Abstract
Background and aims: Chemokine receptors are key determinants of leucocyte trafficking. While the chemokine receptor CCR9 and its chemokine ligand CCL25 (TECK) mediate lymphocyte homing to the healthy small intestine, the chemokine receptors important for recruitment during intestinal inflammation are undefined. Animal studies have suggested potential roles for CCR2 and CCR5 in inflammatory bowel disease (IBD). The aim of this study was to understand the role of CCR2 in human IBD.
Methods: Resections of ileum or colon were obtained from patients undergoing surgery for small bowel Crohn’s disease (SBCD; n = 10), Crohn’s colitis (n = 5), ulcerative colitis (n = 6), and non-IBD related conditions (control ileum n = 11; control colon n = 11). Expression of CCR2 by lamina propria lymphocytes (LPLs) was determined by both flow cytometry and immunohistochemistry. As a functional correlate, chemotaxis assays using the CCR2 ligand, CCL2 (MCP-1), were performed. Expression of CCR2 by peripheral blood lymphocytes was determined by flow cytometry.
Results: There were greater than 30-fold more CCR2+ LPLs in SBCD than in control ileum (29.3% (19.9–55.1) v 0.9% (0.4–11.5); p = 0.0007). Specifically, CCR2+CD4+ LPLs were increased (p = 0.002) whereas CCR2+CD8+ LPLs were not. Increased expression included both memory (CD45RO+; p = 0.005) and naïve (CD45RO−; p = 0.01) CCR2+ populations. The increase in CCR2+ LPLs in SBCD was confirmed by both immunohistochemistry (p = 0.0002) and enhanced chemotactic responses to CCL2. CCR2 expression was not increased in the peripheral blood of patients with SBCD, suggesting ongoing recruitment of the CCR2+ population to the ileum. In contrast with SBCD, there was no significant increase in CCR2+ LPLs in Crohn’s colitis or ulcerative colitis samples.
Conclusions: The chemokine receptor CCR2 appears to be an important contributor to accumulation of CD4+ T lymphocytes in the ileum in small bowel Crohn’s disease. Blockade of CCR2 may provide a novel therapeutic alternative.
Keywords: inflammatory bowel disease, mucosal immunology, T cell trafficking, chemokine receptors, CCR2
Chemokines are a family of small (8–14 kDa) structurally related heparin binding proteins which, through chemoattraction and activation of leucocytes, are important in both homing of leucocytes under physiological conditions and in recruitment of leucocytes to sites of inflammation.1,2 These proteins play an important role in the pathogenesis of several diseases, such as human immunodeficiency virus,3 rheumatoid arthritis,4 and multiple sclerosis.5,6 Chemokines mediate their actions through seven transmembrane spanning G protein coupled receptors on the surface of target cells. Once activated by the chemokine ligand, target cells migrate along a soluble concentration gradient in the circulation (chemotaxis) or along a glycosaminoglycan bound gradient in tissues (haptotaxis).
Chemokines and chemokine receptors important for maintenance of the mucosal immune system of the gastrointestinal tract and in gastrointestinal inflammation continue to be defined. The most widely explored is the chemokine CCL25 (TECK) and its associated receptor CCR9, with mounting evidence suggesting a role for this receptor/ligand pair in homing to the small intestine.7–14
In gastrointestinal inflammation, and specifically in inflammatory bowel disease (IBD) however, the critical chemokines and receptors which regulate leucocyte traffic are not clear. In human small bowel Crohn’s disease (SBCD), expression of CCR9 by lamina propria lymphocytes is reduced and expression of CCL25 is altered in distribution rather than upregulated.10 Specifically, in normal small bowel, CCL25 is expressed by both crypt epithelial cells and small intestinal endothelial cells11,12 whereas in SBCD, CCL25 is not expressed by small bowel endothelial cells and is expressed in a patchy distribution by the intestinal crypts.10 Chemokines documented to have increased expression in human IBD (CD, ulcerative colitis (UC), or both) include CXCL8 (IL-8),15–18 CCL5 (RANTES),19,20 CCL3 (MIP-1α),15,20 CCL4 (MIP-1β),15,20 CXCL10 (IP-10),18,20 CCL2 (MCP-1),15,18,19,21,22 CCL8 (MCP-2),15 and CCL7 (MCP-3).15,18,23 Little work however has been done on the corresponding chemokine receptors and their potential involvement in IBD.24–26 Given the importance of pathogenic lymphocyte recruitment to the intestine in IBD, chemokine receptors uniquely involved in this process offer significant potential as novel therapeutic targets.
The aim of this study was to define the potential role of a putative inflammatory chemokine receptor, CCR2, in human IBD. Using the techniques of flow cytometry and immunohistochemistry, the pattern of expression of CCR2 by lamina propria lymphocytes (LPLs) was examined in human SBCD, Crohn’s colitis, and UC, and compared with control ileum and colon. The likely functional significance of the expression patterns was verified using in vitro chemotaxis assays with the CCR2 ligand CCL2. This is the first study to document a significant increase in expression of CCR2 by LPLs in human SBCD.
METHODS
Subjects
All subjects with IBD included in this study had undergone surgery for treatment of IBD. Involved intestine, either terminal ileum (SBCD) or colon (UC or Crohn’s colitis), was collected at the time of surgery. Control ileum and colon samples were obtained from patients undergoing surgery for non-IBD related conditions, primarily colorectal carcinoma. The segment of intestine used for analysis was greater than 5 cm from the site of the carcinoma. Peripheral blood was collected from all subjects. Clinical data for each subject were recorded, including: sex, age, IBD history, and recent medication. All patients gave written informed consent to participate in the study. The study was approved by the relevant institutional human research ethics committees.
Intestinal mucosal disaggregation and isolation of lamina propria mononuclear cells (LPMCs) and peripheral blood mononuclear cells
Enzymatic disaggregation of intestinal mucosa and isolation of LPMCs were performed as previously described.27 In brief, the mucosa was stripped from the muscularis mucosae and washed several times at 37°C in calcium and magnesium free Hanks’ balanced salt solutions (HBSS; Sigma, St Louis, Missouri, USA) with 0.75 mM EDTA (BDH, Poole, UK) (20 ml of media/g of tissue) with a final wash in Hanks’ balanced salt solution alone. Mucosal tissue was then finely minced and incubated overnight in a disaggregation solution containing RPMI (Gibco, Grand Island, New York, USA), 10% heat inactivated fetal calf serum, 8 U/ml collagenase (Calbiochem, La Jolla, California, USA), 10 U/ml DNAse II (Calbiochem), 2–4 mM l-glutamine (Trace Scientific, Melbourne, Australia), 20 mM Hepes (pH 8.0), 0.1 U/ml Penstrep (Trace Scientific), and 0.05 mg/ml gentamicin (Sigma) (20 ml of solution/g of tissue). The digest was filtered and LPMCs isolated using density gradient centrifugation (Nycomed Pharm As, Oslo, Norway). The resultant mononuclear cell preparation was >90% viable by 0.1% Trypan blue exclusion.
