Abstract
Background: Fistulae are a common complication in up to 35% of all patients with Crohn’s disease. Their therapy is difficult and frequently unsatisfactory. To date, no histological comparison of Crohn’s disease fistulae with non-inflammatory bowel disease fistulae has been performed. In addition, Crohn’s disease fistulae have not been well characterised morphologically.
Methods: Eighty four fistulae from Crohn’s disease patients were compared with 13 fistulae from controls. Haematoxylin-eosin staining, electron microscopy, and immunohistochemistry for panCytokeratin (epithelial cells), CD20 (B cells), CD45R0 (T cells), and CD68 (macrophages) were performed according to standard techniques. In addition, histopathological findings were compared with clinical and laboratory data.
Results: In 31.0% of controls and 27.4% of Crohn’s disease specimens, fistulae had a lining of flattened intestinal epithelium without goblet cells or, in the case of cutaneous/perianal disease, narrow squamous epithelium. Non-epithelialised fistulae were covered by a thin layer of (myo)fibroblasts, focally forming a new basement membrane, as demonstrated by electron microscopy. All fistulae were surrounded by granulation tissue. Crohn’s disease fistulae presented with central infiltration by CD45R0+ T cells, followed by a small band of CD68+ macrophages and dense accumulation of CD20+ B cells. In contrast, in controls, there was dense infiltration by CD68+ macrophages with only few CD20+ B cells and CD45R0+ T lymphocytes.
Conclusions: Fistulae in Crohn’s disease differ markedly from non-Crohn’s disease fistulae with regard to their cellular composition. The presence of an epithelial lining in a subgroup of fistulae may be important for the therapeutic approach and healing process.
Keywords: Crohn’s disease, fistulae, histology, immunohistochemistry, electron microscopy
Fistulae are a frequent problem in patients with Crohn’s disease (CD). The reported incidence ranges from 17% up to 50%.1–5 This may be due to a centre bias as fistulising CD is seen more frequently in centres for inflammatory bowel disease (IBD). However, population based studies also show a high incidence of fistulae in CD.6,7
In the perianal area, formation of fistulae can be easily identified clinically and are sometimes associated with abscesses.8 Enteroenteric fistulae on the other hand are difficult to diagnose and can be the cause of malabsorption in CD patients but they are also less frequent.8 In a recent population based study, fistulae were reported in up to 35% of patients with CD, with perianal fistulae occurring in approximately 20% of cases.6,9 The cumulative incidence of fistulising CD in the study was 33% after 10 years and increased up to 50% after 20 years, making it a major clinical problem of disease management. Two thirds of those patients however had only a single fistula episode: 34% of these patients had recurrent fistulae. Recurrent fistulae occurred less frequently in patients who received maintenance therapy with an immunosuppressive agent.
In approximately 10% of patients suffering from CD, perianal fistulisation is the initial presentation of disease.1 Fistulae may precede the onset of intestinal disease by several years. Patients with colonic disease, especially with involvement of the rectum, have a higher incidence of perianal fistulae. Internal fistulae have been classified clinically into two types: those which form an internal connection between two bowel layers or segments, and those that occur between the intestine and other organs, such as enterovesical or abdominal wall fistulae.10,11
The pathogenesis of fistulae formation is still unknown. It is clear that fistulae in the perianal area do not arise from the small intestine but develop locally.11,12 However, their histological features have never been investigated in detail.
Fistulae are an important problem in the management of CD. A number of patients will develop an abscess, which either drains spontaneously or must be drained surgically. Surgical excision of fistulae has not proved to be very successful.13–15 Seton drainage seems to be more helpful but the frequency of complete healing of fistulae is low.16,17 Medical therapy is based on treatment with antibiotics or immunosuppressants such as azathioprine, cyclosporin, tacrolimus, or infliximab.1,3,8,18,19 Permanent closure of fistulae can only be achieved in approximately 20–30% of patients. Therefore, close cooperation between surgeons and gastroenterologists is usually recommended.
