Abstract
Background: Because hepatic cirrhosis is a major risk factor for hepatocellular carcinoma, recent guidelines by the European Association for the Study of the Liver (EASL) on clinical management of hepatocellular carcinoma recommend periodic ultrasound surveillance of cirrhotic patients with immediate workup for nodules >1 cm; an increase in the frequency of screening is considered sufficient for smaller lesions.
Aims: To determine the actual risk of hepatocellular carcinoma associated with the latter lesions and to assess the role of ultrasound guided-fine needle biopsy in their diagnosis
Patients and methods: Data were analysed for 294 new nodular lesions <20 mm, including 48 that were <10 mm, detected during a prospective multicentre study involving ultrasound surveillance of 4375 patients with hepatic cirrhosis. In the absence of α fetoprotein (AFP) levels diagnostic of hepatocellular carcinoma, ultrasound guided-fine needle biopsy was performed (n = 274). AFP and fine needle biopsy diagnoses of malignancies (hepatocellular carcinoma and lymphoma) were considered definitive. Non-malignant fine needle biopsy diagnoses (dysplastic or regenerative nodule) were verified by a second imaging study. Diagnoses of hepatocellular carcinoma based on this study were considered definitive; non-malignant imaging diagnoses were considered definitive after at least one year of clinical and ultrasound follow up.
Results: Overall, 258/294 (87.6%) nodules proved to be hepatocellular carcinoma, including 33/48 (68.7%) of those ⩽10 mm. Overall typing accuracy of ultrasound guided-fine needle biopsy was 89.4%, and 88.6% for lesions ⩽10 mm.
Conclusions: In a screening population, well over half of very small nodules arising in cirrhotic livers may prove to be hepatocellular carcinoma, and approximately 90% of these malignancies can be reliably identified with ultrasound guided-fine needle biopsy.
Keywords: hepatocellular carcinoma, liver cirrhosis, ultrasound, liver biopsy
Hepatocellular carcinoma (HCC) is one of the most common malignant neoplasms in the world,1 and its incidence is expected to rise in the future.2 Most HCCs arise in cirrhotic livers,3 with an annual incidence of 1–6%.1 Because cirrhosis is the main risk factor for this type of cancer, cirrhotic patients are often enrolled in surveillance programmes based on periodic ultrasound (US) scans and serum α1 fetoprotein (AFP) determinations. This practice increases the chances of early detection of small asymptomatic HCCs which are still amenable to curative treatment by means of percutaneous ethanol injection,4,5 percutaneous radiofrequency ablation,6,7 surgical resection,5,8 orthotopic liver transplantation,9 or transcatheter intra-arterial chemoembolisation.10 Semiannual or annual surveillance programmes have had equally positive effects on survival of cirrhosis patients with HCC and significantly increased the percentage of cases that can be treated with liver transplantation.11
Not all focal lesions found in cirrhotic livers prove to be HCC. Indeed, many of the very small nodules detected today are still in the preneoplastic stage (dysplastic nodules) or are benign lesions (for example, regenerative nodules). The risk of malignancy increases however with the dimensions of the nodule. For this reason, the surveillance scheme proposed by the recent European Association for the Study of the Liver (EASL) conference on “clinical management of hepatocellular carcinoma”12 distinguishes between lesions measuring less than 1 cm and those with larger diameters. While the latter are to be subjected to immediate workup, the recommended policy for smaller lesions is simply an increase in the frequency of US surveillance (every three months instead of every six months). The rationale behind this position is based on the high probability that lesions of this size are not malignant and the low probability of obtaining a reliable diagnosis at this stage based on the diagnostic tools currently at our disposal.
Our longstanding experience in the diagnosis and treatment of cirrhosis patients with HCC tends to contrast with the recommendations of the conference for management of these very small nodules. We recently completed a prospective multicentre study on HCC screening, which involved more than 4000 patients with newly diagnosed cirrhosis from various parts of Italy and lasted nine years. During this time, 688 new focal hepatic lesions were detected by regular US examination, including 294 with diameters of 20 mm or less, which is the current definition of “small” liver nodules.13 These 294 nodules are the primary focus of the present report. The objectives of our analysis were to quantify the actual risk of malignancy (that is, HCC) of small nodular lesions detected by US screening of patients with cirrhosis and to evaluate the accuracy of US guided-fine needle biopsy (FNB) in their diagnosis; a pre-established protocol until a definitive diagnosis of HCC emerged (details below). In most cases, cirrhosis was attributed to hepatitis C (antibodies detected by third generation ELISA and confirmed with third generation recombinant immunoblot assays), hepatitis B, or alcohol abuse. Less common aetiologies were combined hepatitis B and C infections, hepatitis C plus alcohol abuse, haemochromatosis, and coinfections with the hepatitis delta and B viruses. In a small percentage of patients, the aetiology of cirrhosis could not be determined.
PATIENTS AND METHODS
Patient recruitment
This prospective multicentre study was conducted over a nine year period (1 January 1992 to 31 December 2001) in four large healthcare centres in various parts of Italy. Patients were recruited until December 2000. Inclusion criteria were: (1) newly diagnosed hepatic cirrhosis; (2) absence of focal liver lesions on the initial pre-enrolment US examination; and (3) willingness to take part in a follow up surveillance programme aimed at early detection of HCC, which involved periodic US examination and evaluation of serum AFP levels.
