Abstract
Background: Lateral spreading tumours are superficial spreading neoplasms now increasingly diagnosed using chromoscopic colonoscopy. The clinicopathological features and safety of endoscopic mucosal resection for lateral spreading tumours (G-type “aggregate” and F-type “flat”) has yet to be clarified in Western cohorts.
Methods: Eighty two patients underwent magnification chromoscopic colonoscopy using the Olympus CF240Z by a single endoscopist. All patients had received a previous colonoscopy where an endoscopic diagnosis of lateral spreading tumour was made. All lesions were examined initially using indigo carmine chromoscopy to delineate contour followed by crystal violet for magnification crypt pattern analysis. A 20 MHz “mini probe” ultrasound was used if T2 disease was suspected. Following endoscopic mucosal resection, patients were followed up at 3, 6, 12, and 24 months using total colonoscopy.
Results: Eighty two lateral spreading tumours were diagnosed in 80 patients (32% (26/82) F-type and 68% (56/82) G-type). G-type lesions were larger than F-type (G-type mean 42 (SD 14) mm v F-type 24 (6.4) mm; p<0.01). F-type lesions were more common in the right colon (F-type 77% (20/26) compared with G-type 39% (22/56); p<0.01) and more often associated with invasive disease (stage T2) (66% (10/15) v 33% (5/15); p<0.001). Fifty eight lesions underwent endoscopic mucosal resection (G-type 64% (37/58)/F-type 36% (21/58)). Local recurrent disease was detected in 17% of patients (10/58), all within six months of the index resection. Piecemeal resection and G-type morphology were significantly associated with recurrent disease (p<0.1). Overall “cure” rates for lateral spreading tumours using endoscopic mucosal resection at two years of follow-up was 96% (56/58).
Conclusions: Endoscopic mucosal resection for lateral spreading tumours, staged as T1, is a safe and effective treatment despite their large size. Endoscopic mucosal resection may be an alternative to surgery in selected patients.
Keywords: lateral spreading tumour, chromoscopy, cancer, colonoscopy
Lateral spreading tumours (LSTs) of the colorectum are defined as lesions greater than 10 mm in diameter with a low vertical axis that extend laterally along the luminal wall.1 Previously, such lesions have been termed “carpet form”, granular cluster type, assembled nodular type, short villous tumour, and IIa cluster type.2 Okamoto’s description of two distinct clinicopathological and phenotypic types now groups these lesions into two categories: granular-type (G-LST), which endoscopically consist of numerous nodules having a homogenous colour in comparison with the surrounding colonic mucosa and flat-type (F-LST).3 Such lesions are now increasingly reported in the West due to the introduction of chromoscopic and magnification colonoscopy that facilitates detection while permitting in vivo anticipation of histological characteristics and invasive depth.4,5
Although the clinicopathological characteristics and efficacy of endoscopic mucosal resection (EMR) of LSTs have been defined in Japanese cohorts,3,6–9 no such data exist in the West where surgical resection still remains the mainstay of treatment. We therefore conducted a prospective clinicopathological and endoscopic evaluation of LSTs in a large Western cohort to establish the safety and efficacy of EMR in this group.
METHODS
Total colonoscopy was performed on 82 patients using the Olympus CF240Z magnifying colonoscope (Olympus, Tokyo, Japan), from January 1999 to November 2003 by a single endoscopist. All patients had received a previous colonoscopic assessment where an endoscopic diagnosis of LST was made. Full ethics approval for the study was granted from the South Sheffield Research Ethics Committee with written informed consent given by each patient prior to the procedure. Colonic preparation comprised 2–4 litres of hypertonic polyethylene glycol solution (Kleanprep; Helix Bio-pharma Corp, Aurora, Ontario, Canada) 24 hours prior to the procedure. Conscious sedation using intravenous midazolam (1–10 mg) was used if required. All patients received a single 20 mg intravenous bolus dose of hyoscine-N-butylbromide (Buscopan; Boehringer, Ingelheim, Germany) at initiation of the procedure unless contraindicated. Caecal intubation was verified by identification of the tri-radiate fold, appendix orifice, ileocaecal valve, and small bowel biopsy at terminal ileal intubation.
