Abstract
Background: It is now generally accepted that coeliac disease (CD) is caused by inflammatory T cell responses to gluten peptides bound to HLA-DQ2 or -DQ8 molecules. There is overwhelming evidence that CD patients can mount T cell responses to peptides found in both α-gliadin and γ-gliadin molecules. Assays that would detect the presence or absence of such peptides in food would thus be accurate indicators of safety for consumption by CD patients.
Aims: The development of a sensitive method to detect T cell stimulatory epitopes of α-gliadin and γ-gliadin molecules in food products.
Methods: Monoclonal antibodies (mAb) were raised against peptides encoding the T cell stimulatory epitopes of α-gliadin (amino acids (aa) 59–71) and aa γ-gliadin (aa 142–153 and aa 147–159). These mAb competition assays were developed that quantitatively detect T cell stimulatory epitopes present on both intact proteins and peptides of sizes recognisable by CD4+ T cells.
Results: With the mAb based competition assays, T cell epitopes were detected in pepsin/trypsin digests of wheat proteins and ethanol extracts of various food products, with detection levels lower than those reached with gluten specific T cells. Moreover, the presence of T cell stimulatory epitopes was also detected in preparations of barley, rye, and triticale, other cereals known to be toxic for CD patients.
Conclusions: A new antibody based method has been developed, detecting the presence of T cell stimulatory gluten peptides. This can be used to further ensure the safety of food consumed by CD patients.
Keywords: coeliac disease, T cell epitopes, α-gliadin, γ-gliadin, monoclonal antibodies, competition assay
Coeliac disease (CD) is a permanent intolerance of wheat gluten proteins, a complex mixture of storage proteins.1 Similar proteins are present in other cereals, such as barley, rye, oats, and triticale (a hybrid of wheat and rye). Typical symptoms observed in CD patients are chronic diarrhoea, malnutrition, anaemia, fatigue, and growth retardation. These symptoms are the result of a lesion in the small intestine characterised by (sub) total villous atrophy and increased numbers of intraepithelial lymphocytes.2
It is now generally accepted that CD is an immune disease caused by T cells recognising gluten derived peptides presented by HLA-DQ2 or -DQ8 molecules. Such gluten specific, CD4+, HLA-DQ2 or HLA-DQ8 restricted, T lymphocytes can be isolated from small intestinal biopsies of patients but not controls.3–7 T cell stimulatory peptides have been identified by us and others, and these originate from proline and glutamine rich regions in α-gliadin, γ-gliadin, and low (LMW) and high (HMW) molecular weight glutenins.8–13 Modification of these peptides, due to the activity of the enzyme tissue transglutaminase (tTG) is, in the majority of cases, required for or enhances the gluten specific T cell response. tTG activity converts glutamine residues in gluten peptides into glutamic acid which facilitates gluten peptide binding to HLA-DQ2 or -DQ8.14–17 This provides an explanation for the observation that the presence of these molecules predisposes to disease development.3,6,7,18–21
Omission of gluten from the diet of CD patients leads to disappearance of CD symptoms and full recovery of the small intestine. The Codex Alimentarius defines gluten free foods as those whose gluten content is below 200 parts per million (ppm) (200 mg gluten/100 g of food), which is equivalent to 100 ppm of gliadins. In order to further ensure the safety of CD patients, it has been proposed to decrease this level to 20 ppm.22
Accurate detection of gluten however is complicated as gluten is composed of two different protein families: LMW and HMW glutenins and gliadins. The latter can be further subdivided into α-, γ-, and ω-gliadins. Moreover, each gliadin subgroup consists of a mixture of highly similar but distinct proteins (for a recent review see Shewry and Halford23). Another complication of detection of gluten proteins is that they can be present in food products both as intact protein and as small protein fragments. For use in gluten free food products, wheat starch with a remaining low protein content is hydrolysed chemically or enzymatically. During this process, gluten proteins are digested into small peptides and amino acids. When this hydrolysis is incomplete, protein fragments will remain large enough to stimulate T cells. Moreover, protein hydrolysates are widely used in the food industry, including hydrolysates originating from wheat that may also contain small protein fragments.
For the detection of gluten, two commercially available methods are currently available, both based on a sandwich enzyme linked immunosorbent assay (ELISA) system. In one assay, ω-gliadins24 are detected while in the other assay α-, γ-, and ω-gliadins are detected.25 However, when used for screening of food used by CD patients, the methods have two major disadvantages. Firstly, the assays are not specific for detection of T cell stimulatory sequences in gluten. Secondly, the methods based on a sandwich ELISA are not suitable for detection of small peptides (that is, of sizes recognisable by T cells). Consequently, there is an urgent need for better assays that detect those sequences in gluten that stimulate gluten specific T cells in the intestine of CD patients for both intact proteins and peptides of a size that can be recognised by T cells.
In the present study, a new test has been developed which detects the presence of two known T cell stimulatory peptides originating from α-gliadin and γ-gliadin in both intact proteins and peptides. Using the European gliadin reference IRMM-480,26 assays for α- and γ-gliadins have a detection limit that allows detection of gluten in food extracts below the new threshold of 20 ppm proposed by the Codex Alimentarius.22
MATERIALS AND METHODS
Synthetic peptides
Peptides were synthesised by standard Fmoc chemistry on a SyroII peptide synthesiser. The integrity of the peptides was checked by reverse phase high performance liquid chromatography and mass spectrometry. The S-acetyl-mercaptoacetic acid (SAMA) group was introduced into the resin bound peptides by coupling of a sixfold equimolar mixture of SAMA N-hydroxysuccinimide ester and 1-hydroxybenzotriazole in NMP over two hours. Biotin was introduced into the resin bound peptides by a two hour coupling with a sixfold equimolar preactivating mixture of biotin and PyBop.