Of the approximately 5000 newly diagnosed cirrhotic patients admitted to our referral centres during the recruitment period, 4581 agreed to participate in the study. In most cases, cirrhosis had been diagnosed based on clinical findings (hepatosplenomegaly, spider angiomata, ascites, evidence of portosystemic collaterals); blood chemistry data (decreased albumin and total cholesterol serum levels; prolonged prothrombin time; increased γ-globulin, aspartate aminotransferase, alanine aminotransferase, and total bilirubin serum levels; low platelet count), and US findings (evidence of nodular liver surface, caudate lobe hypertrophy, “coarse” liver tissue pattern). In a small percentage of patients, cirrhosis was detected at liver biopsy performed to confirm a suspicion of chronic active hepatitis.
Two hundred six of 4581 candidates (4.5%) were excluded from the surveillance study and referred for appropriate workup and/or treatment of pre-existent nodular lesions revealed by the initial US screening. The remaining 4375 (2928 men, 1447 women, aged 28–86 years; mean 64.1 (SD 11.1) years), whose initial examination revealed no US evidence of liver nodules, were enrolled in the surveillance programme.
The study protocol was approved by the medical ethics committees of the four healthcare institutions participating in the study. Written informed consent was obtained for all procedures and for use of collected data for research purposes.
HCC surveillance protocol and diagnostic criteria
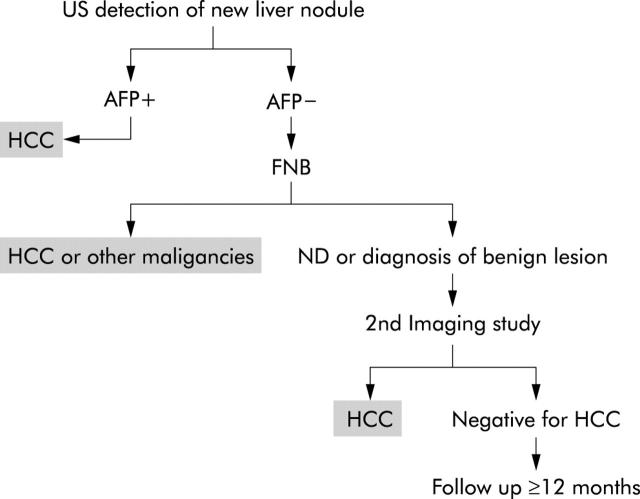
An overview of the diagnostic protocol used in the surveillance programme is shown in fig 1 ▶. The programme was based on hepatic US examination (performed with the following scanners: Toshiba SSA 250 A scanner with a 3.75 MHz convex probe, Toshiba Tosbee scanner with a 3.75 MHz convex probe, Toshiba Eccocee scanner with a multifrequency 3.5–6.0 MHz convex probe, Hitachi AU 560 scanner with 3.5 and 5 MHz convex probes, and ATL 3000 scanner with a multifrequency 2–4 MHz convex probe) and serum AFP determinations at 4–6 month intervals. When one or more focal hepatic lesions were found and AFP levels were >400 ng/ml, which is widely considered diagnostic of HCC,14 the diagnosis was considered definitive. The patient was excluded from further surveillance and referred for appropriate treatment.
Figure 1.
Protocol for diagnosis of new hepatic nodules detected during the hepatocellular carcinoma (HCC) surveillance programme. Patients underwent hepatic ultrasonography (US) and serum α fetoprotein (AFP) determinations every 4–6 months. New nodules detected on US that were associated with AFP levels >400 ng/ml were definitively diagnosed as HCC. All other new nodules were subjected to fine needle biopsy (FNB). If biopsy findings were consistent with HCC or other malignancies, according to the criteria in table 1 ▶, the diagnosis was considered definitive. If the specimen was inadequate for diagnosis (ND) or consistent with benign lesions, a second imaging study (contrast enhanced computed tomography or contrast enhanced magnetic resonance imaging) was performed. Imaging diagnosis of HCC at this point was considered definitive (see text for criteria). If imaging criteria were not met for HCC diagnosis, the non-malignant diagnosis based on FNB was tentatively accepted but an increase in the frequency of follow up was recommended (US, AFP, and clinical evaluation every 3–4 months) for the next 12 months. If there were no signs of malignancy during this period, the non-malignant diagnosis was considered definitive, and HCC surveillance was resumed at the original frequency. Patients with definitive diagnoses (indicated by shaded boxes) of HCC (or other malignancy) were immediately referred for treatment and excluded from the surveillance study.
If AFP levels were not diagnostic, the lesion was subjected to US guided FNB after verification of adequate coagulation (platelet counts >40 000/mm3 and prothrombin activity >40% (INR<2)). All biopsies were performed by experienced gastroenterologists using fine cutting needles and non-cutting spinal needles. The choice of technique was left to the individual examiner, based on personal experience and preference (see results for details). In general, all biopsies collected in a single centre were performed using the same technique, regardless of nodule size.
Biopsy diagnoses were based on the criteria shown in table 1 ▶. When histological and/or cytological findings met the criteria for HCC (or other malignancies shown in table 1 ▶), the diagnosis was considered definitive, and the patient was excluded from further surveillance and referred for treatment.
Table 1.