Endoscopic definitions and technique
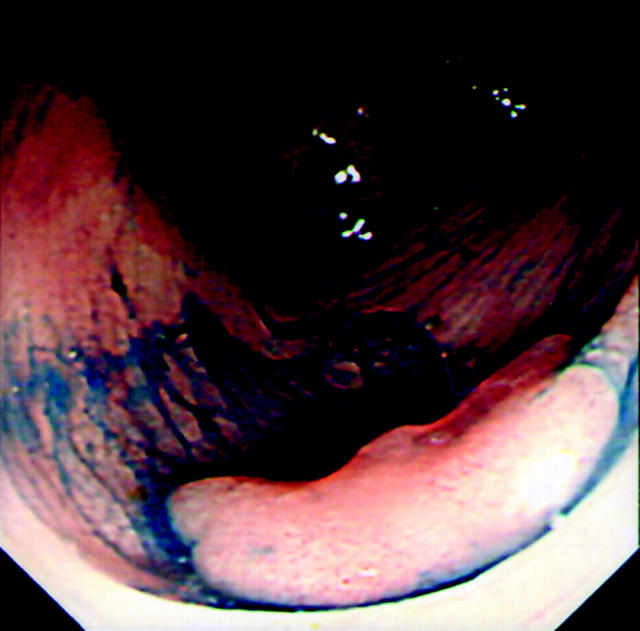
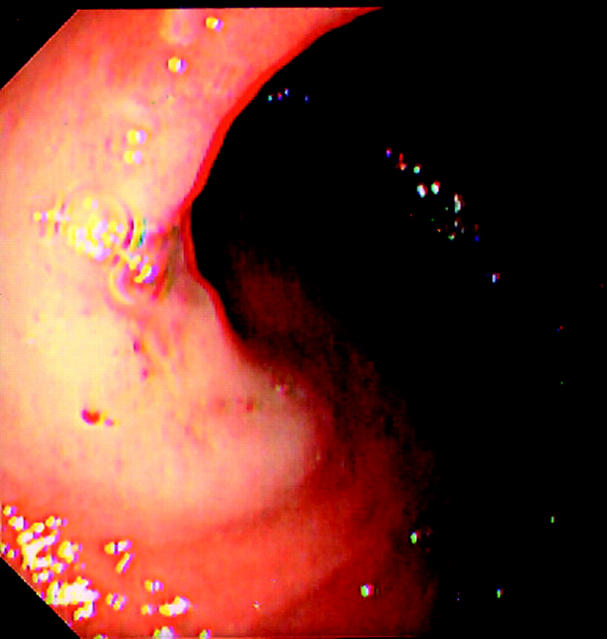
Lesions were initially identified using conventional colonoscopic views prior to further characterisation using 0.5% indigo carmine (IC) chromoscopy (Am. Reagent Laboratories Inc., New York, USA). A targeted chromoscopic technique was used by flushing 3–5 ml of IC down the side port of the colonoscope followed by a 10 ml air “flush”.10 This non-vital staining method allowed detailed morphology of the LST to be delineated, with particular reference to central depression (defined as a constant concavity in the lesion regardless of whether air is insufflated or deflated).11 Based on the criteria of Okamoto and colleagues,3 LSTs were defined as G-type when composed of superficial spreading aggregates of nodules forming a flat broad based lesion with a granulonodular and uneven surface (fig 1 ▶). F-type lesions were defined as lesions with a flat smooth surface in the absence of granulonodular formation (fig 2 ▶). The width of each lesion was estimated using a standard fully opened 4 mm biopsy forcep (Bard “Direct Bite”; Bard Ltd, Crawley, West Sussex, UK) or by using an “endo-rule” placed adjacent to the lesion. The height of each lesion was estimated by placing a closed biopsy forcep tip (2.1 mm) at the margin of the lesion.
Figure 1.
A granular-type lateral spreading tumour composed of spreading aggregates of nodules with a broad base.
Figure 2.
A flat type lateral spreading tumour of the caecum showing a flat smooth surface in the absence of granulonodular formation.
Following IC morphological assessment, all lesions underwent high magnification chromoscopic (HMC) crypt pattern analysis12 (applying the modified crypt guidelines of Kudo et al) using 0.05% crystal violet (CV) (Amino AG, Neuenhoff, Switzerland) which accurately identifies the invasive crypt pattern (Kudo type V(n)).12 Staining with CV was applied using a non-traumatic steel tipped catheter (2.45 mm PW4V-1; Olympus, Keymed, UK) following washing using locally applied N-acetyl-cysteine (2 mg/ml) to remove any adherent mucous.13 High frequency (20 MHz) “mini probe” ultrasound (HFUS) was subsequently performed when submucosal invasion was suspected from the above endoscopic assessment (that is, central depression at IC chromoscopy or identification of a type V crypt pattern at HMC).12 Lesions were excluded from EMR if one or more of the following criteria were met:
HFUS appearances suggestive of submucosal layer 3 invasion or beyond (that is, invasion of the muscularis)/or evidence of local lymph node metastasis.