To investigate the pathophysiology and develop better therapeutic approaches for fistulising CD, it is necessary to understand the histological features of Crohn’s fistulae. However, a detailed histological investigation of Crohn’s fistulae has not been published previously. Therefore, in this study, histological differences between CD and non-CD fistulae were characterised.
MATERIALS AND METHODS
Patients
In this retrospective study, 97 fistula specimens from 78 patients were examined. The series was obtained from unselected cases at the Institute of Pathology, University of Regensburg. Specimens had been surgically removed between August 1993 and May 2003. Eighty four fistula specimens were derived from 67 CD patients and 13 fistulae from 11 controls with no IBD. The diagnosis of CD was based on established clinical, endoscopic, and radiological parameters.20,21 In addition to fistula characterisation, histopathological, electron microscopic, and immunohistochemical characteristics were compared between both groups of patients.
Patients with CD comprised 33 males and 34 females; mean age was 36.9 years (range 13–75). Six controls were male and five were female; mean age was 48.4 years (range 21–79). Fifty two fistulae (53.6%) were diagnosed in males and 45 (46.4%) in females. Clinical data are presented in tables 1 ▶–3 ▶.Most CD fistulae were located in the small or large intestine whereas most control fistulae were perianal or cutaneous fistulae. Eleven patients with CD and two control patients had more than one fistula. Details on fistula distribution are shown in tables 1 ▶–3 ▶.
Table 1.
Clinical data on patients in the control group
| No | Sex | Age at surgery (y) | Fistula location | Clinical diagnosis |
| 1 | M | 49 | Skin, cervical | Recurring cervical fistulae after squamous cell carcinoma of the tongue |
| 2 | M | 40 | Perianal | Recurring perianal fistulae, exclusion of CD |
| 3 | F | 79 | Skin, abdominal | Fistula of the skin, abdominal |
| 4 | M | 34 | Perianal | Sinus peronidalis with recurring perianal fistulae |
| 5 | F | 51 | Stomach | Fistula of the stomach after ulcus ventriculi |
| 6 | M | 63 | Oral cavity | Dental abscess with recurring fistulae of the oral cavity |
| 7 | F | 42 | Perianal | Fistula after periproctitic abscess, since 1994 until 2003 no CD, 2003 breast cancer |
| 42 | Perianal | |||
| 8 | M | 47 | Skin, thoracal | Fistula after thoracal abscess |
| 47 | Skin, thoracal | |||
| 9 | F | 73 | Vaginal | Vaginal fistula after radiation of vaginal squamous cell carcinoma |
| 10 | M | 41 | Perianal | Sinus pilonidalis with recurring perianal fistulae |
| 11 | F | 21 | Perianal | Sinus pilonidalis with perianal fistula |
Table 3.
Clinical data on Crohn’s disease patients
| Patients | |
| Sex (M/F) | 34/33 |
| Age at surgery (y) | 36.7 (4.0) |
| Time between diagnosis of fistula and surgery (months) | 4.0 (37.9) |
| Prior medical treatment for fistular disease | |
| Antibiotics | 10 (11.9%) |
| Azathioprine | 5 (6%) |
| Oral 5-ASA derivatives | 6 (7.1%) |
| Systemic glucocorticoids | 12 (14.3%) |
| Topical 5-ASA derivates | 1 (1.2%) |
| Cyclophosphamide | 1 (1.2%) |
| Cyclosporin | 1 (1.2%) |
| Infliximab | 1 (1.2%) |
| Mycofenolat mofetile | 1 (1.2%) |
| Present medication | |
| Antibiotics | 29 (34.5%) |
| Azathioprine | 10 (11.9%) |
| Oral 5-ASA derivatives | 27 (32.1%) |
| Systemic glucocorticoids | 42 (50%) |
| Topical 5-ASA derivatives | 2 (2.4%) |
| Topical glucocorticoids | 0 (0%) |
| Cyclosporin | 0 (0%) |
| E coli Nissle | 1 (1.2%) |
| Infliximab | 0 (0%) |
| Methotrexate | 1 (1.2%) |
| Location of fistula | |
| Ileum | 50 (59.5%) |
| Colon | 26 (31%) |
| Perianal | 8 (9.5%) |
| Concomitant complication | |
| None | 27 (32%) |
| Abscess | 26 (31%) |
| Conglomerate tumour | 24 (28.6%) |
| Peritonitis | 7 (8.3%) |
Specimen preparation
Tissue specimens were fixed in 4% buffered formalin for at least 24 hours and embedded in paraffin. Sections of approximately 2–3 μm thickness were cut from tissue blocks and stained with haematoxylin and eosin, according to standard protocols.