Peripheral blood mononuclear cells were isolated using density gradient centrifugation (Nycomed Pharm).
Flow cytometry
To reduce non-specific staining, cells were initially incubated for 15 minutes at room temperature with human serum (pooled from normal donors) and mouse serum. Cells were subsequently stained using monoclonal antibodies directed against CCR2 (R&D Systems, Minneapolis, USA), CD4, CD8, CD19 (Dako, Glostrup, Denmark), CD45RO, and β7 integrin (Pharmingen, Becton Dickinson, Singapore) as well as their corresponding isotype control antibodies (Dako).
All antibodies were directly conjugated, apart from anti-β7 integrin antibody. Antibody staining was performed in the dark, on ice, and for 20 minute incubation periods. After staining, red blood cells were lysed with FACS lysing solution (Becton Dickinson, San Jose, California, USA). Cells were then washed twice in phosphate buffered saline/0.01% azide/2% bovine serum albumin and fixed with 1% paraformaldehyde (BDH). For β7 integrin staining, cells were washed twice with phosphate buffered saline/0.01% azide/2% bovine serum albumin between primary and secondary antibody staining steps (rabbit anti-rat Ig-FITC) (Dako) and before addition of other antibodies (CD4, CCR2). Data were acquired using a FACScan flow cytometer (Becton Dickinson) and analysed using CellQuest (Becton Dickinson).
Chemotaxis assay
Chemotaxis assays were performed as previously described.28 Chemotaxis was assessed in 48 well microchemotaxis chambers (Neuroprobe, Gaithersburg, Maryland, USA) using fibronectin coated (Sigma) polyvinylpyrrolidone free 5 μm pore size membranes (Poretics Products, Livermore, California, USA). Migration in response to recombinant human CCL2 or CXCL12 proteins (R&D Systems) was allowed to continue for three hours at 37°C in 5% CO2. The membrane was then removed and the upper surface washed with distilled water, scraped, fixed, and stained with haematoxylin and Scott’s blue. Results were expressed using a chemotaxis index (mean number of cells per high power field for chemoattractant dilution/mean number of cells per high power field for medium).
Immunohistochemistry
A section of the intestinal segment retrieved at surgery was used for immunohistochemistry. The tissue was fixed in 10% formalin for 48 hours and then embedded in paraffin. Sections (4 μm) were cut and placed on silane coated glass slides. Antigen retrieval was carried out by heating the slides in a solution of Tris base, EDTA, and trisodium citrate for 15 minutes in a 750W microwave. Endogenous peroxidase activity was blocked by treating the slides with 3% hydrogen peroxide for 10 minutes. Non-specific staining was reduced by incubation with 20% non-immune goat serum (Vector Laboratories, California, USA) for 40 minutes. Slides were incubated at 37°C for 60 minutes with the primary antibody monoclonal (IgG2B) mouse antihuman-CCR2 (R&D Systems) at a final concentration of 20 μg/ml. Non-immune mouse monoclonal IgG2B (Dako) was used as a negative control antibody. After three washes in 0.0075% brij (Sigma), slides were incubated for 30 minutes at room temperature with a biotinylated goat antimouse antibody (Vector Laboratories) followed by treatment with streptavidin conjugated horseradish peroxidase (Vector Laboratories) for 60 minutes. Slides were stained with 3,3′-diaminobenzidine tetrahydrochloride (Sigma) for four minutes, washed in double distilled H2O, counterstained with Meyer’s haematoxylin (Dako) and Scott’s blue, dehydrated, and coverslipped.
The percentage of CCR2 positive cells was determined by counting the number of positive mononuclear cells and the total number of nuclei in 10 random fields of lamina propria per slide. Cells were counted under ×1000 magnification (with oil immersion).
Histological grading of ileal tissue
All SBCD and control ileal samples were graded histologically so that immunohistochemistry findings could be correlated with disease severity. The histological grading system was adapted a priori for this study from that of D’Haens et al (table 1 ▶).29 An additional category of inflammatory infiltrate in the muscularis propria was included to allow a more comprehensive assessment of the degree of transmural inflammation, while the categories of epithelial damage and erosions/ulcers were combined because of the overlap between these two features. Similarly, the category of architectural changes was omitted to avoid redundancy as the greater the degree of inflammatory infiltrate and epithelial damage the greater the architectural changes. Granulomas were regarded as a diagnostic marker of CD rather than an indicator of disease severity and so were also excluded.
Table 1.
Histological scoring system for Crohn’s disease
| A | Continuity of surface epithelium | 0 | Normal |
| 1 | Patchy/discontinuous | ||
| 2 | Ulcers | ||
| B | Infiltration of polymorphonuclear leucocytes in the lamina propria and epithelium | 0 | Normal |
| 1 | Moderate increase | ||
| 2 | Severe increase | ||
| C | Infiltration of mononuclear leucocytes in the lamina propria | 0 | Normal |
| 1 | Moderate increase | ||
| 2 | Severe increase | ||
| D | Inflammatory infiltrate in the submucosa | 0 | Normal |
| 1 | Moderate increase | ||
| 2 | Severe increase | ||
| E | Inflammatory infiltrate in the muscularis propria | 0 | Normal |
| 1 | Moderate increase | ||
| 2 | Severe increase |
*A moderate increase defined as being up to twice the normal number of cells; severe increase more than twice the normal number.
Each sample was graded histologically by two independent observers, masked to the clinical details and experimental results. Where different scores were allocated, the mean of the two was used.
Statistical analysis
Non-parametric statistical analysis of the results was carried out using the Mann-Whitney U test in Graph Pad Prism for Windows (version 3.0). All data are expressed as median and interquartile ranges (25th percentile–75th percentile). Statistical correlations and inter-rater variability (for example, for histological grading) were analysed by Spearman’s correlation coefficient using SPSS for Windows (version 10.0.5).
RESULTS
Terminal ileum was retrieved from 10 patients with ileal CD and from 11 control subjects having undergone a right hemicolectomy for carcinoma of the ascending colon. Colons from six patients with UC, five with Crohn’s colitis, and 11 control colons were available. One patient with UC had active disease but underwent a proctocolectomy due to the discovery of a synchronous descending colon carcinoma while the others had surgery for failed medical therapy alone. Nine of the 10 subjects with SBCD were female whereas in the control ileum group males predominated (seven of 11 subjects). In subjects with Crohn’s colitis, females also predominated (four of five subjects). In the UC group, there were equal numbers of each sex; similarly in the controls (six of 11 were male). Both UC and CD patients were significantly younger than the control groups: 70 years (63.5–75) in the control colon subjects versus 45 years (31.5–57) in UC patients (p = 0.001), and 30 years (21–52) in Crohn’s colitis patients (p = 0.002); 73 years (63–78) in the control ileum group versus 31 years (23–37) in SBCD patients (p = 0.0003).