Lesions showing an asymmetrical lift at submucosal injection (indicating tethering to the underlying muscularis mucosa where EMR can be complicated by perforation and incomplete resection).
Presence of an invasive type V pit pattern.
Lesions where anatomical position made endoscopic resection inaccessibility for complete EMR (that is, behind folds).
Spreading of lesions over two consecutive folds or occupying more than one third of the luminal circumference.
Non-correctable coagulopathy (prothrombin time >14 seconds or a platelet count <90×109/l).
Lesions excluded from EMR were biopsied only and an adjacent submucosal tattoo placed using the Indian ink “bleb” technique for future localisation. Surgical referral was then made.
Endoscopic mucosal resection technique
EMR using the strip biopsy method, as described by Karita et al, was used for all resections.14 Prior to EMR, mucosal cautery markings were placed around the circumference of the lesion so visualisation of the tumour margins could still be seen after submucosal saline injection.15 A barbed snare (N110; Olympus, Keymed, UK) was used for all EMRs with an “endo-coag” mode 25 W ERBE diathermy (ERBE Medical UK Ltd, Morley, Leeds; UK-Equipment ICC 200-International version). Resected tissue was retrieved using a disposal retrieval net (US Endoscopy Group Inc., Ohio, USA) or a grasping forcep (5202; TeleMed Systems Inc.,USA). Lesions <20 mm in diameter were resected en bloc with those >20 mm undergoing piecemeal resection. Following initial EMR, the lesion was again sprayed with IC so the presence of any residual neoplastic tissue could be identified and extended resection undertaken if required. Any lesion undergoing a piecemeal resection was classified as lateral margin positive for follow up purposes.
Complications of each resection were recorded. Bleeding was defined as “procedural” if it occurred during EMR, “immediate” if occurring within 24 hours of the procedure, and “delayed” if bleeding occurred >24 hours post EMR. Procedural bleeding was defined as that requiring endoscopic haemostatic therapy, with immediate or delayed being identified by the passage of fresh blood per rectum.
Histopathological evaluation
A single consultant histopathologist examined retrieved tissue. Tissue was fixed in 10% buffered formalin solution and examined using haematoxylin and eosin staining. Dysplasia was defined according to the Vienna criteria as either low (LGD) or high (HGD) grade (Vienna 3 and 4, respectively).16 When there was evidence of neoplastic cells involving the muscularis propria, lesions were classed as invasive advanced disease (stage T2 or beyond).16 Lesions were defined histologically as undergoing complete resection only when no remnant tissue was present at any point on the lateral or deep cut margin.
Post EMR follow up
Following “index” EMR, patients were followed up endoscopically at 3, 6, 12, and 24 months. Follow up colonoscopy was performed in an identical manner to the index examination with particular reference made to the previous resection site. On identification of the localising tattoo and previous EMR scar, CV was applied to the previous resection site and high magnification views obtained. Recurrent neoplastic disease was identified at HMC as a type IIIS, IIIL, IV, or V crypt pattern and defined as per the criteria of Higaki and colleagues7:
Tumour evident at the previous EMR site (fig 3 ▶).
Tumour (with no convergent folds) adjacent to a clearly defined EMR scar (1–2 mm proximity).
Tumour evident with fold convergence.
Figure 3.
Recurrent tumour at the previous endoscopic mucosal resection site (note the mucosal tattoo).
Based on the above surveillance criteria, local recurrent or residual neoplastic disease was diagnosed and subjected to a further extended EMR. Patients with histology showing invasive disease (infiltration of the submucosal layer 3 or muscularis mucosa) at the index EMR however were referred for direct surgical resection and excluded from long term endoscopic follow up.
Statistical analysis
The clinicopathological features of all lesions were recorded (including diameter, anatomical location, and histological diagnosis). Statistical differences were analysed by the χ2 test of independence (employing Yates’ correction for continuity) and the Mann-Whitney U test for continuous data between groups. The method of Bonferroni was utilised to recognise there was multiple testing of outcome data arising from individual patients. Calculations were made using the SPSS statistics package for Macintosh (System OsX-Microsoft Corp, USA). The University of Sheffield Statistics Unit provided statistical support.