Chemical cross linking of synthetic peptides to tetanus toxoid (TTd) or bovine serum albumin (BSA)
For cross linking to TTd or BSA (125 mg), an N-terminal SAMA group was coupled to the 5 mg peptides. The carrier proteins TTd and BSA were desalted and equilibrated in 1.8 ml of 100 mM NaH2PO4/Na2HPO4, pH 7.8. In the carrier proteins, bromoacetyl groups were introduced by adding 50 μl of a solution of 23.6 mg/ml succinimidylbromoacetate in 100 mM N,N-dimethylacetamide. After one hour, the reaction mixture was desalted and equilibrated in 3 ml of 100 mM NaH2PO4/Na2HPO4, 5 mM EDTA, pH 6.0. SAMA-peptides were solubilised using 50 μl of 10% sodium dodecyl sulphate and diluted in 200 μl of 100 mM NaH2PO4/Na2HPO4, 5 mM EDTA, pH 6.0. To the peptide solution 2 ml of the bromo-acetylated carrier protein mixture was added together with 25 μl of hydroxylamine. After 24 hours at room temperature the reaction was stopped by adding 150 μl of 38 mM 2-aminoethanethiol. Finally, the reaction mixture was desalted and equilibrated in 3.3 ml of 10 mM NaH2PO4/Na2HPO4, 150 mM NaCl, pH 7.4.
Production and purification of monoclonal antibodies (mAbs) against T cell stimulatory epitopes in gluten proteins
BALB/c mice were immunised intraperitoneally with 150 μg of peptides chemically cross linked to TTd (table 1 ▶) suspended in complete Freund adjuvant (Sigma-Aldrich, Zwijndrecht, the Netherlands). This was followed by three subsequent injections of 150 μg of the protein suspended in incomplete Freund adjuvant (Sigma-Aldrich) at four week intervals. Fusion of the spleen and lymph node cells with mouse myeloma Ag8 cells was performed according to standard procedures.27 Culture supernatant from the resulting hybridomas was screened for the presence of antipeptide mAb using the peptide chemically cross linked to BSA with a different linker (table 1 ▶). The supernatant of selected Ab producing hybridomas was concentrated by Hemoflow dialysis units (Fresenius Medical Care, Nieuwkuijk, the Netherlands). Thereafter, mAbs were purified by protein G affinity chromatography (Pharmacia Fine Chemicals, Uppsala, Sweden) according to the manufacturer’s instructions.
Table 1.
Peptides used for immunisation of mice and detection of antibody producing hybridomas (X = aminohexanoyl spacer)
| Gluten epitope | Peptide | Conjugated |
| Glia-α2/α9 | ||
| (61–71) | DDDXFPQPLPYPQP-amide | TTd |
| (59–69) | DDDXQPFPQPQLPYP-amide | TTd |
| (61–71) | RGRGRFPQPQLPYPQP-amide | BSA |
| (59–69) | RGRGRQPFPQPQLPYP-amide | BSA |
| Glia-γ1 | ||
| (142–153) | DDDXPQQSFPQQQRPF-amide | TTd |
| (147–158) | DDDXPQQQRPFIQPSL-amide | TTd |
| (149–159) | DDDXQQRPFIQPSLQ-amide | TTd |
| (142–159) | RGRGRPQQSFPQQQRPFIQPSLQ-amide | BSA |
ELISA for screening of mAbs producing hybridomas
Peptides cross linked to BSA (table 1 ▶) were incubated at a concentration of 2 μg/ml in 0.1 M sodium carbonate/bicarbonate buffer, pH 9.6, for 16 hours at 4°C in microtitre plates (Nunc Maxisorb Immunoplate; Nunc, Copenhagen, Denmark) (100 μl/well). Plates were then washed with phosphate buffered saline (PBS)/0.02% (w/v) Tween-20. An identical washing procedure was performed after each incubation step, which consisted of 100 μl, except for the blocking step (150 μl). After coating, residual binding sites were blocked by 30 minutes of incubation with PBS/1% skim milk (Fluka, Zwijndrecht, the Netherlands). Supernatant of the hybridomas was diluted 1:100 in PBS/0.1%Tween-20 and incubated for one hour. Next, plates were incubated with an excess of biotinylated rabbit-antimouse antiserum for one hour, followed by a 30 minute incubation with streptavidin polymerised horseradish peroxidase (CLB, Amsterdam, the Netherlands). Bound peroxidase was visualised by incubation with a solution of 3,3′,5,5′-tetramethylbenzidine (Sigma-Aldrich). Finally, absorbance at 620 nm was read on a Multiscan plate reader (Wallac, Turku, Finland).
Competition assays for quantitative detection of T cell stimulatory epitopes of gluten proteins
Microtitre plates (Nunc) were incubated overnight with 2–5 μg/ml mAb in 0.1 M sodium carbonate/bicarbonate buffer, pH 9.2, at room temperature. Plates were washed in PBS/0.02% Tween-20 and residual binding sites were blocked with PBS/ 1% skim milk powder (Fluka). Of the gluten containing samples, different dilutions were made in PBS/0.1% Tween-20/0.1% skim milk and these were mixed with either a biotinylated α- or γ-gliadin T cell epitope encoding peptides (table 2 ▶). For quantification, a standard curve was made using the European gliadin reference IRMM-48026 in the concentration range 100 μg/ml to 3 ng/ml mixed with biotinylated indicator peptides. The mixtures were incubated on plates for 1.5 hours at room temperature. Next, plates were washed and incubated for 30 minutes with streptavidin conjugated horseradish peroxidase in PBS/0.1% skim milk. Thereafter, bound peroxidase was visualised as described previously.
Table 2.
Overview of peptides used in the competition assays for detection of α- and γ-gliadin derived T cell stimulatory epitopes (B = biotin, X = aminohexanoyl spacer)
| T cell epitope | Biotinylated competitor peptide |
| Glia-α2/9 | BXKAKAKAKAXQPFPQPQLPYPQP-amide |
| Glia-γ1 | BXAKAKAKAKXPQQSFPQQQRPFIQPSLQ-amide |
Commercial gluten detection kit
The gluten specific ELISA was performed according to the manufacturer’s instructions.28
Gliadin standard
The new European gluten reference26 IRMM-480 was used as a standard. The standard was dissolved in 40% aqueous ethanol and stored at 4°C.
Preparation and measurement of gluten containing starch and food samples
For measurement of the gluten content of starch and food sample, 1 g of the material was incubated with 10 ml of 40% ethanol to extract the gluten. After addition of ethanol, samples were incubated for one hour at room temperature in a rotary shaker with recurrent vortexing for 30 seconds. Subsequently, samples were centrifuged at 2500 g for 10 minutes at room temperature and supernatants were transferred to eppendorf tubes. Extraction and analysis were performed on the same day.
Preparation of gluten containing samples from different cereals
Samples of different cereals, barley, oat, wheat, rye, and triticale (hybrid between wheat and rye) were grinded and a trypsin/pepsin digest was prepared as described previously.11 A control sample was prepared from a commercial gliadin preparation (Fluka Chemie, Zwijndrecht, the Netherlands) using the same protocol.