Histological grading of inflammation
From a maximum possible score of 10, the median histological score for the SBCD samples was 8 (3.8–10). There was a high degree of correlation between the scores from the two observers (r = 0.947; p<0.01). All control samples had a histological score of 0.
Flow cytometry
In SBCD (n = 9), significantly more of the total LPL population expressed CCR2 than in control ileum (n = 7) (29.3% in SBCD (19.9–55.1) compared with 0.9% in control ileum (0.4–11.5); p = 0.0007) (fig 1 ▶). The expanded CCR2+ lymphocyte population was predominantly CD4+ rather than CD8+ lymphocytes. CCR2+CD4+ lymphocytes were significantly increased in SBCD (21.8% (5.7–24.7) v 0.4% (0.3–7.7) in control ileum; p = 0.002) whereas CCR2+CD8+ lymphocytes were not increased (6.6% (1.0–15.5) in SBCD v 2.5% (0.2–43.6) in control ileum; NS) (fig 2 ▶). As shown in previous studies,24,30 there was no significant difference in the total CD4+ population between the two groups (48.3% (34.5–51.8) in SBCD compared with 36.5% (34.3–44.4) in control samples; NS). CCR2+CD19+ populations were also not significantly different between cases and control subjects. Both memory CCR2+CD45RO+ lymphocytes and naïve CCR2+CD45RO− lymphocyte populations were significantly expanded in SBCD compared with control ileum (fig 3 ▶). In CD, 23.5% (7.6–28.4) of total LPLs were CCR2+CD45RO+ while in control ileum the percentage was 0.5% (0.3–13.3) (p = 0.005). In SBCD, 5.0% of LPLs were CCR2+CD45RO− (1.3–18.8) while in control ileum 0.3% were CCR2+CD45RO− (0.1–2.1) (p = 0.01). Similarly, CCR2+CD4+CD45RO+ and CCR2+CD4+CD45RO− populations were increased in SBCD (fig 4 ▶). In CD patients, 16.8% of LPLs were CCR2+CD4+CD45RO+ (5.3–21.4) while in control ileum 0.3% were CCR2+CD4+CD45RO+ (0.2–7.5; p = 0.002). Of the LPLs in SBCD, 0.9% (0.4–2.3) were CCR2+CD4+CD45RO− while in the control ileum 0.02% (0–0.3) were CCR2+CD4+CD45RO− (p = 0.0007).
Figure 1.
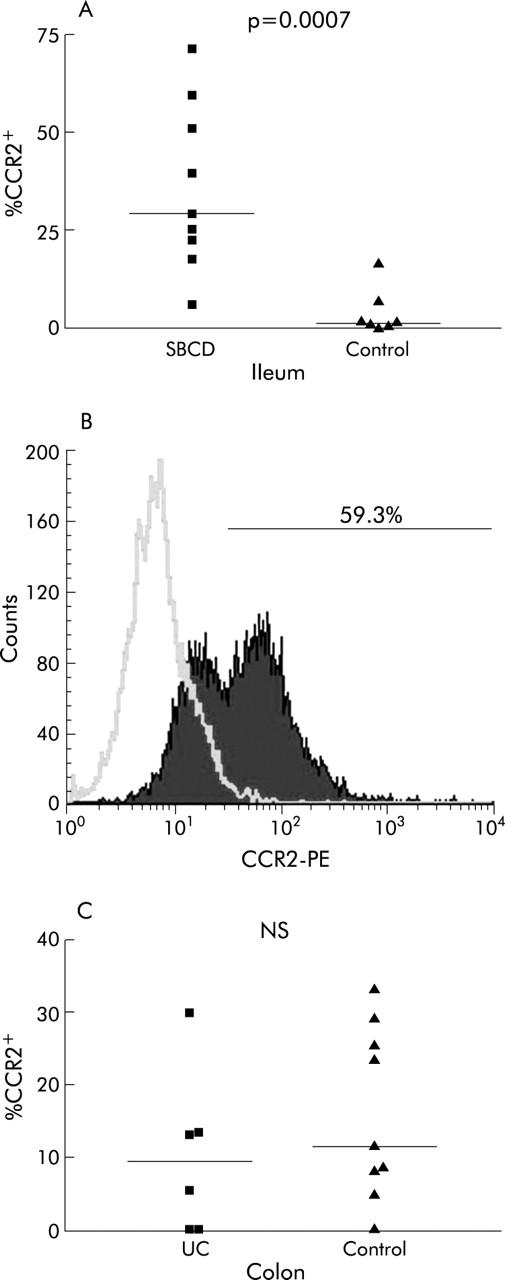
Percentage of CCR2+ lamina propria lymphocytes (LPLs) in small bowel Crohn’s disease (SBCD) and ulcerative colitis (UC), as determined by flow cytometry. Statistics were determined by the Mann-Whitney U test. Medians are represented by horizontal bars. (A) Percentage of CCR2+ LPLs in SBCD and control ileum. (B) A representative histogram from an SBCD patient showing percentage of LPLs expressing CCR2. (C) Percentage of CCR2+ LPLs in UC compared with control colon.
Figure 2.
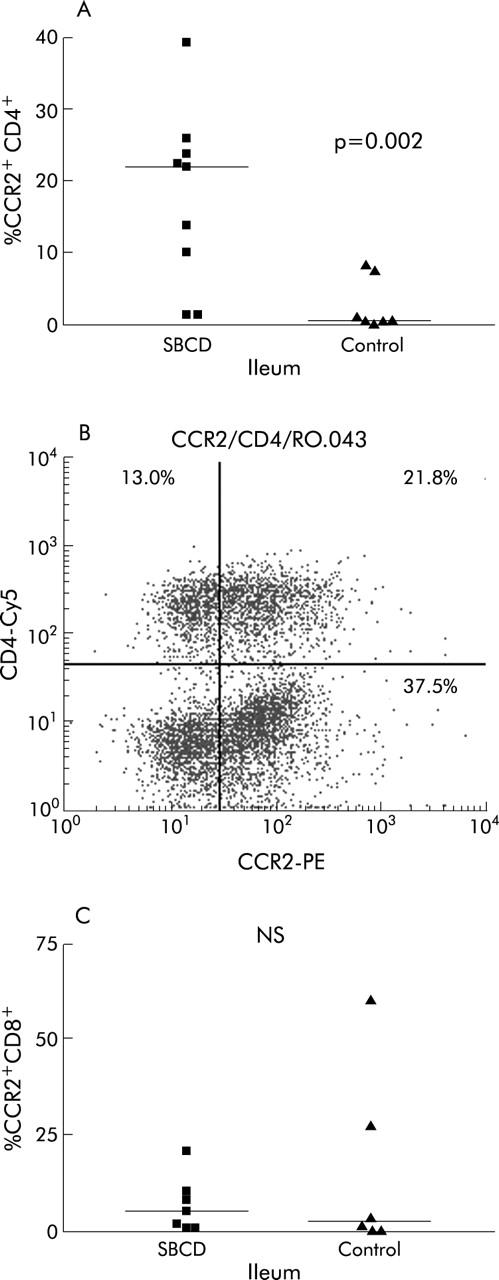
Percentage of CCR2+CD4+ and CCR2+CD8+ lamina propria lymphocytes (LPLs) in small bowel Crohn’s disease (SBCD) and control ileum, as determined by flow cytometry. Statistics were determined by the Mann-Whitney U test. Horizontal bars represent medians. (A) Percentage of CCR2+CD4+ LPLs in SBCD and control ileum. (B) Representative dot plot of CCR2+CD4+ LPLs in SBCD. (C) Percentage of CCR2+CD8+ LPLs in SBCD and control ileum.