RESULTS
Eighty patients underwent total colonoscopy using the CF240Z magnifying colonoscope. Caecal intubation or completion to the anastomosis in patients with a previous right hemicolectomy was achieved in 80 (100%). Terminal ileal intubation with confirmatory small bowel biopsy was achieved in 76 (95%) patients. Males represented 55% (44) of the cohort. Median age at index colonoscopy was 59 years (range 39–86). Sedation using intravenous midazolam (median dose 3 mg (range 1–8)) was required in 35 (44%) cases. One patient had received a previous right hemicolectomy for Dukes’ “A” adenocarcinoma two years previously.
Lesion demographics (table 1 ▶)
Table 1.
Histopathological characteristics, endoscopic classification, and anatomical location of all lateral spreading tumour
| Endoscopic class | Histology | Anatomical location | |||
| Adenoma | Carcinoma (stage T1) | Invasive carcinoma (stage T2 or beyond) | Right colon | Left colon | |
| G-type (n = 56, 68%) | 27 | 24 | 5 | 22 (39%) | 34 (61%) |
| F-type (n = 26, 32%) | 3 | 13 | 10 | 20 (77%) | 6 (23%) |
| Total (n = 82, 100%) | 30 | 37 | 15 | 44 | 40 |
A total of 82 LSTs were diagnosed in 80 patients, of which 56 (68%) and 26 (32%) were G-type and F-type, respectively. G-type lesions were significantly larger than F-type (G-type mean 42 (SD 14) mm v F-type 24 (6.4) mm; p<0.01). F-type lesions were more commonly diagnosed in the right colon (77% (20/26)) compared with G-type lesions (39% (22/56); p<0.01) and were more often associated with submucosal layer 3 invasion (66% (10/15) v 33% (5/15); p<0.001) respectively. Of the 15 invasive adenocarcinomas diagnosed, 11 (73%) had an area of central depression with 13 (87%) having a Kudo type V crypt pattern at HMC. The remaining two invasive lesions (13%) had a combination of crypt type IIIs and V. Ninety three per cent (14/15) of invasive lesions (T2) were correctly diagnosed using 20 MHz HFUS assessment. One lesion at the hepatic flexure could not be adequately assessed using HFUS due to positional difficulties and air artefact. However, using the combination of HMC and HFUS, all invasive carcinomas were correctly diagnosed. EMR was used to resect 58 (71%) lesions with 24 (29%) referred for surgery (15 staged as T2 at HMC/HFUS, two secondary to non-lifting at submucosal injection, and seven where anatomical location made endoscopic access difficult). There was no significance difference observed between the size of lesions treated by surgical resection and EMR (38 (12) mm v 36 (16) mm), respectively (p>0.5). Histopathological characteristics, endoscopic classification, and anatomical location of all lesions are summarised in table 1 ▶.
Endoscopic mucosal resection group
Of the 58 lesions resected by EMR, 64% (37/58) and 36% (21/58) were G-type and F-type LSTs, respectively. Histology confirmed adenoma in 24 (17 LGD/7 HGD), four villous adenomas, and 30 carcinomas (stage T1) without breach of the submucosal layer 3 or muscularis propria (stage T1). Thirty six lesions (62% (36/58)) were resected using a piecemeal technique with 38% (22/58) en bloc. EMR using piecemeal resection was used more often for G-type (62% (23/37)) than for F-type (18% (4/21)) LSTs (p<0.02). The total number of margin negative EMRs was 36% (21/58) where all lesions resected using a piecemeal technique had margin positive status histologically.
Bleeding complications occurred in six (10%) patients undergoing EMR (four procedural/two immediate). There were no delayed procedural bleeding complications with none requiring surgical intervention. All bleeding complications were controlled by “endo-clip” application (Olympus, Keymed, UK HX-200U-13K) where a focal bleeding point was identified in 5/6 cases using the “strawberry and cream” technique of Fujishiro and colleagues.17 There was no significant difference observed regarding the frequency of bleeding complications, size of lesion, morphological type, or choice of resection (that is, piecemeal versus en bloc). There were no perforations or other EMR related complications.