Histology
Two gastrointestinal pathologists (FB, PR) performed the histopathological examinations without knowledge of the clinical data. Specimens were examined in random order. The configuration of inflammation (acute/chronic), granulation tissue, capillarity, deepness of fistula, formation of abscesses, and presence of lining epithelium were evaluated separately.
Electron microscopy
Small tissue (primarily fixed in buffered 4% formalin for LM) samples were deparaffinised in xylol, hydrated in descending ethanols, additionally fixed in 0.1 M cacodylate buffered 4% glutaraldehyde (overnight), postfixed in 1% osmium tetroxide (two hours) at pH 7.3, dehydrated in graded ethanols, and embedded in the EmBed-812 epoxy resin (all reagents from Science Services, Munich, Germany). After 48 hours of heat polymerisation at 60°C, semithin (0.8 µm) sections were cut, stained with toluidine blue, and after selection of appropriate areas of interest the Epon block was trimmed for ultrathin sectioning. Ultrathin (80 nm) sections were cut with a diamond knife on a Reichert Ultracut-S ultramicrotome and double contrasted with aqueous 2% uranyl acetate and lead citrate solutions for 10 minutes each. Sections were examined in a LEO912AB electron microscope operating at 80 kV. Details of the investigated fistulae are shown in table 4 ▶.
Table 4.
Epithelialised and non-epithelialised fistulae of Crohn’s disease (CD) patients and controls by electron microscopy
| Sex | Age at surgery (y) | Location of fistula | Epithelialised/non-epithelialised | Disease |
| Male | 31 | Sigma | Epithelialised | CD |
| Male | 33 | Terminal ileum | Epithelialised | CD |
| Male | 71 | Perianal | Non-epithelialised | CD |
| Female | 42 | Perianal | Epithelialised | Non-CD |
| Female | 73 | Sigma | Epithelialised | Non-CD |
| Male | 34 | Perianal | Non-epithelialised | Non-CD |
Immunohistochemistry
Immunohistochemical studies for expression of CD20, CD45RO, CD68, and panCytokeratin were performed on 21 fistulae of patients with CD and 13 controls using an avidin-biotin peroxidase method with diaminobenzidine (DAB) chromogen. After antigen retrieval (microwave treatment of formalin fixed paraffin embedded 2-3 µm tissue sections for 40 minutes at 240 W in citrate buffer, pH 6.0) immunohistochemistry was carried out in a NEXES immunostainer (Ventana Medical System, Tucson, Arizona, USA) according to the manufacturer’s instructions. As primary antibody, mouse monoclonal antibodies were used at a dilution of 1:50 for panCytokeratin (panCK; Coulter-Immunotech Diagnostics, Hamburg, Germany), 1:100 for CD20 (clone L26, DakoCytomation, Hamburg, Germany) and CD45RO (clone UCHL T cell; Dako), and at a dilution of 1:200 for CD68 (clone KP1; Dako). After incubation for 24 minutes at 37°C, slides where rinsed in phosphate buffered saline and incubated with the secondary antibody (rabbit-antimouse,1:500 dilution in phosphate buffered saline; Ventana Medical System) for two hours at room temperature. Antibody binding was visualised with 0.05% DAB (Ventana Medical System) and 0.01% hydrogen peroxide. The material was rinsed in phosphate buffered saline and counterstained with haematoxylin. Cells were regarded as expressing panCytokeratin, CD20, CD45RO, and CD68 when there was cytoplasmic staining.