Figure 3.
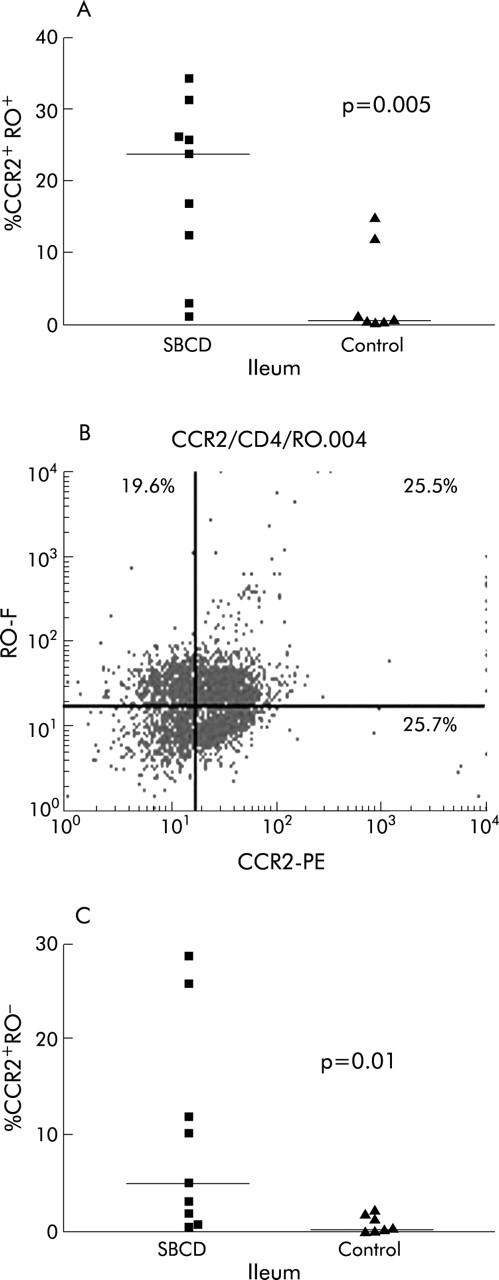
Percentage of CCR2+RO+ and CCR2+RO- lamina propria lymphocytes (LPLs) in small bowel Crohn’s disease (SBCD) and control ileum, as determined by flow cytometry. Medians are represented by horizontal bars. (A) Percentage of CCR2+RO+ LPLs in SBCD and control ileum. (B) Representative dot plot of CCR2+RO+ LPLs in SBCD. (C) Percentage of CCR2+RO− LPLs in SBCD and control ileum.
Figure 4.
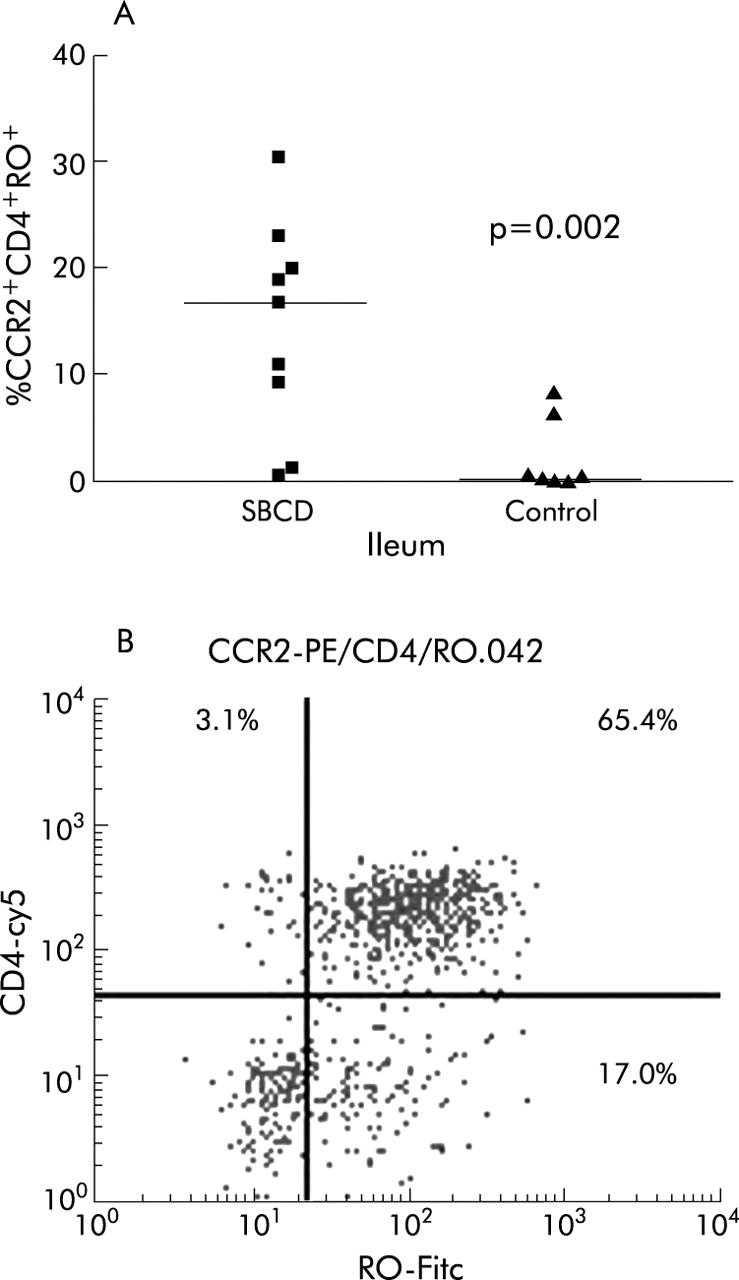
Percentage of CCR2+CD4+RO+ lamina propria lymphocytes (LPLs) in small bowel Crohn’s disease (SBCD) and control ileum, as determined by flow cytometry. (A) Statistics were determined by the Mann-Whitney U test. Medians are represented by horizontal bars. (B) Representative dot plot from an SBCD patient showing the CD4+RO+ population within the gated population of CCR2+ LPLs.