Post EMR colonoscopic follow up
Fifty eight patients underwent colonoscopic follow up of 58 lesions after their “index” EMR at 3, 6, 12, and 24 months. A post polypectomy scar identified as focal mucosal pallor with an indistinct vascular net pattern7 adjacent to a mucosal tattoo was localised at all follow up examinations (fig 4 ▶). Locally recurrent lesions were detected in 10 patients (17% (10/58)) throughout the two year surveillance programme. Eight of the recurrent lesions were G-type LSTs resected at “index” EMR using a piecemeal technique, with the remaining two (one G-type/one F-type) undergoing en bloc EMR. Piecemeal resection and G-type morphology were significantly associated with recurrent disease (p<0.1). Conventional colonoscopy diagnosed recurrent disease in six cases at follow up with four diagnosed as a type IIIL/IV or IIIs crypt pattern using HMC (fig 5 ▶). All cases of recurrent disease were diagnosed within the first six month follow up interval (see table 2 ▶). Eight patients underwent a further EMR to resect local recurrence with no residual disease present at 24 months of follow up. Histology showed adenoma (HGD), adenoma (LGD), tubulovillous adenoma, and carcinoma in situ in four, two, one, and three patients, respectively. Two lesions were referred for surgical resection due to non-lifting at attempted repeat EMR (one carcinoma (stage T1), one adenoma (LGD)) with no local lymph node metastasis identified at laparotomy. For all recurrent lesions, the “index” lesion was classified as margin positive histologically. Overall “cure” rates for LSTs using EMR at two years of follow up was therefore 96% (56/58).
Figure 4.
Post endoscopic mucosal resection scar. Note the focal mucosal pallor and indistinct vascular net pattern (marked with red arrow).
Figure 5.
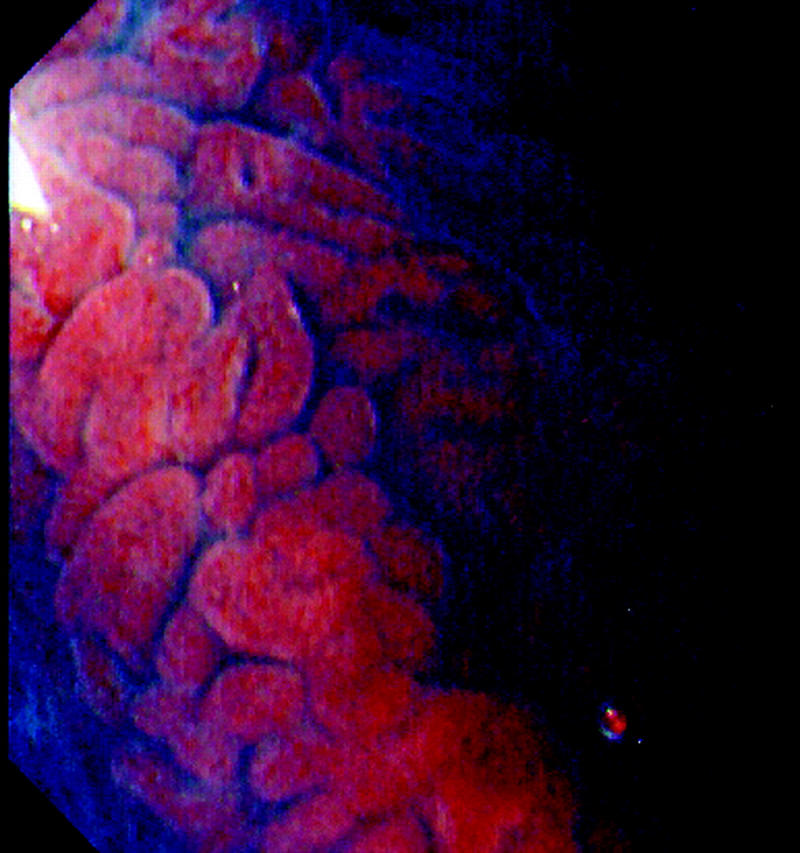
High magnification views (×100) of a recurrent lesion at the resection base of a mid transverse colon lesion. The crypt pattern is a type IV, suggesting tubulovillous adenoma.
Table 2.
Demographics of recurrent lesions following endoscopic mucosal resection (EMR)
| Case | Endoscopic class | Anatomical site | Index EMR method | Index margin status | Histology (recurrence) | Dominant crypt type | Recurrence interval (months) |
| 1 | G | Rectum | Piecemeal | + | Adenoma (LGD) | IIIL | 3 |
| 2 | G | Distal sigmoid | Piecemeal | + | Adenoma (HGD) | IIIL | 3 |
| 3 | G | Descending | Piecemeal | + | TVA | IV | 3 |
| 4 | G | Splenic flexure | Piecemeal | + | Adenoma (LGD) | IIIL | 3 |
| 5 | G | Proximal sigmoid | En bloc | + | Adenoma (HGD) | IIIs | 3 |
| 6 | G | Rectum | Piecemeal | + | Carcinoma (stage T1) | IIIs | 6 |
| 7 | G | Ascending | Piecemeal | + | Carcinoma (stage T1) | IIIs/V(a) | 3 |
| 8 | G | Transverse | Piecemeal | + | Adenoma (HGD) | IIIL | 3 |
| 9 | G | Caecum | Piecemeal | + | Adenoma (HGD) | IIIL | 3 |
| 10 | F | Caecum | En bloc | + | Carcinoma (stage T1) | IIIs/V(a) | 6 |
LGD, low grade dysplasia; HGD, high grade dysplasia.