T cell proliferation assays
To test for the presence of T cell stimulatory epitopes in different wheat varieties, two different T cell clones (one recognising both the glia-α2 and -9 T cell epitopes and one recognising the glia-γ1 T cell epitope) were used. The clones originate from gluten specific T cell lines generated from small intestinal biopsies from two different CD patients.
Proliferation assays were performed in triplicate in 150 μl of Iscove’s modified Dulbecco’s medium (BioWhittaker, Verviers, Belgium) with 10% pooled normal human serum in 96 well flat bottom plates using 104 gluten specific T cells stimulated with 105 irradiated HLA-DQ2 matched allogeneic peripheral blood mononuclear cells (3000 rad) in the presence or absence of antigen (1–10 μg/ml). After two days, 3H-thymidine (1 μCi/well) was added to the cultures, and 18–20 hours thereafter cells were harvested. 3H-thymidine incorporation into T cell DNA was counted on a liquid scintillation counter (1205 Betaplate Liquid Scintillation Counter; LKB Instruments, Gaithersburg, Maryland, USA).
RESULTS
Competition assay for the detection of T cell stimulatory epitopes in gluten
BALB/c mice were immunised with TTd coupled peptides encoding either a T cell stimulatory peptide present in α-gliadin or γ-gliadin. The spleens of the immunised mice were fused to a myeloma cell line to generate antibody secreting hybridoma cells. In this way, for both T cell stimulatory peptides, several specific mAbs were obtained (fig 1 ▶). The mAbs were used to develop a competition assay. In this competition assay, the sample is mixed with a fixed concentration of a biotinylated synthetic 20-mer indicator peptide encoding the T cell epitope of either α-/or γ-gliadin. When added to an immobilised mAb, the T cell epitopes present in the sample will compete with the T cell epitopes encoded in the biotinylated indicator peptide for binding to the mAb. Depending on the gluten content of the sample, more or less biotinylated indicator peptide will bind to the mAb which can be visualised with peroxidase conjugated streptavidin and 3,3′,5,5′-tetramethylbenzidine (TMB) (fig 2 ▶). Two mAbs were selected that proved the most sensitive in the competition assays. For the α-gliadin T cell epitope, a mAb was selected that was obtained after immunisation with peptides encoding amino acids 59–69 of α-gliadin. This mAb is referred to as anti-glia-α2/9 hereafter. For γ-gliadin, a mAb was selected that was obtained after immunisation with amino acids 147–159 of γ-gliadin. This mAb is referred to as anti-glia-γ1 hereafter. For both assays, the detection limit was determined using the European gliadin reference (IRMM-480)26 as standard. In this way, sensitive assays were developed in which the gliadin reference was detected in the range 100 μg/ml to 12 ng/ml for both the glia-α2/9 T cell epitope and the glia-γ1 T cell epitope (fig 3 ▶). The detection limit of 12 ng/ml is routinely reached in the competition assays performed in our laboratory (results not shown). As a consequence, with the generally accepted method of food analysis for the presence of gliadins, extraction of 2 g food/20 ml of 40% aqueous ethanol, and a minimal sample dilution of 1:20, a gliadin content as low as 2.4 ppm (or 4.8 ppm gluten) can be detected.
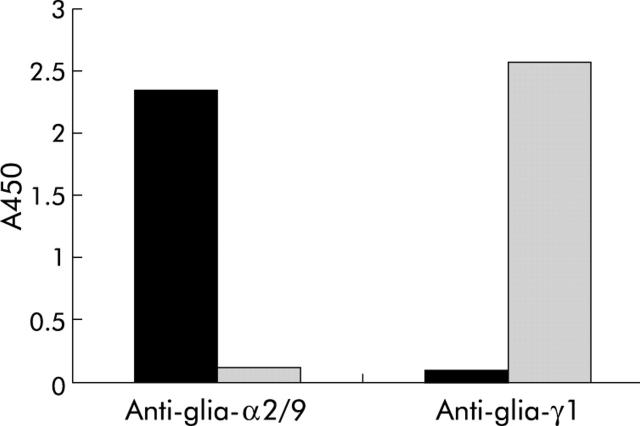
Figure 1.
Specificity of the monoclonal antibodies (mAbs) for the different T cell epitopes. Wells of an ELISA plate were coated with 0.5 μg/ml of peptides glia-α2/9 (amino acids 59–71) and glia-γ1 (amino acids 142–159) coupled to bovine serum albumin. Next, plates were incubated with the anti-glia-α-2/9 mAb and anti-glia-γ1 mAb. Binding of the mAb was detected with a peroxidase coupled rabbit-antimouse polyclonal antibody and visualised with 3,3′,5,5′-tetramethylbenzidine.
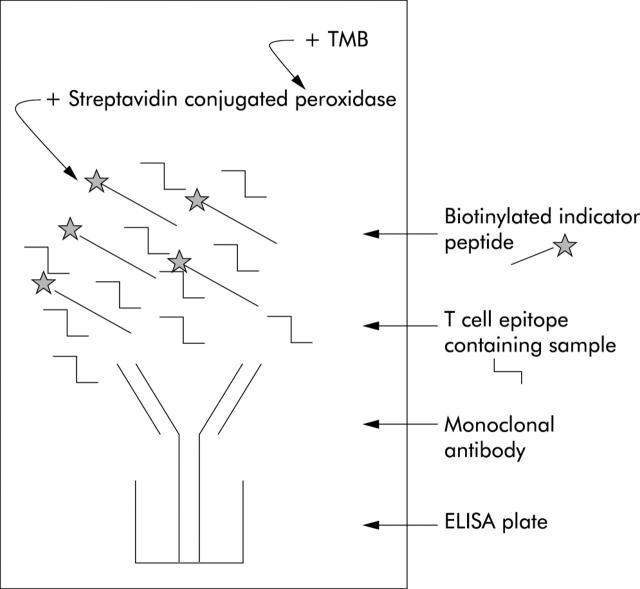
Figure 2.
Schematic representation of a competition experiment. In a competition experiment, the gluten containing sample is mixed with a fixed concentration of biotinylated indicator peptide encoding a T cell stimulatory peptide. This mixture was added to an ELISA plate previously coated with a monoclonal antibody (mAb) specific for the biotinylated indicator peptide. During incubation, T cell epitopes present in the sample compete with the biotinylated indicator peptide for binding to the mAb. After washing, bound indicator peptide was visualised with peroxidase conjugated streptavidine and 3,3′,5,5′-tetramethylbenzidine (TMB).