Diagnostic criteria used to type 274 focal liver lesions subjected to fine needle biopsy
| Diagnosis | Pathological criteria (reference) | Other requirements |
| HCC (histology) | Multiple liver cell plates in trabecular, solid, or acinar patterns surrounded by a network of sinusoidal vessels with stroma; scanty reticular architecture; invasion of veins in portal tract15 | None |
| HCC (cytology) | Nuclear hyperchromasia; increased nuclear-cytoplasmic ratio; trabecular pattern; nucleolar prominence; atypical naked nuclei; bizarre mitotic figures16 | None |
| Dysplastic nodules (mild dysplasia) | Plates ⩽2 cells thick; intact reticular architecture; no infiltrative edge; large cell dysplasia in some cases17 | Confirmation by second imaging study*+clinical and US follow up |
| Dysplastic nodules (severe dysplasia) | Plates ⩽3 cells thick; foci of decreased reticulum staining; irregular edges; small cell dysplasia and/or isolated glandular structures in some cases17 | Confirmation by second imaging study*+clinical and US follow up |
| Regenerative nodules | Hyperplastic hepatocytes relative or similar to the surrounding liver, generally uniform in structure, with elements of portal tracts regularly distributed inside; absence of cellular and nuclear atypia18 | Confirmation by second imaging study*+clinical and US follow-up |
| Non-Hodgkin’s lymphoma | Atypical lymphoid cells, mono- or polymorphic, whose monoclonal origin was confirmed by immunocytochemical techniques19 | None |
| Haemangioma | Abundant endothelial cells surrounded by stroma in the absence of other types of cells | Confirmation by second imaging study*+clinical and US follow up |
*Contrast enhanced computed tomography or contrast enhanced magnetic resonance (see methods for criteria and details).
HCC, hepatocellular carcinoma; US, ultrasound.
In contrast, patients whose biopsies were non-diagnostic and those with biopsy diagnoses of non-malignant lesions (table 1 ▶) underwent a second imaging study using standard techniques.20 In all participating centres, the vast majority of these patients underwent contrast enhanced computed tomography (CT). CT diagnosis of HCC was based on demonstration of lesion hypervascularity (that is, hyperattenuation of the nodule against a background of minimally enhanced liver tissue during the early phase of contrast medium administration, with hypoattenuation during the late portal venous phase).21 In one centre, contrast enhanced conventional spin echo magnetic resonance imaging (MRI) was used instead of CT for all patients studied between 1999 and 2001. On MRI studies, the following nodule characteristics were considered indicative of HCC: on conventional spin echo scans, high intensity signals in T1 weighted images and hyper- or isointensity on T2 weighted images; and high signal intensity during the arterial phase of triple phase dynamic GRE imaging.22 When CT or MRI criteria for HCC were met, the non-malignant FNB diagnosis was considered to be a false negative, the nodule was definitively diagnosed as HCC, and the patient was referred for pretreatment workup and appropriate therapy.
If the results of the second imaging study were not indicative of HCC (for example, CT findings of nodule hypoattenuation during contrast administration with isoattenuation during the late portal venous phase, characteristic of dysplastic or regenerative nodules23; or MRI findings compatible with haemangioma—that is, conventional spin echo scans showing lesion hypointensity on T1 weighted images with hyperintensity on T2 weighted images22) the non-malignant FNB diagnosis was tentatively accepted but the patient was followed closely for at least 12 months with clinical evaluation, hepatic US, and serum AFP determinations every 3–4 months. If there were no changes in the size or echostructure of the nodule and AFP levels remained unchanged, the non-HCC diagnosis based on FNB and second imaging findings was considered definitive, and HCC surveillance was resumed at a frequency of 4–6 months.
Typing accuracy of FNB was calculated as the percentage of pathological diagnoses that corresponded to the definitive diagnoses, as defined in table 1 ▶.
RESULTS
Nodules detected during surveillance
The 4375 cirrhotic patients enrolled in the surveillance programme were followed for a mean of 35.8 (21.8) months (range 13–108). During the study, a total of 688 new focal liver lesions (diameters 6–42 mm; mean diameter 22 (SD 10) mm) were detected by US. It is important to recall that none of these lesions had been visualised on examinations performed 3–4 months earlier. At the time of detection, 117 (17%) of the nodules had diameters >30 mm, and 277 others (40.3%) measured 21–30 mm. The percentages definitively diagnosed as HCC were, respectively, 94.9% and 97.1%.
The remaining 294 (42.7%) nodules, which are the primary focus of this report, were small single lesions with diameters ⩽20 mm (mean 13.6 (SD 4) mm; range 6–20). These nodules were found in 294 patients (216 men, 78 women; mean age 67.3 (SD 9.1) years). The aetiologies of cirrhosis in these patients were similar to those of the total study population: hepatitis C (219 cases, 74.5%); hepatitis B (37 cases, 12.6%); alcohol abuse (18 cases; 6.1%); hepatitis B and C (five cases; 1.7%); coinfection with the hepatitis delta and B viruses (three cases, 1%); hepatitis C and alcohol (two cases; 0.7%); haemochromatosis (two cases; 0.7%); and unidentified factors in eight patients (2.7%).
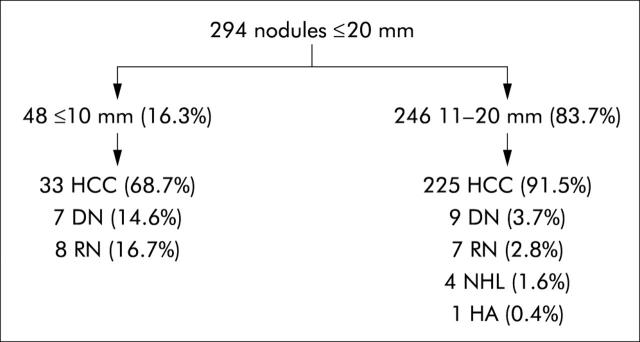
Figure 2 ▶ shows the definitive diagnoses made for these 294 small single hepatic nodules. In total, 258/294 (87.6%) proved to be HCC, based on the criteria for a definitive diagnosis of these lesions used in our study: (1) serum AFP levels >400 ng/ml or (2) FNB findings (cytology and/or histology) consistent with the diagnostic criteria for HCC listed in table 1 ▶ or (3) CT or MRI findings consistent with the imaging criteria for a diagnosis of HCC, described in the methods section. Because of their size, most of these lesions were treated with less invasive procedures, such as percutaneous ethanol injections or radiofrequency ablation. For the 10 small HCC nodules that were treated surgically (eight with hepatic resection, two with liver transplant), the preoperative diagnosis was consistently confirmed by surgical pathology. As fig 2 ▶ shows, the frequency of HCC diagnosis was considerably higher among nodules with diameters >10 mm. None the less, more than half of the nodules measuring ⩽10 mm (68.7%) were in fact HCCs, and almost 15% were confirmed as preneoplastic.