Clinical and serological parameters
Clinical and laboratory data were retrieved by chart review. Data collected included duration between diagnosis and resection, medication at time of surgery, previous medical therapy, last C reactive protein (CRP) level before surgery, and white blood cell count before resection.
Statistics
Associations between histopathological features, clinicopathological parameters, clinical data, and patient sex and age were analysed statistically in univariate fashion by χ2 testing, Mann-Whitney U test, or Fisher’s exact test, where appropriate. Statistics were computed using SPSS software (SPSS, Inc.; Chicago, Illinois, USA). All statistical tests were performed two sided with a p value <0.05 considered statistically significant.
RESULTS
Histology
Histologically, all studied fistulae had a central fissure in common, which branched and penetrated through lamina propria and muscularis mucosae deep into the underlying tissue. All fistulae were lined by granulation tissue with conspicuous pale plump histiocytes and a dense network of tender capillaries. According to the duration of the process, the lumen was filled up by nuclear debris, sometimes erythrocytes, and non-specific acute (neutrophils; n = 83) or chronic (lymphocytes; n = 14) inflammation (fig 1 ▶). In 2.0% of fistula specimens there was no acute inflammation, in 12.4% there was mild, in 28.9% moderate, and in 56.7% severe acute inflammation. Independent of the inflammatory infiltrate, 31.0% of control fistulae and 27.4% of CD fistulae had a lining epithelium central in the fistulising inflammation (fig 2 ▶). Depending on the location, the lining epithelium consisted of flattened epithelium of the small intestine or colon without goblet cells, or narrow squamous epithelium in cutaneous or perianal fistulae (fig 2 ▶). There was no association between epithelialisation of fistulae and deepness of infiltration or extent of inflammation.
Figure 1.
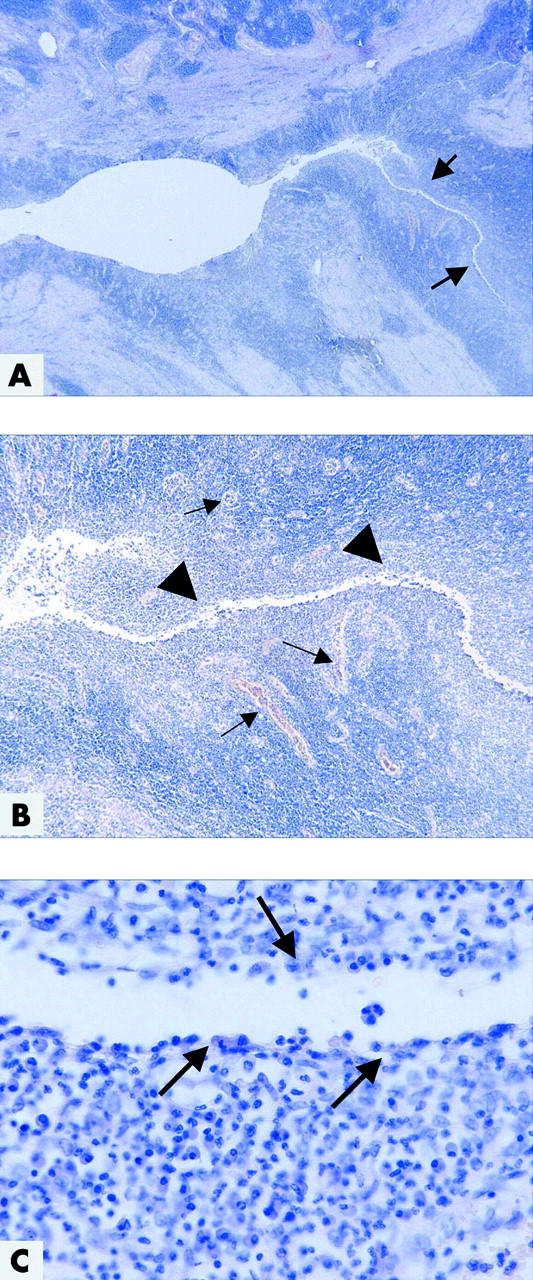
Histopathological picture of colonic Crohn’s disease with a fistula. (A) At low magnification, the mucosa is destroyed, ulcerated, and replaced by granulation tissue, including a dense capillary network. The surface is covered by fibrin and neutrophils. In the right half, there is a deep fistula which infiltrates the muscularis propria (arrows) (haematoxylin-eosin (H&E), ×16). (B) The central fissure (arrowheads) is branching deep into the underlying tissue, lined by neutrophils, granulation tissue with numerous capillaries (arrows), histiocytes, and lymphocytes (H&E, ×50). (C) At the surface of the central fissure, there is a small layer of histiocytes and neutrophils (arrows). Lining epithelium is not detectable (H&E, ×400).