Figure 3.
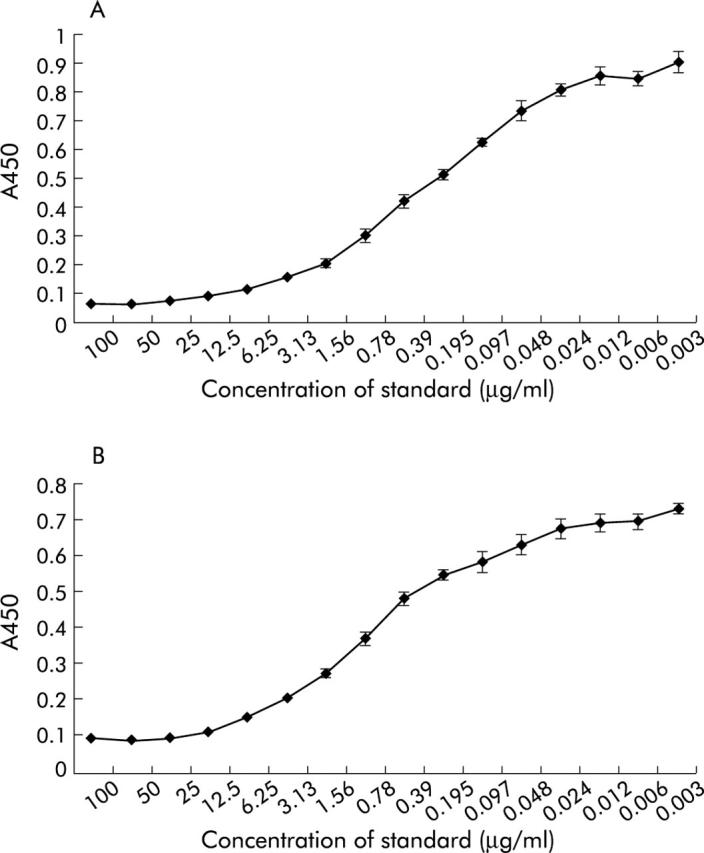
Competition assay for detection of different T cell stimulatory epitopes in gluten containing samples. (A) Competition assay for glia-α2/α9 epitope. (B) Competition assay for glia-γ1 epitope. Dilutions of the European gliadin reference IRMM-48026 were used as standard. Data represent the mean (SD) of five experiments. The detection limit of the competition assay (defined as the concentration of standard showing an optical difference in absorbance compared with absorbance in the absence of the peptide) was 12 ng/ml for both the glia-α2/9 and glia-γ1 T cell epitopes.
Definition of the minimal epitopes recognised by the mAb used in the different competition assays
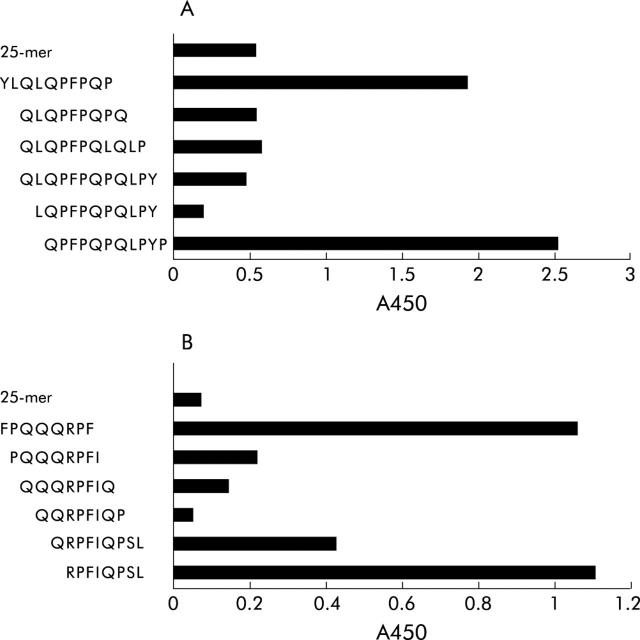
To define the minimal amino acid sequence still recognised by anti-glia-α2/9 and anti-glia-γ1 mAbs, competition experiments were performed. Peptides were synthesised encoding sequences that overlap with the amino acid sequence used for generation of the mAb. The minimal amino acid sequence recognised by the anti-glia-α2/9 mAb was LQPFPQPQ (fig 4A ▶) which covers most of the glia-α9 T cell epitope. Similarly, we found that the minimal sequence recognised by the anti-glia-γ1 mAb was QQRPFI (fig 4B ▶) which overlaps with the C terminal amino acids of the glia-γ1 T cell epitope.9
Figure 4.
Minimal epitope detected by (A) anti-glia-α-2/9 monoclonal antibody (mAb) and (B) anti-glia-γ1 mAb. The minimal epitope was determined by measuring binding of the T cell epitope specific mAb to a set of overlapping peptides covering the entire T cell epitopes. The minimal epitope determined for anti-glia-α2/9 mAb was LQPFPQPQ and for anti-glia-γ1 QQRPFI. However, as generation of the anti-glia-α2/9 mAb immunisations were performed with peptides with an aminohexanoyl group N terminally of proline, the minimal epitope is XQPFPQPQ, in which X represents any amino acid.
Comparison of detection of T cell epitopes in intact gliadin proteins with detection of T cell epitopes in peptides
T cell stimulatory epitopes can be encoded by both small peptide fragments (as low as 11–12 amino acids) and intact gluten proteins. To determine whether the new assays can detect T cell epitopes in both proteins and peptides, competition assays were performed with the European gliadin reference (IRMM-480)26 containing intact gliadin proteins and synthetic peptides of 25 amino acids. For the glia-α2/9 competition assay, a difference was found between detection of the peptides in intact proteins and the 25-mer synthetic peptide (fig 5A ▶). A higher concentration of peptides is required to reach the same level of competition as found for intact proteins. This indicates that the affinity of the anti-glia-α2/9 mAb is lower for peptides than for intact proteins. In contrast, for the glia-γ1 competition assay, no difference was found between detection of the peptide in intact proteins and the 25-mer synthetic peptide (fig 5B ▶). Both were detected with similar sensitivity.
Figure 5.