Figure 2.
Definitive diagnoses of the 294 small liver nodules detected during ultrasound surveillance of patients with cirrhosis. HCC, hepatocellular carcinoma; DN, dysplastic nodule; RN, regenerative nodule; NHL, non-Hodgkin’s lymphoma; HA, haemangioma.
Diagnosis of small nodules
Twenty of 294 small nodules (6.8%), including four with diameters ⩽10 mm, were immediately diagnosed as HCC based on serum AFP levels (>400 ng/ml), and these patients were scheduled for appropriate therapy without further investigation.
The remaining 274 lesions (mean diameter 15.3 (SD 3.8) mm; range 6–20) were subjected to US guided FNB. A total of 114 biopsies were collected “free hand”24 with a cutting needle (Histocut, 20–21 gauge; Sterylab, Rho, Milan, Italy) and samples for both histology and cytology were obtained with a single puncture.25 In the other 160 cases, a needle guide was attached to the side of the US scanner: 136 of these biopsies were performed with cutting needles (Surecut, calibre 21 gauge, or Biomol, calibre 20 gauge, both manufactured by TSK, Tokyo, Japan and purchased from Hospital Service, Rome, Italy); eight were done with spinal needles (Ecojet, calibre 21 gauge; Hospital Service); and in the remaining 16 cases, two consecutive punctures were made with cutting and spinal needles. Of the 274 subjected to US guided FNB, 245 (89.4%) received pathological diagnoses that were ultimately considered to be correct: 210 presented cytological and/or histological findings met our pathological criteria for a definitive diagnosis of HCC. These patients, as well as four others whose biopsies revealed non-Hodgkin’s lymphoma, were immediately referred for specific treatment. The remaining 31 nodules were pathologically diagnosed as non-malignant lesions: dysplastic nodules (n = 16: six with mild dysplasia, 10 with severe dysplasia); regenerative nodules (n = 14); or haemangioma (n = 1), and all diagnoses were subsequently confirmed by the results of a second imaging study and follow up based on clinical, US, and AFP findings (mean 28.4 (13.1) months; range 12–72).
For the remaining 29/274 lesions (10.6%), FNB failed to yield a correct diagnosis. In eight cases (2.9%) the specimens were considered inadequate for diagnosis. The other 21 lesions were incorrectly diagnosed as regenerative (n = 11) or dysplastic (n = 10) nodules on the basis of histology and/or cytology. All 29 of these nodules were subsequently diagnosed as HCC based on contrast enhanced CT (n = 24) or contrast enhanced MRI (n = 5), findings consistent with the criteria specified in the methods section.
The percentages of inadequate specimens collected with the various biopsy techniques used in the study were not significantly different.
Influence of nodule dimensions on FNB typing accuracy
To evaluate the possible influence of nodule size on US guided FNB typing accuracy, 274 nodules that were subjected to biopsy were divided into three groups based on their diameters. FNB typing accuracy was 88.6% for 44 nodules with diameters ⩽10 mm (group A); 86.2% for 80 nodules with diameters of 11–15 mm (group B); and 91.3% for 150 nodules measuring 16–20 mm (group C). Kappa statistics (used by one of the authors (SF) to verify the reliability of the FNB diagnoses in the three groups) were 0.75 for group A, 0.68 for group B, and 0.64 for group C (p significant for all groups).
Diagnosis of nodules ⩽10 mm in diameter
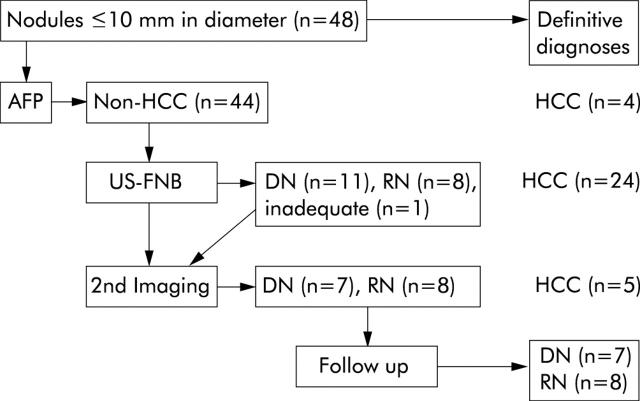
Figure 3 ▶ shows the modalities used to diagnose 48 nodules with diameters ⩽10 mm. Thirty three (68.7%) were ultimately identified as HCCs, the majority (24/33; 72.7%) based on biopsy findings alone. The 20 nodules that could not be definitively diagnosed as HCC based on AFP levels or FNB results were all examined with a second imaging method. Five (four diagnosed pathologically as dysplastic nodules, the other with an inadequate biopsy sample) were ultimately diagnosed as HCC, all based on CT findings. In the remaining 15 cases, the results of the imaging study and subsequent clinical and US follow up data (range 15–64 months; mean 23.6 months) were all fully consistent with the FNB diagnoses of dysplastic or regenerative nodules.
Figure 3.
Methods used to obtain definitive diagnoses of the 48 nodules ⩽10 mm in diameter. HCC, hepatocellular carcinoma; AFP, serum α fetoprotein (non-HCC: AFP <400 ng/ml; HCC: >400 ng/ml); US-FNB, ultrasound guided-fine needle biopsy; DN, dysplastic nodule; RN, regenerative nodule.