There was no significant difference between total β7 integrin expression on LPLs in SBCD (41.3% (31.1–48.9)) compared with control ileum (46.1% (33.6–71.2); NS). Our data (n = 4) also suggested that CCR2+ β7 integrin+ lymphocytes were not significantly increased in SBCD compared with control ileum. In peripheral blood, there was no significant increase in CCR2+ lymphocytes in SBCD compared with control subjects (12.9% (1.5–17.3) v 2.5% (1.1–28.8), respectively) suggesting the increase in CCR2+ lymphocytes in SBCD is localised to the ileum rather than a generalised phenomenon of SBCD.
In contrast with ileal CD, in colonic CD (n = 5) CCR2+ LPLs were not significantly increased above levels seen in control colons (n = 9) (8.5% (4.0–11.4) in Crohn’s colitis compared with 11.4% (6.4–27.3) in control colon; NS).
In UC (n = 6) there was no significant difference in expression of CCR2 by LPLs compared with control colon (n = 9) (9.3% (0–21.6) in UC v 11.4% (6.4–27.3) in control colon; NS). There were also no significant differences in the CCR2+CD4+ populations between the two groups (6.6% (0.5–11.5) in UC compared with 8.0% (4.4–18.3) in control colons; NS).
Immunohistochemistry
The findings on immunohistochemistry corroborated the flow cytometry results (figs 5 ▶, 6 ▶). In SBCD (n = 9) there were significantly more CCR2+ lymphocytes in the lamina propria (44% (40.4–48.2) than in control ileum (n = 7) (31.7% (28.5–34.0); p = 0.0002). There was no significant difference between UC (n = 6) and control colons (n = 10) (53.0% (39.2–59.6) and 47.6% (39.2–55.3), respectively; NS).
Figure 5.
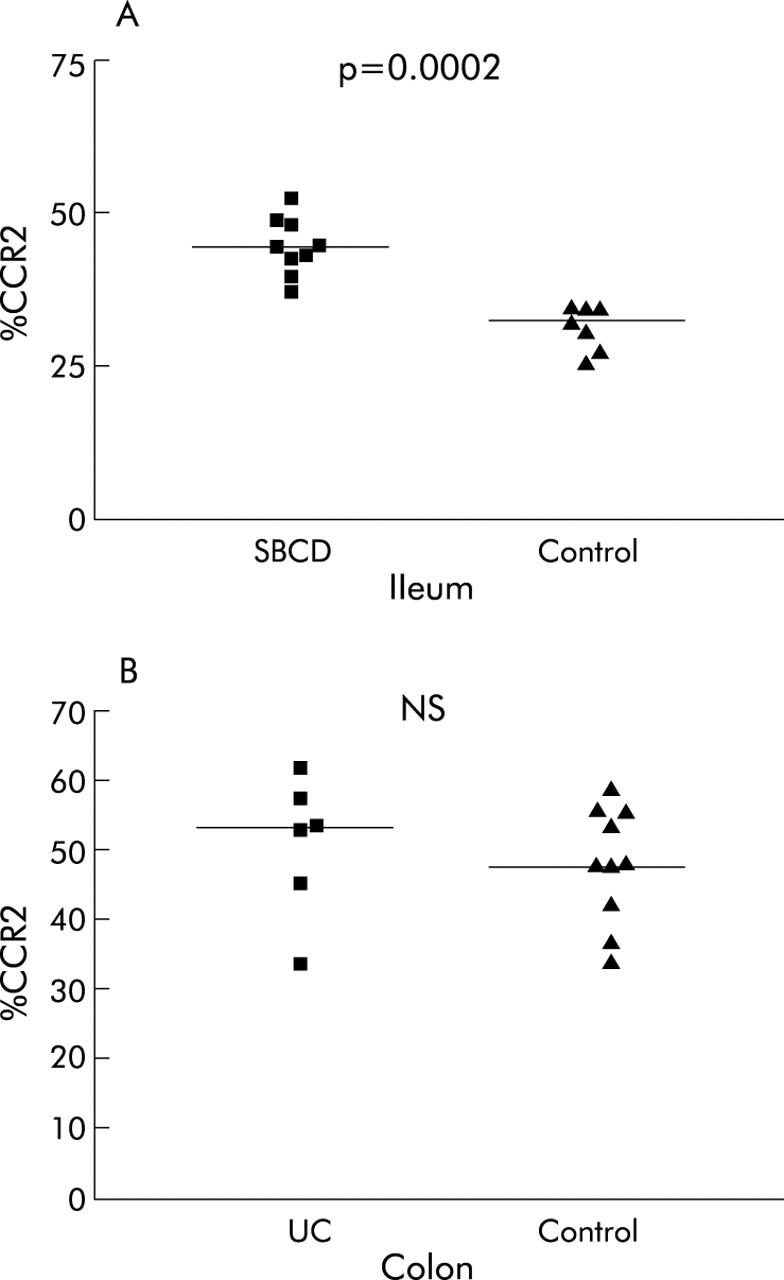
Percentage of CCR2+ lamina propria lymphocytes in small bowel Crohn’s disease (SBCD) and ulcerative colitis (UC), as determined by immunohistochemistry. Statistics were determined by the Mann-Whitney U test. Medians are represented by horizontal bars. (A) SBCD and control ileum. (B) UC and control colon.
Figure 6.
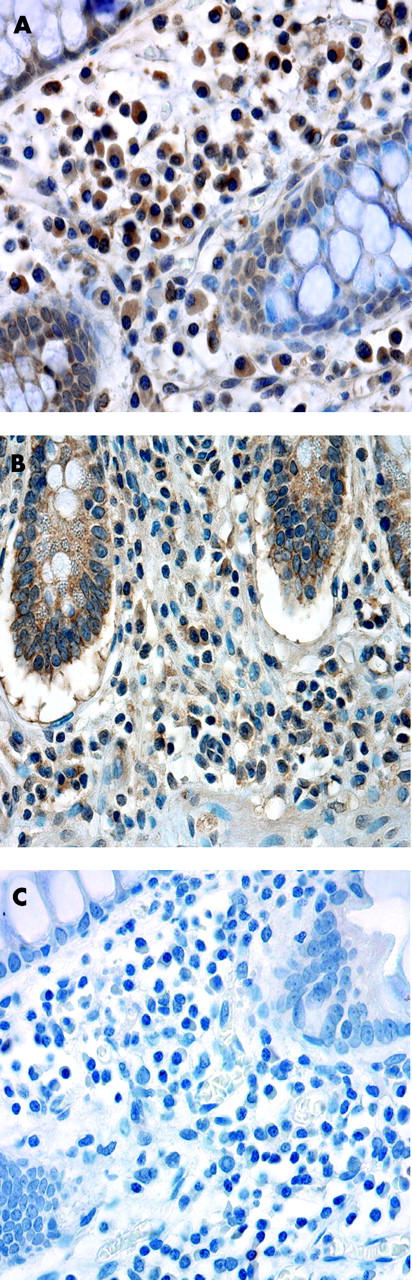
Representative immunohistochemistry showing expression of CCR2 by lamina propria lymphocytes (LPLs) in small bowel Crohn’s disease (SBCD) and control ileum. (A) CCR2 expression by LPLs in SBCD. (B) CCR2 expression by LPLs in control ileum. (C) Isotype control IgG2a in SBCD.