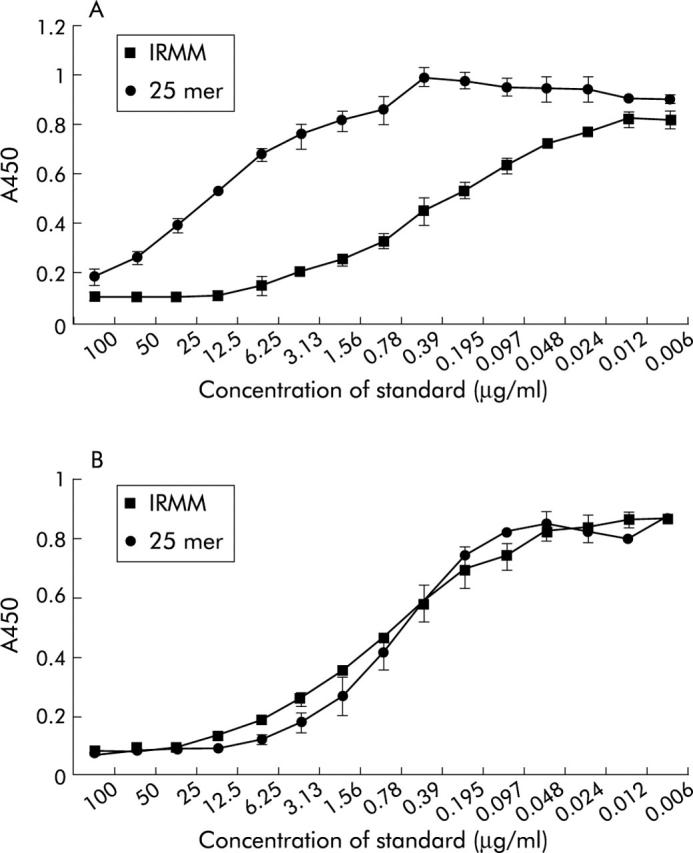
Comparison of the affinity of the (A) anti-glia-α2/9 monoclonal antibody (mAb) and (B) anti-glia-γ1 mAb for T cell epitopes encoded in intact proteins and peptides. Competition experiments were performed with both intact proteins (European gliadin reference) and a 25-mer synthetic peptide. Data represent the mean (SD) of two experiments.
Detection of T cell stimulatory epitopes in cereals by the competition assay and gliadin specific T cell clones
In addition to wheat, other cereals such as barley, rye, and oats also contain storage proteins that are homologues to gliadin. To test whether our method is suitable for the detection of gliadin homologues encoding T cell stimulatory epitopes in cereals other than wheat, protein extracts were made of barley, oats, wheat, rye, and triticale (a hybrid of wheat and rye used in the food industry). Extracts were measured both in competition assays for α- and γ-gliadins and in assays with a glia-α2/9 and a glia-γ1 specific T cell clone. With both techniques a similar reactivity pattern towards the cereal preparations was observed. Both methods detected the glia-α2/9 epitope in protein preparations of barley, wheat, rye, and triticale, but not in oats (fig 6A ▶, B). Both methods however detected the glia-γ1 T cell epitope not only in the cereals barley, wheat, rye, and triticale but also in oats (fig 6A ▶, C). This demonstrates that the new method is suitable for detection of the presence or absence of not only T cell epitopes encoded by wheat gliadin but also of gliadin homologues of other cereals. This is likely to relate to the toxicity of these cereals for CD patients
Figure 6.
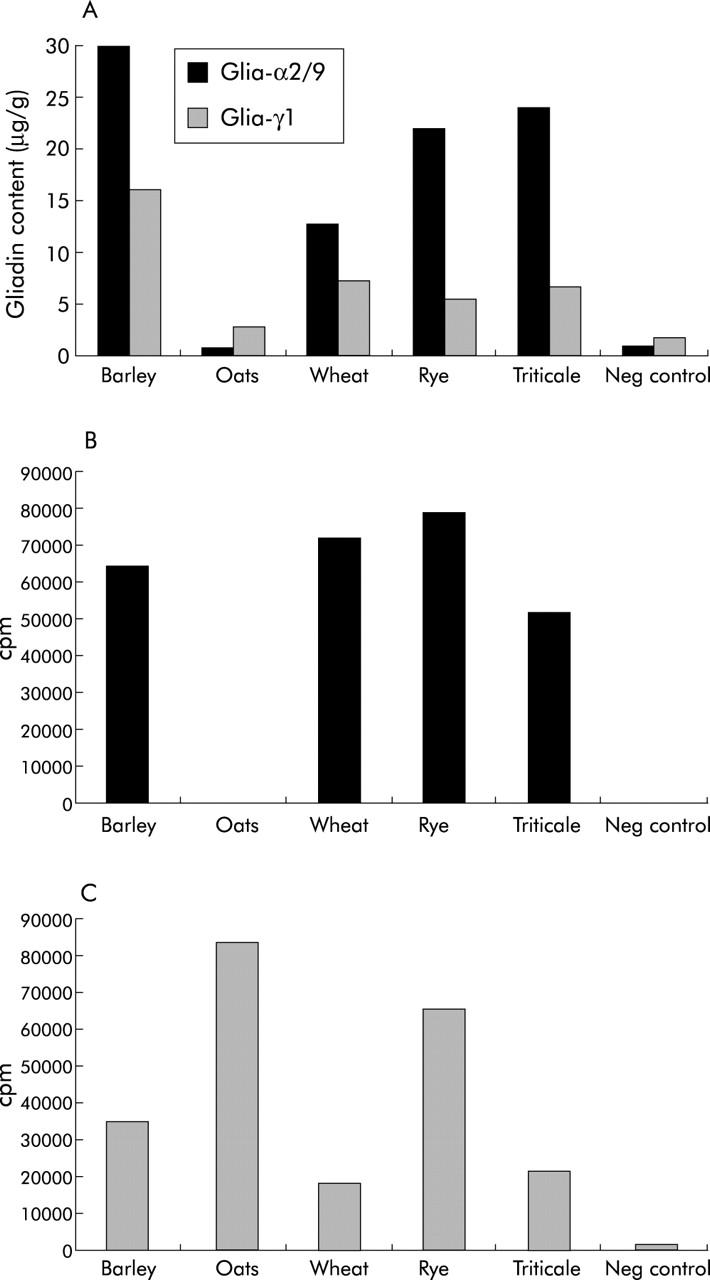
Detection of T cell stimulatory epitopes glia-α2/9 and glia-γ1 in protein preparations of different cereals by (A) competition assay, (B) a glia-α2/9 specific T cell clone, or (C) a glia-γ1 specific T cell clone. Both T cell clones and the competition assay detected peptides in barley, rye, triticale, and oats. As the level of glia-α2/9 epitopes detected by the competition assay in the barley preparation (315 μg/g) was very high compared with levels in other cereals, the bar extends beyond the applied scale of the graph.