DISCUSSION
Early detection of HCC increases the patient’s chances of receiving treatment10 and improves prognosis,26,27 and for this reason serial US surveillance of high risk cirrhosis patients is becoming increasingly widespread. US is probably the most accurate imaging modality for visualising small liver nodules, even compared with more advanced techniques such as helical CT,28 CT during arterial portography, digital subtraction angiography, CT arteriography, and T1/T2 weighted MRI.29 As a result of these surveillance programmes, smaller and smaller HCCs are being found and subjected to early treatment with positive results. As the dimensions of detectable liver nodules decrease however, the percentage that actually prove to be malignant also declines. Indeed, tiny focal lesions are more likely to be premalignant (dysplastic) or even benign (regenerative) nodules, and our ability to distinguish these lesions from those of early stage HCC on the basis of US features (or those of other imaging modalities) remains limited.29–32
In the guidelines that emerged from the recent EASL conference in Barcelona,12 the authors acknowledged that “detection of a hypo- or hyperechoic nodule (in a cirrhotic liver) during follow up US should raise the suspicion of HCC”. However, while further diagnostic workup is indeed recommended for lesions of this type that are >1 cm, an increase in the frequency of US-AFP screening is considered sufficient for smaller nodules. The rationale behind this policy is based on the observations that only half of these small lesions will ultimately prove to be HCC and that, with the tools currently at our disposal, the likelihood of obtaining a correct diagnosis for HCCs of this size is extremely low.12
Our experience in the present study, together with a review of the literature, indicates that a number of reasonable objections can be raised against the “wait and see” policy of the EASL guidelines. As noted, the commission’s decision is justified by the substantial possibility that nodules with diameters <1 cm found in cirrhotic livers are in fact simply regenerative or mildly dysplastic lesions. This is based primarily on the results of post mortem pathological studies33,34 and those of studies comparing pretransplantation sonograms with pathological specimens of explanted livers, which show that lesions of this size (including HCCs) are often missed on US.35–37 In our opinion, however, the significance of a new nodule that appears in a cirrhotic liver during serial US surveillance is likely to be quite different from those found only at autopsy or following liver explantation. Indeed, for many years, the development of a focal lesion (regardless of its size) in a previously nodule free cirrhotic liver has been widely regarded as probable evidence of a neoplastic, or at least preneoplastic, process.38–40 As investigators in Japan41,42 have demonstrated, some dysplastic nodules detected by US show no signs of progression over time, and some may even disappear, but cases of this type are by no means the rule.
In the recent literature, there is little information on the actual nature of the nodules disclosed by US during the follow up of liver cirrhosis. In a recent study,43 all of 287 nodular lesions (range 7–41 mm; mean 18 mm) detected during US surveillance of cirrhotic patients were either neoplastic (93.7% HCCs and 1.8% non-Hodgkin’s lymphomas) or preneoplastic (4.5% dysplastic nodules). In an earlier study30 of 32 small (⩽20 mm) focal hepatic lesions detected in 23 consecutive cirrhotic patients, there were only 15 HCCs (46.9%) and five preneoplastic lesions (15.6%). However, this series included seven lesions that represented focal fatty changes, which usually have a non-nodular US appearance that is by no means typical of HCC. If these lesions are excluded, the frequencies of HCC (15/25; 60%) and preneoplastic nodules (5/25; 20%) increase considerably. Neither of these studies provides specific profiles of the nodular lesions measuring ⩽10 mm but our data indicate that the risk of HCC in these tiny lesions is substantial. More than half (68.7%) of those we examined proved to be HCC, and seven others (14.6%) were found to be preneoplastic (dysplastic nodules). However, our results were obtained in a screening setting, and different results may be encountered in non-screening groups of cirrhotic patients.
The policy recommended in the EASL guidelines also reflects the objective difficulties encountered in the diagnosis of liver nodules of this size. The limitations of imaging studies in this setting are well known.30,32,44 Guided biopsy is often the only way to differentiate between benign and malignant nodules arising on a background of liver cirrhosis.29 As early as 1994, a multicentre Italian survey45 found that US guided FNB yielded correct diagnoses for 87.5% of all liver lesions ⩽10 mm in diameter. This study was not confined to cirrhotic patients, and the lesions diagnosed included HCC as well as metastatic and benign nodules. A study from Japan46 published in 1999 compared the value of digital subtraction angiography and MRI with that of US guided biopsy in the diagnosis of 180 HCC nodules ⩽20 mm in diameter. Only 68% of the lesions measuring 11–20 mm in diameter could be diagnosed by imaging alone; the remaining 32% required biopsy. The contribution of biopsy was even more important for diagnosis of the smaller nodules in this series (⩽10 mm): less than half (45%) could be diagnosed based on imaging findings. Our experience in the present study confirms the diagnostic value of US guided FNB, which displayed an overall typing accuracy of 89.4% for nodules ⩽20 mm in diameter and equally good performance (88.6%) for the 44 lesions with diameters ⩽10 mm. These results are consistent with those of a recent French study47 in which US guided FNB diagnosed HCC nodules in cirrhotic livers with a sensitivity of 91% and, again, its accuracy was not influenced by the size of the nodule.