DISCUSSION
This is the largest prospective study addressing the safety and efficacy of EMR for the treatment of LSTs in a Western cohort. We have shown that EMR can achieve high endoscopic “cure” rates in a selected cohort of lesions that is comparable with the Japanese experience of Tanaka and colleagues,6 Saito and colleagues,8 and Higaki and colleagues.7 These data may change the current management approach of LSTs from that of primary surgical resection to include endoscopic based strategies. The low elevated morphological characteristics of LSTs also makes them ideal candidates for “on table” staging using new technologies such as HMC and HFUS, often not possible in the assessment of sessile and subpedunculated colorectal lesions.18
Our data show a propensity of F-type LSTs for the right colon (77% (20/26)) and also a higher incidence of submucosal invasion (38% (10/26)) compared with G-type lesions (39% (22/56) and 9% (5/56), respectively). Our observations validate Tanaka’s series where there was a higher frequency of submucosal invasion in F-type compared with G-type lesions (p<0.05).6 G-type lesions in our series were also significantly larger than F-type lesions (p<0.01), a finding similar to that of Yoshikane and colleagues,19 Okamoto and colleagues,3 and Saito and colleagues.8 Furthermore, the frequency of invasive carcinoma in G-type LSTs was less than for exophytic lesions of comparable size.20
Fifteen stage T2 carcinomas (invading the muscularis) were diagnosed in our series (five G-type/10 F-type), and were excluded from EMR (18% (15/82) of the cohort). All of these lesions were correctly staged endoscopically using a combination of chromoscopy to accurately delineate morphology, crypt pattern analysis to identify an invasive type V pattern, and localisation of the submucosal layer 3 and muscularis propria using HFUS. Although in this study we correctly predicted 100% of invasive neoplasms by combining these techniques, new technologies such as HMC and HFUS are not available at many centres and carry a significant expense with requirements for further specialised training. However, given this limitation to our study, 13 lesions were diagnosed as stage T2 on the basic chromoscopic criteria of central depression and the non-lifting sign at submucosal injection.21 Therefore, even without HMC and HFUS, 86% (13/15) of T2 lesions would have been correctly anticipated using these basic endoscopic signs.
Although the risk of local nodal metastasis is low (5–10%) for stage T1 G-type and F-type LSTs,10 there remains a risk that endoscopic resection may leave untreated nodal disease in situ. The efficacy of routine preoperative computed tomography (CT) for the preoperative diagnosis of lymph node involvement has however been addressed by McAndrew and Saba.22 In this series of 180 patients undergoing surgery for primary colorectal carcinoma, only 19% of patients with lymph node involvement were correctly staged using CT. Furthermore, the utility of routine preoperative CT scanning in the management of colorectal cancer was shown to be unhelpful by Barton et al with clinical management decisions being definitively altered in only 19% of patients.23 Isbister and al-Sanea also demonstrated similar data in their prospective analysis of preoperative CT scanning in the assessment of colorectal cancer management.24 In this series, definitive management changes were proposed in only 12% of cases.24
In contrast with preoperative CT imaging, Hunerbein et al have recently assessed the efficacy of mini probe ultrasonography for the prediction of lymph node metastasis in patients with gastric and colorectal neoplasia.25 Lymph node status was correctly predicted in 82% of cases (sensitivity 61%/specificity 94%).25 Based on mini probe staging criteria, patients with gastric cancer were accurately selected to undergo EMR, laparoscopic resection, or open surgery in 100%, 91%, and 86% of cases, respectively. Furthermore, in patients with colorectal tumours, the treatment decision analysis showed correct stratification in 90% of patients.25
Our study used combination in vivo staging with HFUS and HMCC, which at present appears to be the optimal staging modality to predict local nodal disease compared with CT imaging. Two lesions (one G-LST/one F-LST) were referred for surgical resection in our series due to recurrent disease at six months of follow up. Both lesions demonstrated the non-lifting sign at follow up EMR but had no evidence of local nodal disease at HFUS, confirmed at subsequent laparotomy. Therefore, in clinic practice the risk of local nodal metastases should be discussed with the patient, where the low risk of leaving local lymph nodes in situ can be debated against the overall risk of surgery on consideration of the wider clinical context (that is, patient age and comorbidity). We therefore propose that EMR of LSTs can be practised safely in both the district general and specialised tertiary referral endoscopic units.