While there was a significant difference in the percentage of CCR2+ lymphocytes between the SBCD and control group, a statistical correlation between the level of CCR2 expression and histological disease severity in the SBCD samples was not seen. There was however a trend towards such a correlation (r = 0.604; p = 0.085).
Chemotaxis assays
As shown in fig 7A ▶, there was greater chemotaxis in response to the CCR2 ligand, CCL2, by LPMCs from CD ileum than from control ileum. The CXCR3 ligand, CXCL10, was used as a positive control as expression of CXCR3 was not significantly different between the two groups (93.4% (86.1–97.8) in control ileum v 96.2% (92.7–98.6) in SBCD; NS). Chemotaxis to CXCL10 was similar in CD and control ileum (fig 7B ▶).
Figure 7.
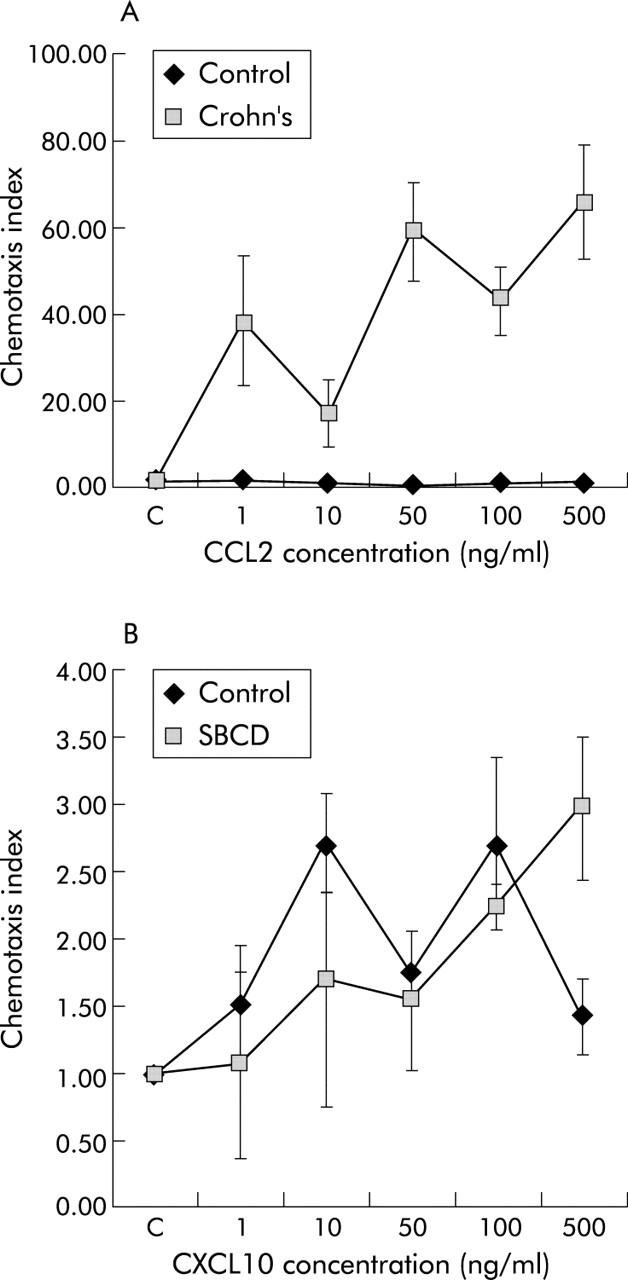
Chemotaxis assays in small bowel Crohn’s disease (SBCD) and control ileum. (A) Chemotaxis to CCR2 ligand, CCL2, in both SBCD and control ileum. (B) Chemotaxis to the CXCR3 ligand, CXCL10, in both SBCD and control ileum. Values are mean (SD) (n = 3).
DISCUSSION
This study has demonstrated for the first time a significant increase in the percentage of CCR2+ LPLs in SBCD. CCR2+ lymphocytes were predominantly CD4+ T cells rather than CD8+ T cells or CD19+ cells (B cells) and included both memory and naïve phenotypes (CD45RO+ and RO−, respectively). The increase in CCR2+ lymphocytes was specific for ileal CD: there was no increase in colonic CD or in UC, and the changes did not reflect a generalised phenomenon of increased circulating CCR2+ T lymphocytes in subjects with SBCD. The functional significance of these findings was confirmed by the demonstration of increased chemotaxis of LPMCs in response to CCL2 in SBCD samples.
The findings in control ileal tissue in this study did not confirm the previously reported level of expression of CCR2 by LPLs in normal small bowel.31 The median percentage of CCR2+ LPLs in control ileum in the study reported here was 0.9%, which was lower than that found by Agace et al (20%).31 Possible reasons for this difference include different tissues of origin of the LPLs (jejunum versus terminal ileum) and different subject groups (morbidly obese versus colorectal carcinoma). Enzymatic disaggregation used to isolate LPLs is unlikely to be the cause: the same disaggregation process was tested on peripheral blood lymphocytes and no effect on CCR2 expression was seen (data not shown).
The increase in CCR2+ lymphocytes in SBCD is likely to be the result of increased recruitment of these cells into the ileum in CD, rather than upregulation of CCR2 on lymphocytes in situ or proliferation of CCR2 positive lymphocytes. Upregulated expression of ligands for CCR2, namely CCL2, CCL7, and CCL8, which have each been documented to be increased in human CD,15,19,21–23 would favour preferential recruitment of CCR2+ lymphocytes into the tissue. Two mouse models of Crohn’s colitis, IL-10−/− mice and Rag-2−/− mice reconstituted with CD4+CD45RBhigh T cells, also documented increases in CCL2.25 The caveats regarding such mouse models as accurate representations of human disease also hold.32
With respect to in situ upregulation of CCR2, the increase in CCR2 ligands documented in CD may in fact potentiate receptor downregulation rather than the converse.33 When lymphocytes are stimulated with anti-CD3 antibody, no upregulation of CCR2 occurs, suggesting that lymphocyte activation, which is a feature of CD, is not likely to result in CCR2 upregulation.34
CCR2 is unlikely to be the sole factor determining LPL recruitment in SBCD. The majority of LPLs did not express CCR2. It is likely that in addition to CCR2, expression of additional chemokine receptors is required for recruitment and fine positioning of T lymphocytes within the lamina propria of the ileum in SBCD. Definition of these additional molecules is a potential area for future research.