The use of US guided FNB (without confirmatory studies) allowed us to diagnose and initiate treatment almost immediately for 24/29 (82.8%) HCC nodules ⩽10 mm. Adherence to the EASL guidelines for these lesions would have led to a delay in treatment of at least three months. The potential prognostic repercussions of such a delay are difficult to identify. On the whole, earlier detection of HCC through screening has been shown to increase the chances of potentially curative treatment and improve survival.10,11 But there are no data that confirm that prompt treatment of HCCs <10 mm offers any advantage (in terms of long term survival or reduced healthcare costs) over somewhat later treatment,48 and this issue was not addressed in our study. In our experience however, a “wait and see” approach with increased frequency US can provoke stress and anxiety in the patient. Furthermore, the growth rate of untreated HCCs varies widely, but doubling of tumour volume within as little as one month as been documented by at least two groups,49,50 suggesting that in some cases at least, a 3–4 month delay in treatment could result in a considerably larger (and prognostically less favourable) lesion. All of the nodules detected in the present study were detected in patients whose livers had presented no evidence of focal lesions in US examinations performed 4–6 months earlier, and 17% of these nodules were already >30 mm in diameter at the time of US detection. Although we cannot exclude the possibility that some of these large nodules had been missed on earlier scans, as a result of the smaller dimensions, it is conceivable that they arose during the interim between the two scans and that their size at the time of detection simply reflects more rapid growth.
Confident exclusion of HCC is admittedly more difficult based on biopsy alone. In fact, the gold standard for diagnosis of regenerative and dysplastic liver nodules is the pathological examination of the whole resected lesion. In our study, FNB of nodules <2 cm in diameter was inadequate for diagnosis or revealed no evidence of malignancy in 29/274 (10.6%) cases. Our experience indicates that non-diagnostic specimens are more common with large nodules, which are more likely to contain areas of necrosis, while false negative biopsies are a greater risk with small nodules which often contain zones of cells that are still well differentiated. In both cases, a second FNB is associated with a fairly limited diagnostic gain.51,52 For this reason, in the present study, nodules whose FNBs were non-diagnostic or indicative of benign lesions were not re-biopsied. Instead, each was subjected to a second imaging, and all non-malignant diagnoses that emerged were considered definitive only after at least 12 months of close clinical and US follow up. This approach is less invasive and reliable. In fact, none of the 31 dysplastic or regenerative nodules diagnosed in this manner have shown any signs of malignant transformation.
The risk of malignant seeding is one of the major objections that has been raised against the use of FNB for diagnosis of suspected HCC lesions.53 Based on data from some of the largest series in the literature,54–57 this risk appears to be extremely low (0.003–0.009%). A somewhat higher rate emerged from the previously cited French study,46 in which two of 137 FNBs (1.6%) were associated with seeding. However, there were no cases of local recurrence after HCC nodule resection. As the authors of this study pointed out, the limited risk of seeding should be considered against that of false positive diagnosis of malignancy based on imaging studies alone. Indeed, in a series of Japanese patients whose focal lesions were evaluated exclusively on the basis of imaging data, 4/160 (2.5%) underwent surgery for lesions erroneously diagnosed as malignant.53 In our series, there were no cases of malignant seeding after FNB of a HCC nodule, and we feel that this risk is more than outweighed by the valuable information provided by US guided FNB.
In conclusion, our data confirm that US follow up of liver cirrhosis can result in very early detection of potentially malignant nodular hepatic lesions (that is, when they are still less than 10 mm in diameter). The probability of HCC undoubtedly increases when nodule diameter exceeds 10 mm (approximately 90%) but well over half (approximately 68%) of the smaller nodules we examined proved to be HCC and about 15% were considered to be premalignant. While US and other imaging techniques (even the more advanced) are of limited use for the diagnosis of these small nodules, close to 90% can be safely, rapidly, and reliably identified by means of US guided FNB. This approach can reduce the expense and patient anxiety associated with continuing US surveillance of possible/probable malignant lesions, but more importantly, it allows prompt treatment of HCC at stages in which the possibility of a cure is still good.
Acknowledgments
The authors wish to thank Ms Marian E Kent for her editorial assistance.
Abbreviations
HCC, hepatocellular carcinoma
EASL, European Association for the Study of the Liver
US, ultrasound
AFP, α fetoprotein
FNB, fine needle biopsy
US-FNB, ultrasound guided-fine needle biopsy
CT, computed tomography
MRI, magnetic resonance imaging
REFERENCES
- 1.Collier J , Sherman M. Screening for hepatocellular carcinoma. Hepatology 1998;27:273–8. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 1999;340:745–50. [DOI] [PubMed] [Google Scholar]
- 3.Simonetti RG, Cammà C, Fiorello F, et al. Hepatocellular carcinoma. A worldwide problem and the major risk factors. Dig Dis Sci 1991;36:862–72. [DOI] [PubMed] [Google Scholar]