Analysis of food samples by the new method
For detection of gliadin in food products and starch samples with known gluten content,28 ethanol extracts were prepared. These starch samples were included in the experiments to compare the results of the new method with those obtained by a commercial ELISA. After a 20–40-fold dilution, the gliadin content of the extracts was measured with the competition assays for the glia-α2/9 and glia-γ1 T cell epitopes.
Gliadin levels detected in commercial starch control samples with the new competition assays for α- and γ-gliadins corresponded with levels found by the commercial ELISA kit (table 3 ▶).28 When measured in the ethanol extract of the food products, high levels of both α- and γ-gliadins were detected in products 11, 12, and 16 whereas low levels were detected in food products 1, 2, 4, 13, 14, and 18. In a product in which maize starch (product No 15) was used, levels of gliadin were very low (γ-gliadin) or not detectable (α-gliadin). Most of the values obtained with the new assays were comparable with those obtained with the commercial gluten detection kit. Moreover, the results indicate that with the new assays, more accurate values can be assigned. Moreover, a distinction can be made between α- and γ-gliadins which is not possible with the commercial gluten detection kit. These results indicate that the new competition assays are suitable for the detection of gluten in ethanol extracts of food samples. Differences in the levels of gliadin detected in a sample by the different assays can be explained by either variation in the amounts of proteins detected by the different assays or by the presence of intact proteins together with small protein fragments.
Table 3.
Detection of the presence of T cell stimulatory epitopes by the new assays for α- and γ-gliadins and by a commercially available gluten detection kit28 in 40% aqueous ethanol extracts of commercial starch controls28 and various food products. Gliadin levels were calculated using the European gliadin reference IRMM-48026 as standard
| Sample No | Content | Glia-α2/9 (μg/g) ppm | Glia-γ1 (μg/g) ppm | Gliadin content detected by commercial ELISA28 (μg/g) ppm |
| Low starch | <0.016% gluten28 (≈<80 ppm of gliadin) | 1 | 7 | <75 |
| Medium starch | 0.02–0.04% gluten28 (≈100–200 ppm of gliadin) | 107 | 83 | 73 |
| High starch | >0.1% gluten28 (≈>500 ppm of gliadin) | 889 | 421 | >1250 |
| 1 | Starch | 4 | 28 | <75 |
| 2 | Wheat starch | 1 | 20 | <75 |
| 4 | Wheat flour | 18 | 51 | 227 |
| 6 | Starch | 224 | 14 | 1174 |
| 11 | Modified starch | 962 | 301 | >1250 |
| 12 | Wheat flour | 11806 | 12593 | >1250 |
| 13 | Modified starch | 13 | 37 | <75 |
| 14 | Modified starch | 20 | 5 | <75 |
| 15 | Modified starch (maize) | nd | 13 | <75 |
| 16 | Wheat flour | 1540 | 1709 | 1711 |
| 18 | Malt aroma | 152 | 59 | <150 |
DISCUSSION
In this study, a new method for the detection of gluten is described by which T cell stimulatory epitopes of α- and γ-gliadins (glia-α2/9 and glia-γ1, respectively) can be detected. This is the first antibody based method that can detect the presence of T cell stimulatory epitopes thought to be involved in the development of CD. Moreover, the method detects the glia-α2/9 and glia-γ1 T cell epitopes separately, and thus allows for discrimination between the presence of α- and γ-gliadin proteins in a sample.
For the development of the method, mAbs were generated specific for the glia-α2/9 and glia-γ1 T cell epitopes. The minimal amino acid sequence recognised by the mAb was found to be smaller than the minimal T cell epitopes.9,29,30 The sequence detected by the anti-glia-γ1 mAb overlaps with the C terminal part of the glia-γ1 T cell epitope. For the anti-glia-α2/9 mAb, the minimal recognition sequence is contained within the T cell epitope and actually resides within the glia-α9 peptide. Although this latter peptide is not recognised by all CD patients,29 the glia-α2 and glia-α9 peptides are partially overlapping and most patients respond to either one of these peptides.10,29 Detection of the sequence within the glia-α9 peptide with our competition assay will thus also indicate the presence of the glia-α2 peptide within the test sample.
With the mAbs, competition assays were developed. In a competition assay, gluten epitopes in a sample compete for binding to a mAb with a biotinylated indicator peptide. Binding of the latter is detected. The advantage of a competition assay, in which only one mAb is used for detection, is that both intact proteins and small protein fragments can be detected. This is not possible with the currently used sandwich ELISA systems.25,28 These sandwich ELISA based system can only detect the presence of intact or rather large gluten fragments25,28 as small fragments cannot simultaneously be bound by two antibodies. These methods are therefore incapable of detecting the small gluten peptides that suffice for T cell stimulation.
Although our method is suitable for the detection of both intact proteins and protein fragments, the affinity of the glia-α2/9 specific mAb is much higher for the intact protein. In contrast, the anti-glia-γ1 mAb has a comparable affinity for intact proteins and protein fragments. Suitable standards must therefore be incorporated to ensure the accurate determination of both α- and γ-gliadins in test samples.
The ability to measure the presence of small gluten fragments is a major advantage as gluten hydrolysates and wheat starch hydrolysates are widely used in the food industry. These hydrolysates are obtained by enzymatic or chemical treatment and result in smaller constituents, primarily sugars, peptides, and amino acids. As peptides with only 11 amino acids are large enough to stimulate T cells, it is important to ensure the absence of such remaining peptides in food products and in gluten free food products in particular.
The competition assay detected both glia-α2/9 and glia-γ1 T cell epitopes in ethanol extracts of food samples containing wheat flour or wheat starch. In food samples in which maize starch was used, no α-gliadin and only a very small amount of γ-gliadin was detected. Gliadin levels measured in the control starch samples were comparable with levels detected by a commercial gluten assay. With a detection limit of 12.5 ng/ml for the European gliadin standard and a minimal dilution of 1:20 for the ethanol extraction samples (1 g/10 ml), our assay can detect both α- and γ-gliadins at a level of 2.5 ppm which corresponds to 5 ppm of gluten. This detection limit is well below the 20 ppm threshold for gluten in gluten free products proposed by the Codex Alimentarius Commission. Therefore, the new method is suitable for screening of food products recommended for a gluten free diet for the presence of T cell stimulatory epitopes.