CD4+ T lymphocytes are thought to play a critical role in the pathogenesis of IBD. Although the proportions of CD4+ T cells in IBD and control mucosa do not differ,30 CD4+ cells in IBD show an increased level of activation.35 Of particular note is the effect of HIV infection which results in complete remission of CD, presumably attributable to the associated CD4+ T cell depletion.36 In the present study, CCR2+ lymphocytes were predominantly CD4+ in phenotype. This supports the potential importance of CCR2 in the pathogenesis of SBCD and the possibility that blockade of CCR2 may be of therapeutic benefit.
The data presented here suggest that the β7 integrin is not an additional determinant for LPL recruitment in SBCD as no significant difference in the percentage of β7 integrin positive LPLs in SBCD was detected when compared with control samples, and initial findings would suggest that there is also no difference in dual positive CCR2+β7 integrin+ LPLs between the two groups. Although β7 integrin heterodimers, comprising α4β7 and αEβ7, play a major role in the homing of T lymphocytes to the normal gastrointestinal tract,37,38 in human Crohn’s colitis, β7 integrin expression in LPLs was significantly reduced.39,40 Apart from this work, there have been no previous published data on β7 integrin expression in SBCD. It appears likely that β7 integrin is critical for homing of LPLs to the normal ileum but not in recruitment of lymphocytes in human SBCD.
While CCR2 was significantly increased in SBCD, there was no significant increase in CCR2+ LPLs in UC compared with control colon. Current dogma suggests that CD is likely to be a Th1 mediated disease, consistent with the prominent granulomatous inflammation,41 whereas in UC a Th2 immune response is thought to predominate, although data on polarisation of the immune response in UC are not as strong as for CD.42 Data on whether CCR2 is best categorised as a Th1 or Th2 associated receptor are conflicting. The weight of evidence suggests that CCR2 expression is more closely associated with Th1 responses43–45 but there are also strong data supporting CCL2 and CCR2 being involved in Th2 inflammation46,47 and other data showing equal expression of CCR2 on both Th1 and Th2 cells.48
Mutations of the recently identified NOD2/CARD15 gene are associated with ileal CD.49,50 It is not known if there is an association between the NOD2/CARD15 mutation and increased CCR2 or CCL2 expression. Such a study would require prohibitively large numbers as the NOD2 mutation is uncommon, with only 6.5% of all Crohn’s patients being homozygous for this allele.51
Chemokine receptors are an essential component of the multistep adhesion cascade. By understanding the combinations of chemokine receptors and adhesion molecules responsible for recruitment of leucocytes in each of the different CD phenotypes, highly targeted biological therapies might be developed. This study suggests that CCR2 is one of the determinants for lymphocyte accumulation and recruitment in SBCD. Thus CCR2 may be a novel and effective therapeutic target.
Acknowledgments
The authors wish to acknowledge the considerable assistance of the surgeons who provided all the resections for this study and ongoing access to potentially appropriate surgical patients: Associate Professor Michael Solomon (Royal Prince Alfred Hospital, Camperdown, NSW, Australia) and Dr Philip Douglas (Prince of Wales Hospital, Randwick, NSW, Australia). This project was supported by a National Health and Medical Research Council of Australia (NHMRC) project grant (113853) and Dr Susan Connor was supported by a NHMRC Postgraduate Medical and Dental Scholarship.
Abbreviations
SBCD, small bowel Crohn’s disease
CD, Crohn’s disease
LPLs, lamina propria lymphocytes
LPMCs, lamina propria mononuclear cells
UC, ulcerative colitis
IBD, inflammatory bowel disease
REFERENCES
- 1.Yoshie O , Imai T, Nomiyama H. Chemokines in immunity. Adv Immunol 2001;78:57–110. [DOI] [PubMed] [Google Scholar]
- 2.Zlotnik A , Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity 2000;12:121–7. [DOI] [PubMed] [Google Scholar]
- 3.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol 1999;17:657–700. [DOI] [PubMed] [Google Scholar]
- 4.Qin S , Rottman JB, Myers P, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest 1998;101:746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balashov KE, Rottman JB, Weiner HL, et al. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci U S A 1999;96:6873–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorensen TL, Tani M, Jensen J, et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest 1999;103:807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zabel BA, Agace WW, Campbell JJ, et al. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J Exp Med 1999;190:1241–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wurbel MA, Philippe JM, Nguyen C, et al. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur J Immunol 2000;30:262–71. [DOI] [PubMed] [Google Scholar]
- 9.Svensson M , Marsal J, Ericsson A, et al. CCL25 mediates the localization of recently activated CD8alphabeta(+) lymphocytes to the small-intestinal mucosa. J Clin Invest 2002;110:1113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papadakis KA, Prehn J, Moreno ST, et al. CCR9-positive lymphocytes and thymus-expressed chemokine distinguish small bowel from colonic Crohn’s disease. Gastroenterology 2001;121:246–54. [DOI] [PubMed] [Google Scholar]
- 11.Papadakis KA, Prehn J, Nelson V, et al. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J Immunol 2000;165:5069–76. [DOI] [PubMed] [Google Scholar]
- 12.Kunkel EJ, Campbell JJ, Haraldsen G, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med 2000;192:761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med 2002;195:135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowman EP, Kuklin NA, Youngman KR, et al. The intestinal chemokine thymus-expressed chemokine (CCL25) attracts IgA antibody-secreting cells. J Exp Med 2002;195:269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banks C , Bateman A, Payne R, et al. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn’s disease. J Pathol 2003;199:28–35. [DOI] [PubMed] [Google Scholar]
- 16.Daig R , Andus T, Aschenbrenner E, et al. Increased interleukin 8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut 1996;38:216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzucchelli L , Hauser C, Zgraggen K, et al. Expression of interleukin-8 gene in inflammatory bowel disease is related to the histological grade of active inflammation. Am J Pathol 1994;144:997–1007. [PMC free article] [PubMed] [Google Scholar]