- 4.Livraghi T . Role of percutaneous ethanol injection in the treatment of hepatocellular carcinoma. Dig Dis 2001;19:292–300. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto J , Okada S, Shimada K, et al. Treatment strategy for small hepatocellular carcinoma: comparison of long-term results after percutaneous ethanol injection therapy and surgical resection. Hepatology 2001;34:707–13. [DOI] [PubMed] [Google Scholar]
- 6.Livraghi T , Goldberg SN, Lazzaroni S, et al. Small hepatocellular carcinoma: treatment with radiofrequency ablation versus ethanol injection. Radiology 1999;210:655–61. [DOI] [PubMed] [Google Scholar]
- 7.Shirato K , Morimoto M, Tomita N, et al. Small hepatocellular carcinoma. Therapeutic effectiveness of percutaneous radio frequency ablation therapy with a Le Veen Needle electrode. J Ultrasound Med 2002;21:67–76. [DOI] [PubMed] [Google Scholar]
- 8.Livraghi T , Bolondi L, Buscarini L, et al. No treatment, resection and ethanol injection in hepatocellular carcinoma: a retrospective analysis of survival in 391 patients with cirrhosis. Cooperative HCC study group. J Hepatol 1995;25:522–6. [DOI] [PubMed] [Google Scholar]
- 9.Mazzaferro V , Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693–9. [DOI] [PubMed] [Google Scholar]
- 10.Yuen MF, Cheng CC, Lauder IJ, et al. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology 2000;31:330–5. [DOI] [PubMed] [Google Scholar]
- 11.Trevisani F , De Notariis S, Rapaccini GL, et al. Semiannual and annual surveillance of cirrhotic patients for hepatocellular carcinoma: effects on cancer stage and patient survival (Italian experience). Am J Gastroenterol 2002;97:734–44. [DOI] [PubMed] [Google Scholar]
- 12.Bruix J , Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona–2000 EASL Conference. J Hepatol 2001;35:421–30. [DOI] [PubMed] [Google Scholar]
- 13.International Working Party. Terminology of nodular hepatocellular lesions. Hepatology 1995;22:983–93. [DOI] [PubMed] [Google Scholar]
- 14.Taketa K . α-Fetoprotein: reevaluation in hepatology. Hepatology 1990;6:1420–32. [DOI] [PubMed] [Google Scholar]
- 15.Bottles K , Cohen MB. An approach to fine-needle aspiration biopsy diagnosis of hepatic masses. Diagn Cytopathol 1991;7:201–4. [DOI] [PubMed] [Google Scholar]
- 16.Kung IT, Chan SK, Fung KH. Fine-needle aspiration in hepatocellular carcinoma. Combined cytologic and histologic approach. Cancer 1991;67:673–80. [DOI] [PubMed] [Google Scholar]
- 17.Ferrell LD, Crawford JM, Dhillon AP, et al. Proposal for standardized criteria for the diagnosis of benign, borderline, and malignant hepatocellular lesions arising in chronic advanced liver disease. Am J Surg Pathol 1993;17:1113–23. [DOI] [PubMed] [Google Scholar]
- 18.Nakanuma Y , Terada T, Ueda K, et al. Adenomatous hyperplasia of the liver as a precancerous lesion. Liver 1993;13:1–9. [DOI] [PubMed] [Google Scholar]
- 19.Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: A proposal from the International Lymphoma Study Group. Blood 1994;5:1361–92. [PubMed] [Google Scholar]
- 20.Choi BI, Takayasu K, Han MC. Small hepatocellular carcinomas and associated nodular lesions of the liver: patology, pathogenesis, and imaging findings. AJR Am J Roentgenol 1993;160:1177–87. [DOI] [PubMed] [Google Scholar]
- 21.Freeny PC, Marks WM. Patterns of contrast enhancement of benign and malignant hepatic neoplasms during bolus dynamic and delayed CT. Radiology 1986;160:613–18. [DOI] [PubMed] [Google Scholar]
- 22.McFarland EG, Mayo-Smith WW, Saini S, et al. Hepatic hemangiomas and malignant tumors: improved differentiation with heavily T2-weighted conventional spin-echo MR imaging. Radiology 1994;193:43–7. [DOI] [PubMed] [Google Scholar]
- 23.Kudo M . Imaging diagnosis of hepatocellular carcinoma and premalignant/borderline lesions. Semin Liver Dis 1999;19:297–309. [DOI] [PubMed] [Google Scholar]
- 24.Caturelli E , Giacobbe A, Facciorusso D, et al. Free-hand technique with ordinary antisepsis in abdominal US-guided fine-needle punctures: three-year experience. Radiology 1996;199:721–3. [DOI] [PubMed] [Google Scholar]
- 25.Caturelli E , Bisceglia M, Fusilli S, et al. Cytological vs. microhistological diagnosis of hepatocellular carcinoma. Comparative accuracies in the same fine-needle biopsy specimen. Dig Dis Sci 1996;41:2326–31. [DOI] [PubMed] [Google Scholar]
- 26.Bolondi L , Sofia S, Siringo S, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut 2001;48:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen MH, Keeffen EB. Screening for hepatocellular carcinoma. J Clin Gastroenterol 2002;35:S86–91. [DOI] [PubMed] [Google Scholar]
- 28.Lim JH, Kim CK, Lee WJ, et al. Detection of hepatocellular carcinomas and dysplastic nodules in cirrhotic livers: accuracy of helical CT in transplant patients. AJR Am J Roentgenol 2000;175:693–8. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka Y , Sasaki Y, Katayama K, et al. Probability of hepatocellular carcinoma of small hepatocellular nodules undetectable by computed tomography during arterial portography. Hepatology 2000;31:890–8. [DOI] [PubMed] [Google Scholar]
- 30.Kanematsu M , Hoshi H, Yamada T, et al. Small hepatic nodules in cirrhosis: ultrasonographic, CT, and MR imaging findings. Abdom Imaging 1999;24:47–55. [DOI] [PubMed] [Google Scholar]
- 31.Lim JH, Cho JM, Kim EY, et al. Dysplastic nodules in liver cirrhosis: evaluation of hemodynamics with CT during arterial portography and CT hepatic arteriography. Radiology 2000;214:869–74. [DOI] [PubMed] [Google Scholar]
- 32.Rode A , Bancel B, Douek P, et al. Small nodule detection in cirrhotic livers: evaluation with US, spiral CT, and MRI and correlation with pathological examination of explanted liver. J Comput Assist Tomogr 2001;25:327–36. [DOI] [PubMed] [Google Scholar]
- 33.Nakashima T , Kojiro M. Hepatocellular carcinoma. Tokyo: Springer-Verlag, 1987.
