von Boyen et al recently reported a study of glial fibrillary acidic protein (GFAP) expression in enteric glia (Gut 2004;53;222–8). Their new data are very interesting and add to our understanding of the possible role of enteric glia in gastrointestinal pathophysiology. However, we must take issue with some of the data presented that show extensive nuclear labelling with S-100 and with the description of the distribution of enteric glia in the colon.
Figure 1 ▶ of their paper shows labelling of enteric glia in the rat colon below the epithelial crypts and is thus presumably labelling of cells in the submucosal plexus. In the paper, this layer is described as the “plexus mucosus”. The plexus mucosus, which is also known as the mucosal plexus,1 has previously been described in humans and rat.2,3 As the name implies, the mucosal plexus is located within the mucosa. Given the position of the crypts, as indicated by the ovals in fig 1 ▶, it would appear that the labelling shown in panels A and B is in fact localised to the submucosal plexus.
Figure 1.
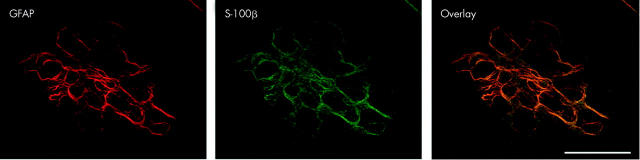
Confocal image revealing colocalisation of glial fibrillary acidic protein (GFAP) and S-100 in enteric glia.
We find extensive colocalisation of GFAP and S-100 in the submucosal plexus. This is illustrated below in fig 1 ▶ in a whole mount preparation of the submucosal plexus from the rat colon. This confocal image reveals colocalisation of GFAP and S-100 in enteric glia (17 µm z stack of 1 µm optical sections; scale bar 50 µm) (fig 1 ▶). S-100 is also found in the cytoplasm of the glial perikarya; there is virtually no nuclear labelling, which was the most obvious element of the staining demonstrated by von Boyen et al.
In fig 1 ▶ of the paper of von Boyen et al, the nature of the GFAP immunoreactivity is not fibrous, but granular, while the predominant labelling of S-100 is nuclear. In our hands this is not the case (see our fig 1 ▶) and so we feel this calls into question whether the extensive nuclear labelling observed in both fig 1 ▶ and fig 2 is really reflective of the distribution of S-100. Moreover, in the paper cited by the authors in support of nuclear localisation, Ferri et al state that “only cytoplasmic localisation (of S-100) was consistently demonstrated in enteric glia”,4 contrary to von Boyen et al’s assertion that S-100 labelling is largely nuclear.
Finally, it should also be noted that GFAP expression in culture may reflect an altered state of differentiation as an adaptation to culturing.5,6 Hence some of the observed changes in GFAP expression may be explained by processes reflecting changes in the culture conditions rather than a pathophysiological response to cytokines.
The issues of glial heterogeneity and the role of enteric glia in inflammation raised in the paper are very interesting, and of considerable importance in understanding the physiology and pathophysiology of the gastrointestinal tract. By analogy with the brain, it is likely that enteric glia play an important role in the function of the gut. However, we feel that the extensive glial heterogeneity suggested in the paper by von Boyen et al may be overestimated and we urge caution in extrapolation of these data based on the immunohistochemistry presented in this manuscript.
References
- 1.Furness JB, Costa M. The enteric nervous system. Edinburgh: Churchill-Livingstone, 1987.
- 2.Wedel T , Roblick U, Gleiss J, et al. Organization of the enteric nervous system in the human colon demonstrated by wholemount immunohistochemistry with special reference to the submucous plexus. Anat Anz 1999;181:327–37. [DOI] [PubMed] [Google Scholar]
- 3.Stach W . Innervation of the small intestinal mucosa of laboratory animals. II. Ultrastructure of neuro-cellular connections. Z Mikrosk Anat Forsch 1979;93:1012–24. [PubMed] [Google Scholar]
- 4.Ferri GL, Probert L, Cocchia D, et al. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature 1982;297:409–10. [DOI] [PubMed] [Google Scholar]
- 5.Juurlink BH, Hertz L. Plasticity of astrocytes in primary cultures: an experimental tool and a reason for methodological caution. Dev Neurosci 1985;7:263–77. [DOI] [PubMed] [Google Scholar]
- 6.Hauck SM, Suppmann S, Ueffing M. Proteomic profiling of primary retinal Müller glia cells reveals a shift in expression patterns upon adaptation to in vitro conditions. Glia 2003;44:251–63. [DOI] [PubMed] [Google Scholar]