Of the 58 lesions undergoing EMR, 10% (6/58) were complicated by bleeding but there were no perforations. These data represent a higher bleed rate compared with Higaki’s series7 (4% (1/23)) but lower than that reported by Tanaka et al (16% (13/78)).6 Such data are comparable with those of Walsh and colleagues20 and Bedogni and colleagues26 who reported a bleed frequency (procedural and delayed) of 3–12% for snare resection of giant colorectal polyps. However, all bleeding complications in both of our series and others were managed successfully using endoscopic clipping with no requirement for surgical intervention.
Endoscopic follow up in our study was performed at similar time intervals to Higaki’s series7 (3, 6, 12, and 24 months post index resection) with those in Tanaka’s cohort undergoing a mean follow up of 60.8 (SD 20.1) months.6 Of the 10 recurrent lesions in our series, all were diagnosed at the first six month follow up colonoscopy and all but one were adenomatous lesions accompanying the index lesion. In the single exception, a carcinoma (stage T1) was identified as a flat 3 mm lesion at the resection base of a 20 mm F-type LST with a type IIIs crypt pattern. These data are comparable with those of Tanaka’s series where 83% (5/6) of cases of recurrent disease were diagnosed within six months of the index EMR.6 Only one patient in Tanaka’s cohort developed a recurrent adenoma at 13.5 months post index resection of a 20 mm G-type ascending colonic lesion.6 Our data therefore suggest that recurrence rates are low following EMR (either piecemeal or en bloc) for either G- or F-type LSTs, where recurrence in Brooker’s series of sessile polypectomy without argon plasma coagulation was in excess of 50% at 12 months post polypectomy.27
In conclusion, EMR of both G- and F-type LSTs is a safe and effective management for stage T1 lesions of the colorectum, even in large lesions. We recommend that colonoscopic follow up is performed at six and 12 months post index resection to assess recurrent disease status where a further EMR can be performed if required. Chromoscopy and HMC may be helpful tools given this scenario. Accordingly, EMR may provide an alternative to surgery in selected patients.
Acknowledgments
The study received grant support from the following: Smith and Nephew Research Foundation (UK), BRET Research Foundation (UK), Butterfield “Sasakawa” Research Foundation (UK), Mason Medical Research Foundation (UK), Keymed (UK), Special Trustees to the Sheffield Central University Hospitals, and the Peel Medical Research Foundation.
Abbreviations
LST, lateral spreading tumour
G-LST, granular-type LST
F-LST, flat-type LST
EMR, endoscopic mucosal resection
LGD, low grade dysplasia
HGD, high grade dysplasia
HFUS, high frequency ultrasound
HMC, high magnification chromoscopy
IC, indigo carmine
CV, crystal violet
CT, computed tomography
REFERENCES
- 1.Kudo S . Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy 1993;25:455–61. [DOI] [PubMed] [Google Scholar]
- 2.Hurlstone D , Korulla C, Lobo A. Colorectal laterally spreading tumors: Clinical evaluation and endoscopic strategies updated. J Gastroenterol Hepatol 2002;17:1344–5. [DOI] [PubMed] [Google Scholar]
- 3.Okamoto T , Tanaka S, Haruma K, et al. Clinicopathological evaluation on colorectal laterally spreading tumor (LST) (in Japanese with English abstract). Nippon Geka Gakkai Zasshi 1996;93:83–9. [PubMed] [Google Scholar]
- 4.Hurlstone DP, Fujii T, Lobo AJ. Early detection of colorectal cancer using high-magnification chromoscopic colonoscopy. Br J Surg 2002;89:272–82. [DOI] [PubMed] [Google Scholar]
- 5.Hurlstone DP, Cross SS, Adam I, et al. Efficacy of high magnification chromoscopic colonoscopy for the diagnosis of neoplasia in flat and depressed lesions of the colorectum: a prospective analysis. Gut 2004;53:284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka S , Haruma K, Oka S, et al. Clinicopathological features and endoscopic treatment of superficial spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc 2001;54:62–6. [DOI] [PubMed] [Google Scholar]