- 34.Nakashima T , Kojiro M. Pathologic characteristics of hepatocellular carcinoma. Semin Liver Dis 1986;6:259–66. [DOI] [PubMed] [Google Scholar]
- 35.Mion F , Grozel L, Boillot O, et al. Adult cirrhotic liver explants: precancerous lesions and undetected small hepatocellular carcinomas. Gastroenterology 1996;111:1587–92. [DOI] [PubMed] [Google Scholar]
- 36.Kim CK, Lim JH, Lee WJ. Detection of hepatocellular carcinomas and dysplastic nodules in cirrhotic liver: accuracy of ultrasonography in transplant patients. J Ultrasound Med 2001;20:99–104. [DOI] [PubMed] [Google Scholar]
- 37.Bennett GL, Krinsky GA, Abitbol RJ, et al. Sonographic detection of hepatocellular carcinoma and dysplastic nodules in cirrhosis: correlation of pretransplantation sonography and liver explant pathology in 200 patients. AJR Am J Roentgenol 2002;179:75–80. [DOI] [PubMed] [Google Scholar]
- 38.Arakawa M , Kage M, Suguhara S, et al. Emergence of malignant lesions within an adenomatous hyperplastic nodule in a cirrhotic liver. Gastroenterology 1986;91:198–208. [DOI] [PubMed] [Google Scholar]
- 39.Rapaccini GL, Pompili M, Caturelli E, et al. Focal ultrasound lesions in liver cirrhosis diagnosed as regenerating nodules by fine-needle biopsy. Dig Dis Sci 1990;35:422–7. [DOI] [PubMed] [Google Scholar]
- 40.Caturelli E , Fusilli S, Costarelli L, et al. Focal ultrasound lesions in cirrhotic liver diagnosed as regenerative nodules by biopsy. A morphometric analysis. J Clin Gastroenterol 1993;17:67–72. [DOI] [PubMed] [Google Scholar]
- 41.Kondo F , Ebara M, Sugiura N, et al. Histological features and clinical course of large regenerative nodules: evaluation of their precancerous potentiality. Hepatology 1990;12:592–8. [DOI] [PubMed] [Google Scholar]
- 42.Seki S , Sakaguchi H, Kitada T, et al. Outcomes of dysplastic nodules in human cirrhotic liver: a clinicopathological study. Clin Cancer Res 2000;6:3469–73. [PubMed] [Google Scholar]
- 43.Caturelli E , Bartolucci F, Biasini E, et al. Diagnosis of liver nodules observed in chronic liver disease patients during ultrasound screening for early detection of hepatocellular carcinoma. Am J Gastroenterol 2002;97:397–405. [DOI] [PubMed] [Google Scholar]
- 44.Krinsky GA, Lee VS, Theise ND, et al. Hepatocellular carcinoma and dysplastic nodules in patients with cirrhosis: prospective diagnosis with MR imaging and explantation correlation. Radiology 2001;219:445–54. [DOI] [PubMed] [Google Scholar]
- 45.Fornari F , Filice C, Rapaccini GL, et al. Small (⩽3 cm) hepatic lesions. Results of sonographically guided fine-needle biopsy in 385 patients. Dig Dis Sci 1994;39:2267–75. [DOI] [PubMed] [Google Scholar]
- 46.Horigome H , Nomura T, Saso K, et al. Limitations of imaging diagnosis for small hepatocellular carcinoma: comparison with histological findings. J Gastroenterol Hepatol 1999;14:559–65. [DOI] [PubMed] [Google Scholar]
- 47.Durand F , Regimbeau JM, Belghiti J, et al. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol 2001;35:254–8. [DOI] [PubMed] [Google Scholar]
- 48.Ryder SD. Guidelines for the diagnosis and treatment of hepatocellular carcinoma in adults. Gut 2003;52:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheu JC, Sung JL, Chen DS, et al. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology 1985;89:259–66. [DOI] [PubMed] [Google Scholar]
- 50.Ebara M , Ohto M, Shinagawa T, et al. Natural history of minute hepatocellular carcinoma smaller than three centimeters complicating cirrhosis. A study in 22 patients. Gastroentrology 1986;90:289–98. [DOI] [PubMed] [Google Scholar]
- 51.Brunetti E , Bruno R, Marangio A, et al. Is second biopsy helpful in the diagnosis of hepatocellular carcinoma with a first negative biopsy? Am J Gastroenterol 2000;95:3688–9. [DOI] [PubMed] [Google Scholar]
- 52.Caturelli E , Biasini E, Bartolucci F, et al. Diagnosis of hepatocellular carcinoma complicating liver cirrhosis: utility of repeat ultrasound-guided biopsy after unsuccessful first sampling. Cardiovasc Intervent Radiol 2002;25:295–9. [DOI] [PubMed] [Google Scholar]
- 53.Torzilli G , Minagawa M, Takayama T, et al. Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology 1999;30:889–93. [DOI] [PubMed] [Google Scholar]
- 54.Weiss H , Duntsch U, Weiss A. Risiken der feinnadelpunktion. Ergebnisse einer umfrage in der BRD (DEGUM-Umfrage). Ultraschall 1988;9:121–7. [DOI] [PubMed] [Google Scholar]
- 55.Smith EH. Complications of percutaneous abdominal fine-needle biopsy. Radiology 1991;178:253–8. [DOI] [PubMed] [Google Scholar]
- 56.Weiss H , Duntsch U. Komplikationen der feinnadelpunktion (DEGUM-Umfrage II). Ultraschall 1996;17:118–30. [DOI] [PubMed] [Google Scholar]
- 57.Livraghi T , Torzilli G, Lazzaroni S, et al. Biopsia percutanea con ago sottile delle lesioni focali. In: Torzilli G, Olivari N, Livraghi T, et al, eds. Ecografia in Chirurgia. Milan: Poletto Editore, 1997:167–90.