Moreover, when protein preparations of different cereals were tested with our new method, different levels of both α- and γ-gliadins could be detected in barley, wheat, rye, triticale, and oats. These results indicate that the mAbs not only detect the T cell epitopes present in gliadin but also those present in other homologous proteins such as the hordeins of barley, the secalins of rye, and the avenins of oats. This broad cross reactivity of the mAbs is a major advantage compared with already existing gluten detection methods as the mAbs used in a commercial assay for the detection of ω-gliadin have only low cross reactivity with hordeins and no cross reactivity with avenins.24 Moreover, with an assay detecting α-, γ- and ω-gliadins, no cross reactivity with avenin was detected.25
Until recently, oats was considered relatively safe for CD patients. This was because of the predicted low number of T cell stimulatory sequences31–33 and studies which showed that most CD patients tolerate the introduction of oats into their diet.31,33–41 In the present study, however, we demonstrate that both with the new method and with gliadin specific T cell clones, T cell stimulatory epitopes are not only abundant in barley, wheat, rye, and triticale, but some epitopes can also be found in oats (in particular the glia-γ1 T cell epitope). Together with recent reports on reactions of CD patients to oats, our results42,43 suggest that oats may not be safe for all CD patients.
Finally, our study further emphasises the need for new cereals lacking T cell stimulatory epitopes. Although there is no evidence for the existence of cereals that lack toxicity for CD patients, our new method may be a useful tool for the selection of wheat varieties that contain less of the harmful T cell stimulatory gluten peptides. Such varieties may open the way to the breeding and/or development of cereals that are safer for consumption by patients.
In conclusion, we have developed a mAb based method for the detection of T cell stimulatory epitopes known to be involved in CD. The new method has many advantages compared with existing methods for the detection of gluten as it is the first method that can detect: (i) T cell stimulatory epitopes; (ii) α- and γ-gliadins separately; (iii) T cell stimulatory epitopes present on gliadin homologues present in other cereals also known to be involved in CD; and (iv) T cell stimulatory epitopes on both intact proteins and small protein fragments. The new method will be a valuable tool in the screening of food products that are intended to be used in the gluten free diet of CD patients.
Acknowledgments
We thank Willemien Benckhuijsen and Peter de Koning for peptide synthesis and Peter van Veelen for help with database searches. TTd was a generous gift from Dr Peter Hoogerhout of the Netherlands Vaccine Institute. The different cereals used in this study were a kind gift of the plant breeding station Wiersum (Dronten the Netherlands) and Applied Plant Research (PPO, Lelystad, the Netherlands). This study was supported by a grant from the European Community (QLRT-2000-00657).
Abbreviations
aa, amino acids
HLA, human leucocyte antigen
CD, coeliac disease
mAb, monoclonal antibody
tTG, tissue transglutaminase
HMW, high molecular weight
LMW, low molecular weight
ppm, parts per million
TTd, tetanus toxoid
BSA, bovine serum albumin
PBS, phosphate buffered saline
TMB, 3,3′,5,5′-tetramethylbenzidine
ELISA, enzyme linked immunosorbent assay
SAMA, S-acetyl-mercaptoacetic acid
REFERENCES
- 1.Dicke WK, Weijers HA, Van de Kamer JH. Coeliac disease II. The presence in wheat of a factor having a deleterious effect in cases of coeliac disease. Acta Paediatr 1952;42:34–42. [DOI] [PubMed] [Google Scholar]
- 2.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 1992;102:330–54. [PubMed] [Google Scholar]
- 3.Molberg O , Kett K, Scott H, et al. Gliadin specific, HLA DQ2-restricted T cells are commonly found in small intestinal biopsies from coeliac disease patients, but not from controls. Scand J Immunol 1997;46:103–9. [DOI] [PubMed] [Google Scholar]
- 4.Jensen K , Sollid LM, Scott H, et al. Gliadin-specific T cell responses in peripheral blood of healthy individuals involve T cells restricted by the coeliac disease associated DQ2 heterodimer. Scand J Immunol 1995;42:166–170. [DOI] [PubMed] [Google Scholar]
- 5.Lundin KE, Scott H, Fausa O, et al. T cells from the small intestinal mucosa of a DR4, DQ7/DR4, DQ8 celiac disease patient preferentially recognize gliadin when presented by DQ8. Hum Immunol 1994;41:285–91. [DOI] [PubMed] [Google Scholar]
- 6.Gjertsen HA, Lundin KE, Sollid LM, et al. T cells recognize a peptide derived from alpha-gliadin presented by the celiac disease-associated HLA-DQ (alpha 1*0501, beta 1*0201) heterodimer. Hum Immunol 1994;39:243–52. [DOI] [PubMed] [Google Scholar]
- 7.Lundin KE, Scott H, Hansen T, et al. Gliadin-specific, HLA-DQ(alpha 1*0501,beta 1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J Exp Med 1993;178:187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paulsen G , Lundin KE, Gjertsen HA, et al. HLA-DQ2-restricted T-cell recognition of gluten-derived peptides in celiac disease. Influence of amino acid substitutions in the membrane distal domain of DQ beta 1*0201. Hum Immunol 1995;42:145–53. [DOI] [PubMed] [Google Scholar]
- 9.Sjostrom H , Lundin KE, Molberg O, et al. Identification of a gliadin T-cell epitope in coeliac disease: general importance of gliadin deamidation for intestinal T-cell recognition. Scand J Immunol 1998;48:111–15. [DOI] [PubMed] [Google Scholar]
- 10.Vader W , Kooy Y, van Veelen P, et al. The gluten response in children with celiac disease is directed toward multiple gliadin and glutenin peptides. Gastroenterology 2002;122:1729–37. [DOI] [PubMed] [Google Scholar]
- 11.Van de Wal Y , Kooy YM, van Veelen PA, et al. Small intestinal T cells of celiac disease patients recognize a natural pepsin fragment of gliadin. Proc Natl Acad Sci U S A 1998;95:10050–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van de Wal Y , Kooy YM, van Veelen P, et al. Glutenin is involved in the gluten-driven mucosal T cell response. Eur J Immunol 1999;29:3133–9. [DOI] [PubMed] [Google Scholar]