- 18.Uguccioni M , Gionchetti P, Robbiani DF, et al. Increased expression of IP-10, IL-8, MCP-1, and MCP-3 in ulcerative colitis. Am J Pathol 1999;155:331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzucchelli L , Hauser C, Zgraggen K, et al. Differential in situ expression of the genes encoding the chemokines MCP-1 and RANTES in human inflammatory bowel disease. J Pathol 1996;178:201–6. [DOI] [PubMed] [Google Scholar]
- 20.Grimm MC, Doe WF. Chemokines in inflammatory bowel disease mucosa: expression of RANTES, macrophage inflammatory protein (MIP)-1alpha, MIP-1beta, and gamma-interferon-inducible protein-10 by macrophages, lymphocytes, endothelial cells, and granulomas. Inflamm Bowel Dis 1996;2:88–96. [PubMed] [Google Scholar]
- 21.Grimm MC, Elsbury SKO, Pavli P, et al. Enhanced expression and production of monocyte chemoattractant protein-1 in inflammatory bowel disease mucosa. J Leukoc Biol 1996;59:804–12. [DOI] [PubMed] [Google Scholar]
- 22.Reinecker HC, Loh EY, Ringler DJ, et al. Monocyte-chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease mucosa. Gastroenterology 1995;108:40–50. [DOI] [PubMed] [Google Scholar]
- 23.Wedemeyer J , Lorentz A, Goke M, et al. Enhanced production of monocyte chemotactic protein 3 in inflammatory bowel disease mucosa. Gut 1999;44:629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan YH, ten Hove T, The FO, et al. Chemokine receptor CXCR3 expression in inflammatory bowel disease. Inflamm Bowel Dis 2001;7:281–6. [DOI] [PubMed] [Google Scholar]
- 25.Scheerens H , Hessel E, de Waal-Malefyt R, et al. Characterization of chemokines and chemokine receptors in two murine models of inflammatory bowel disease: IL-10−/− mice and Rag-2−/− mice reconstituted with CD4+CD45RBhigh T cells. Eur J Immunol 2001;31:1465–74. [DOI] [PubMed] [Google Scholar]
- 26.Andres PG, Beck PL, Mizoguchi E, et al. Mice with a selective deletion of the CC chemokine receptors 5 0r 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J Immunol 2000;164:6303–12. [DOI] [PubMed] [Google Scholar]
- 27.Golder JP, Doe WF. Isolation and preliminary characterization of human intestinal macrophages. Gastroenterology 1983;84:795–802. [PubMed] [Google Scholar]
- 28.Grimm MC, Ben-Baruch A, Taub DD, et al. Opiates transdeactivate chemokine receptors: delta and mu opiate receptor - mediated heterologous desensitization. J Exp Med 1998;188:317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Haens GR, Geboes K, Peeters M, et al. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology 1998;114:262–7. [DOI] [PubMed] [Google Scholar]
- 30.Hirata I , Berrebi G, Austin LL, et al. Immunohistological characterization of intraepithelial and lamina propria lymphocytes in control ileum and colon and in inflammatory bowel disease. Dig Dis Sci 1986;31:593–603. [DOI] [PubMed] [Google Scholar]
- 31.Agace WW, Roberts AI, Wu L, et al. Human intestinal lamina propria and intraepithelial lymphocytes express receptors specific for chemokines induced by inflammation. Eur J Immunol 2000;30:819–26. [DOI] [PubMed] [Google Scholar]
- 32.Strober W , Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol 2002;20:495–549. [DOI] [PubMed] [Google Scholar]
- 33.Franci C , Gosling J, Tsou CL, et al. Phosphorylation by a G protein-coupled kinase inhibits signaling and promotes internalization of the monocyte chemoattractant protein-1 receptor. Critical role of carboxyl-tail serines/threonines in receptor function. J Immunol 1996;157:5606–12. [PubMed] [Google Scholar]
- 34.Rabin RL, Park MK, Liao F, et al. Chemokine receptor responses on T cells are achieved through regulation of both receptor expression and signaling. J Immunol 1999;162:3840–50. [PubMed] [Google Scholar]
- 35.Schreiber S , MacDermott RP, Raedler A, et al. Increased activation of isolated intestinal lamina propria mononuclear cells in inflammatory bowel disease. Gastroenterology 1991;101:1020–30. [DOI] [PubMed] [Google Scholar]
- 36.James SP. Remission of Crohn’s disease after human immunodeficiency virus infection. Gastroenterology 1988;95:1667–9. [DOI] [PubMed] [Google Scholar]
- 37.Schon MP, Arya A, Murphy EA, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol 1999;162:6641–9. [PubMed] [Google Scholar]
- 38.Berlin C , Berg EL, Briskin MJ, et al. Alpha4beta7 integrin mediates lymphocyte binding to the mucosal vascular addressin MadCAM-1. Cell 1993;74:185–95. [DOI] [PubMed] [Google Scholar]
- 39.Elewaut D , De Keyser F, Cuvelier C, et al. Distinctive activated cellular subsets in colon from patients with Crohn’s disease and ulcerative colitis. Scand J Gastroenterol 1998;33:743–8. [DOI] [PubMed] [Google Scholar]
- 40.Meenan J , Spaans J, Grool TA, et al. Altered expression of alpha 4 beta 7, a gut homing integrin, by circulating and mucosal T cells in colonic mucosal inflammation. Gut 1997;40:241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shanahan F . Crohn’s disease. Lancet 2002;359:62–9. [DOI] [PubMed] [Google Scholar]
- 42.Farrell RJ, Peppercorn MA. Ulcerative colitis. Lancet 2002;359:331–40. [DOI] [PubMed] [Google Scholar]
- 43.Boring L , Gosling J, Chensue SW, et al. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest 1997;100:2552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang DR, Wang J, Kivisakk P, et al. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med 2001;193:713–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Traynor TR, Kuziel WA, Toews GB, et al. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J Immunol 2000;164:2021–7. [DOI] [PubMed] [Google Scholar]
- 46.Gu L , Tseng S, Horner RM, et al. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature 2000;404:407–11. [DOI] [PubMed] [Google Scholar]
- 47.Karpus WJ, Kennedy KJ, Kunkel SL, et al. Monocyte chemotactic protein 1 regulates oral tolerance induction by inhibition of T helper cell 1-related cytokines. J Exp Med 1998;187:733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonecchi R , Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med 1998;187:129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmad T , Armuzzi A, Bunce M, et al. The molecular classification of the clinical manifestations of Crohn’s disease. Gastroenterology 2002;122:854–66. [DOI] [PubMed] [Google Scholar]
- 50.Cuthbert AP, Fisher SA, Mirza MM, et al. The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease. Gastroenterology 2002;122:867–74. [DOI] [PubMed] [Google Scholar]
- 51.Hampe J , Cuthbert A, Croucher PJ, et al. Association between insertion mutation in NOD2 gene and Crohn’s disease in German and British populations. Lancet 2001;357:1925–8. [DOI] [PubMed] [Google Scholar]