- 7.Higaki S , Hashimoto S, Harada K, et al. Long-tern follow-up of large flat colorectal tumours resected endoscopically. Endoscopy 2003;35:845–9. [DOI] [PubMed] [Google Scholar]
- 8.Saito Y , Fujii T, Kondo H, et al. Endoscopic treatment for laterally spreading tumors in the colon. Endoscopy 2001;33:682–6. [DOI] [PubMed] [Google Scholar]
- 9.Koba I , Yoshida S, Fujii T, et al. Diagnostic findings in endoscopic screening of superficial colorectal neoplasia: results froma prospective study. Jpn J Clin Oncol 1998;28:542–5. [DOI] [PubMed] [Google Scholar]
- 10.Kudo S , Kashida H, Tamura T, et al. Colonoscopic diagnosis and management of nonpolypoid early colorectal cancer. World J Surg 2000;24:1081–90. [DOI] [PubMed] [Google Scholar]
- 11.Kudo S , Tamure S, Nakajima T, et al. Depressed type of colorectal cancer. Endoscopy 1995;27:54–7. [DOI] [PubMed] [Google Scholar]
- 12.Kudo S , Rubio CA, Teixeira CR, et al. Pit pattern in colorectal neoplasia: endoscopic magnifying view. Endoscopy 2001;33:367–73. [DOI] [PubMed] [Google Scholar]
- 13.Kudo S , Kashida H, Nakajima T, et al. Endoscopic diagnosis and treatment of early colorectal cancer. World J Surg 1997;21:694–701. [DOI] [PubMed] [Google Scholar]
- 14.Karita M , Tada M, Okita K, et al. Endoscopic therapy for early colon cancer: the strip biopsy resection technique. Gastrointest Endosc 1991;37:128–32. [DOI] [PubMed] [Google Scholar]
- 15.Hurlstone DP. Superficial spreading type colorectal tumors. World J Surg 2003;27:1340–1. [DOI] [PubMed] [Google Scholar]
- 16.Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal neoplasia. Gut 2000;47:251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujishiro M , Ono H, Gotoda T, et al. Usefulness of Maalox for detection of the precise bleeding point position and confirmation of hemostasis on gastrointestinal hemorrhage. Endoscopy 2001;33:196. [PubMed] [Google Scholar]
- 18.Saitoh Y , Obara T, Einami K, et al. Efficacy of high-frequency ultrasound probes for the preoperative staging of invasion depth in flat and depressed colorectal tumors. Gastrointest Endosc 1996;44:34–9. [DOI] [PubMed] [Google Scholar]
- 19.Yoshikane H , Hidano H, Sakakibara A, et al. Endoscopic resection of laterally spreading tumours of the large intestine using a distal attachment. Endoscopy 1999;31:426–30. [DOI] [PubMed] [Google Scholar]
- 20.Walsh RM, Ackroyd FW, Shellito PC. Endoscopic resection of large sessile colorectal polyps. Gastrointestinal Endoscopy 1992;38:303–9. [DOI] [PubMed] [Google Scholar]
- 21.Saitoh Y , Obara T, Watari J, et al. Invasion depth diagnosis of depressed type early colorectal cancers by combined use of videoendoscopy and chromoendoscopy. Gastrointest Endosc 1998;48:362–70. [DOI] [PubMed] [Google Scholar]
- 22.McAndrew MR, Saba AK. Efficacy of routine preoperative computed tomography scans in colon cancer. Am Surg 1999;65:205–8. [PubMed] [Google Scholar]
- 23.Barton JB, Langdale LA, Cummings JS, et al. The utility of routine preoperative computed tomography scanning in the management of veterans with colon cancer. Am J Surg 2002;183:499–503. [DOI] [PubMed] [Google Scholar]
- 24.Isbister WH, al-Sanea O. The utility of pre-operative abdominal computerized tomography scanning in colorectal surgery. J R Coll Surg Edinb 1996;41:232–4. [PubMed] [Google Scholar]
- 25.Hunerbein M , Handke T, Ulmer C, et al. Impact of miniprobe ultrasonography on planning of minimal invasive surgery for gastric and colonic tumours. Surg Endosc 2004;18:601–5. [DOI] [PubMed] [Google Scholar]
- 26.Bedogni G , Bertoni G, Ricci H, et al. Colonoscopic excision of large and giant colorectal polyps. Technical implications and results over eight years. Dis Colon Rectum 1986;29:831–5. [DOI] [PubMed] [Google Scholar]
- 27.Brooker JC, Saunders BP, Shah SG, et al. Treatment with argon plasma coagulation reduces recurrence after piecmeal resection of large sessile colonic polyps: a randomised trial and recommendations. Gastrointest Endosc 2002;55:371–5. [DOI] [PubMed] [Google Scholar]