- 13.Molberg O , Solheim FN, Jensen T, et al. Intestinal T-cell responses to high-molecular-weight glutenins in celiac disease. Gastroenterology 2003;125:337–44. [DOI] [PubMed] [Google Scholar]
- 14.Van de Wal Y , Kooy Y, van Veelen P, et al. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J Immunol 1998;161:1585–8. [PubMed] [Google Scholar]
- 15.Vader LW, De Ru A, Van der Wal Y, et al. Specificity of tissue transglutaminase explains cereal toxicity in celiac disease. J Exp Med 2002;195:643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleckenstein B , Molberg O, Qiao SW, et al. Gliadin T cell epitope selection by tissue transglutaminase in celiac disease. J Biol Chem 2002;277:34109–16. [DOI] [PubMed] [Google Scholar]
- 17.Molberg O , McAdam S, Lundin KE, et al. T cells from celiac disease lesions recognize gliadin epitopes deamidated in situ by endogenous tissue transglutaminase. Eur J Immunol 2001;31:1317–23. [DOI] [PubMed] [Google Scholar]
- 18.Molberg O , Kett K, Scott H, et al. Gliadin specific, HLA DQ2-restricted T cells are commonly found in small intestinal biopsies from coeliac disease patients, but not from controls. Scand J Immunol 1997;46:103–8. [PubMed] [Google Scholar]
- 19.Spurkland A , Ingvarsson G, Falk ES, et al. Dermatitis herpetiformis and celiac disease are both primarily associated with the HLA-DQ (alpha 1*0501, beta 1*02) or the HLA-DQ (alpha 1*03, beta 1*0302) heterodimers. Tissue Antigens 1997;49:29–34. [DOI] [PubMed] [Google Scholar]
- 20.Spurkland A , Sollid LM, Ronningen KS, et al. Susceptibility to develop celiac disease is primarily associated with HLA-DQ alleles. Hum Immunol 1990;29:157–65. [DOI] [PubMed] [Google Scholar]
- 21.Sollid LM, Markussen G, Ek J, et al. Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J Exp Med 1989;169:345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Report of the 25th Session of the Codex Alimentarius Committee on Nutrition and Foods For Special Dietary Uses. Bonn 2003.
- 23.Shewry PR, Halford NG. Cereal seed storage proteins: structures, properties and role in grain utilization. J Exp Bot 2002;53:947–58. [DOI] [PubMed] [Google Scholar]
- 24.Skerritt JH, Hill AS. Enzyme immunoassay for determination of gluten in foods: collaborative study. J Assoc Off Anal Chem 1991;74:257–64. [PubMed] [Google Scholar]
- 25.Valdes I , Garcia E, Llorente M, et al. Innovative approach to low-level gluten determination in foods using a novel sandwich enzyme-linked immunosorbent assay protocol. Eur J Gastroenterol Hepatol 2003;15:465–74. [DOI] [PubMed] [Google Scholar]
- 26.Eckert R Van . The PWG gliadin, a new refernce material. Proceedings of the 16th meeting Working Group on Prolamin Analysis and Toxicity. Spain: Sitges, 2000.
- 27.Hack CE, Paardekooper J, Smeenk RJ, et al. Disruption of the internal thioester bond in the third component of complement (C3) results in the exposure of neodeterminants also present on activation products of C3. An analysis with monoclonal antibodies. J Immunol 1988;141:1602–9. [PubMed] [Google Scholar]
- 28.Tepnel Biosystems. Gluten assay kit, for the quantitative determination of gluten in food products by enzyme immunoassay. Tepnel Biosystems, UK.
- 29.Arentz-Hansen H , Korner R, Molberg O, et al. The intestinal T cell response to alpha-gliadin in adult celiac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase. J Exp Med 2000;191:603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fraser JS, Engel W, Ellis HJ, et al. Coeliac disease: in vivo toxicity of the putative immunodominant epitope. Gut 2003;52:1698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffenberg EJ, Haas J, Drescher A, et al. A trial of oats in children with newly diagnosed celiac disease. J Pediatr 2000;137:361–6. [DOI] [PubMed] [Google Scholar]
- 32.Janatuinen EK, Kemppainen TA, Pikkarainen PH, et al. Lack of cellular and humoral immunological responses to oats in adults with coeliac disease. Gut 2000;46:327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janatuinen EK, Kemppainen TA, Julkunen RJ, et al. No harm from five year ingestion of oats in coeliac disease. Gut 2002;50:332–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janatuinen EK, Pikkarainen PH, Kemppainen TA, et al. A comparison of diets with and without oats in adults with celiac disease. N Engl J Med 1995;333:1033–7. [DOI] [PubMed] [Google Scholar]
- 35.Picarelli A , Di Tola M, Sabbatella L, et al. Immunologic evidence of no harmful effect of oats in celiac disease. Am J Clin Nutr 2001;74:137–40. [DOI] [PubMed] [Google Scholar]
- 36.Storsrud S , Hulthen LR, Lenner RA. Beneficial effects of oats in the gluten-free diet of adults with special reference to nutrient status, symptoms and subjective experiences. Br J Nutr 2003;90:101–7. [DOI] [PubMed] [Google Scholar]
- 37.Storsrud S , Olsson M, Arvidsson LR, et al. Adult coeliac patients do tolerate large amounts of oats. Eur J Clin Nutr 2003;57:163–9. [DOI] [PubMed] [Google Scholar]
- 38.Janatuinen EK, Kemppainen TA, Julkunen RJ, et al. No harm from five year ingestion of oats in coeliac disease. Gut 2002;50:332–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Picarelli A , Di Tola M, Sabbatella L, et al. Immunologic evidence of no harmful effect of oats in celiac disease. Am J Clin Nutr 2001;74:137–40. [DOI] [PubMed] [Google Scholar]
- 40.Janatuinen EK, Kemppainen TA, Pikkarainen PH, et al. Lack of cellular and humoral immunological responses to oats in adults with coeliac disease. Gut 2000;46:327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srinivasan U , Leonard N, Jones E, et al. Absence of oats toxicity in adult coeliac disease. BMJ 1996;313:1300–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lundin KE, Nilsen EM, Scott HG, et al. Oats induced villous atrophy in coeliac disease. Gut 2003;52:1649–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hollen E , Hogberg L, Stenhammar L, et al. Antibodies to oat prolamines (avenins) in children with coeliac disease. Scand J Gastroenterol 2003;38:742–6. [DOI] [PubMed] [Google Scholar]