.Yei S , Mittereder N, Tang K, et al. Adenovirus-mediated gene transfer for cystic fibrosis: quantitative evaluation of repeated in vivo vector administration to the lung. Gene Ther 1994;1:192–200. [PubMed] [Google Scholar]
- 5.Dong J-Y, Wang D, Van Ginkel FW, et al. Systematic analysis of repeated gene delivery into animal lungs with a recombinant adenovirus vector. Hum Gene Ther 1996;7:319–31. [DOI] [PubMed] [Google Scholar]
- 6.D’Ambrosio E , Del Grosso N, Chicca A, et al. Neutralizing antibodies against 33 human adenoviruses in normal children in Rome. J Hyg 1982;89:155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piedra PA, Poveda GA, Ramsey B, et al. Incidence and prevalence of neutralizing antibodies to the common adenoviruses in children with cystic fibrosis: implication for gene therapy with adenovirus vectors. Pediatrics 1998;101:1013–9. [DOI] [PubMed] [Google Scholar]
- 8.Rosenecker J , Harms K-H, Bertele RM, et al. Adenovirus infection in cystic fibrosis patients: implications for the use of adenoviral vectors for gene transfer. Infection 1996;24:5–8. [DOI] [PubMed] [Google Scholar]
- 9.Weinberg A , Fink MC, Takimoto S, et al. Enzyme linked immunosorbent assay: determination of anti-adenovirus antibodies in an infant population. Rev Inst Med Trop São Paulo 1989;31:336–40. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura Y , Wakimoto H, Abe J, et al. Adoptive immunotherapy with murine tumor-specific T lymphocytes engineered to secrete interleukin 2. Cancer Res 1994;54:5757–60. [PubMed] [Google Scholar]
- 11.Miyake S , Makimura M, Kanegae Y, et al. Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc Natl Acad Sci USA 1996;93:1320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuriyama S , Yoshikawa M, Ishizaka S, et al. A potential approach for gene therapy targeting hepatoma using a liver-specific promoter on a retroviral vector. Cell Struct Funct 1991;16:503–10. [DOI] [PubMed] [Google Scholar]
- 13.Kuriyama S , Tominaga K, Kikukawa M, et al. Transient cyclophosphamide treatment before intraportal readministration of an adenoviral vector can induce re-expression of the original gene construct in rat liver. Gene Ther 1999;6:749–57. [DOI] [PubMed] [Google Scholar]
- 14.Kuriyama S , Tominaga K, Mitoro A, et al. Immunomodulation with FK506 around the time of intravenous re-administration of an adenoviral vector facilitates gene transfer into primed rat liver. Int J Cancer 2000;85:839–44. [DOI] [PubMed] [Google Scholar]
- 15.Nakatani T , Kuriyama S, Tominaga K, et al. Assessment of efficiency and safety of adenovirus mediated gene transfer into normal and damaged murine livers. Gut 2000;47:563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y , Greenough K, Wilson JM. Transient immune blockade prevents formation of neutralizing antibody to recombinant adenovirus and allows repeated gene transfer to mouse liver. Gene Ther 1996;3:412–20. [PubMed] [Google Scholar]
- 17.Tsujinoue H , Kuriyama S, Tominaga K, et al. Intravenous readministration of an adenoviral vector performed long after the initial administration failed to induce re-expression of the original transgene in rats. Int J Oncol 2001;18:575–80. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan JM, Smith AE. Transient immunosuppression with deoxyspergualin improves longevity of transgene expression and ability to readminister adenoviral vector to the mouse lung. Hum Gene Ther 1997;8:1095–104. [DOI] [PubMed] [Google Scholar]
- 19.Kozarsky KF, McKinley DR, Austin LL, et al. In vivo correction of low density lipoprotein receptor deficiency in the Watanabe Heritable hyperlipidemic rabbit with recombinant adenoviruses. J Biol Chem 1994;269:13695–702. [PubMed] [Google Scholar]
- 20.Yang Y , Trinchieri G, Wilson JM. Recombinant IL-12 prevents formation of blocking IgA antibodies to recombinant adenovirus and allows repeated gene therapy to mouse lung. Nat Med 1995;1:890–3. [DOI] [PubMed] [Google Scholar]
- 21.Bennett J , Pakola S, Zeng Y, et al. Humoral response after administration of E1-deleted adenoviruses: immune privilege of the subretinal space. Hum Gene Ther 1996;7:1763–9. [DOI] [PubMed] [Google Scholar]
- 22.Green DR, Ware CF. Fas-ligand: privilege and peril. Proc Natl Acad Sci USA 1997;94:5986–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen P , Kovesdi I, Bruder JT. Effective repeat administration with adenovirus vectors to the muscle. Gene Ther 2000;7:587–95. [DOI] [PubMed] [Google Scholar]
- 24.McClane SJ, Chirmule N, Burke CV, et al. Characterization of the immune response after local delivery of recombinant adenovirus in murine pancreas and successful strategies for readministration. Hum Gene Ther 1997;8:2207–16. [DOI] [PubMed] [Google Scholar]
- 25.Vilquin J-T, Guérette B, Kinoshita I, et al. FK506 immunosuppression to control the immune reactions triggered by first-generation adenovirus-mediated gene transfer. Hum Gene Ther 1995;6:1391–401. [DOI] [PubMed] [Google Scholar]
- 26.Jooss K , Yang Y, Wilson JM. Cyclophosphamide diminishes inflammation and prolongs transgene expression following delivery of adenoviral vectors to mouse liver and lung. Hum Gene Ther 1996;7:1555–66. [DOI] [PubMed] [Google Scholar]
- 27.Smith TAG, White BD, Gardner JM, et al. Transient immunosuppression permits successful repetitive intravenous administration of an adenovirus vector. Gene Ther 1996;3:496–502. [PubMed] [Google Scholar]
- 28.Ilan Y , Jona VK, Sengupta K, et al. Transient immunosuppression with FK506 permits long-term expression of therapeutic genes introduced into the liver using recombinant adenoviruses in the rat. Hepatology 1997;26:949–56. [DOI] [PubMed] [Google Scholar]
- 29.Kuriyama S , Tominaga K, Kikukawa M, et al. Inhibitory effects of human sera on adenovirus-mediated gene transfer into rat liver. Anticancer Res 1998;18:2345–52. [PubMed] [Google Scholar]
- 30.Weiss A . T lymphocyte activation. In: Paul WE, ed. Fundamental immunology. New York: Raven Press, 1993:467–504.