Figure 2.
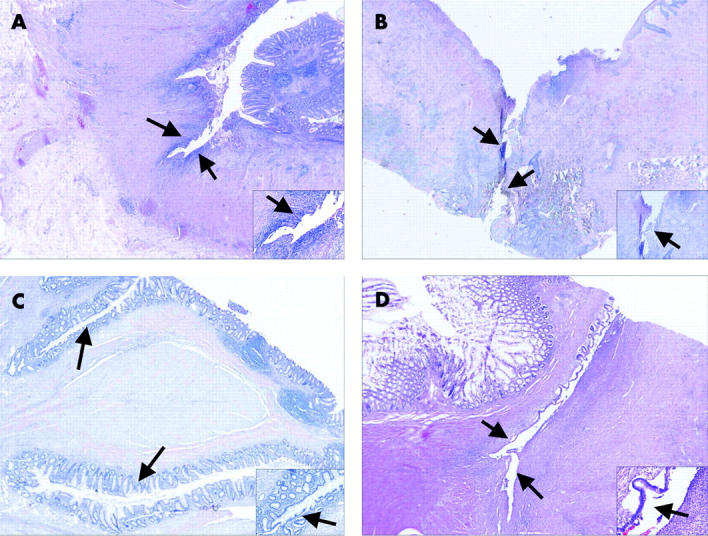
Representative histological pictures of epithelialised and non-epithelialised fistulae in Crohn’s disease (CD) (A, C) and controls (B, D). Depending on the location, the lining epithelium was similar to epithelium of the small intestine, colon, or squamous epithelium. (A) Colonic fistula (arrows) without lining epithelium in a CD patient (haematoxylin-eosin (H&E), ×16 (inlay ×100)). (B) Perianal fistula (arrows) without lining epithelium in a control patient (H&E, ×16 (inlay ×50)). (C) Epithelialised fistula (arrows) of the colon in a CD patient (H&E, ×16 (inlay ×100)). (D) Epithelialised fistula (arrows) of the small intestine in a control patient (H&E, ×16 (inlay ×200)).
For patients with more than one fistula, six CD patients (55%) and two patients in the control group had fistulae with different histological compositions. CD patients had synchronic (n = 7) or metachronic (n = 10) fistulae in the small intestine and colon. In four of six patients with CD, only fistulae of the small intestine had a lining epithelium. Synchronic fistulae in the control group were located perianally or cutaneously, each in a single patient. In both patients, only one of the two fistulae was epithelialised.
Electron microscopy
Fistulae were located in the small intestine, colon, or perianal region (table 4 ▶). Independent of the underlying disease, the lining epithelium consisted of flattened epithelium of the small or large intestine, or narrowed squamous cells. Cells showed tight junctions and a basement membrane. Non-epithelialised CD fistulae were lined in sections with myofibroblasts, with gap junctions to each other. In some areas, a new basement membrane, localised between the myofibroblasts and the underlying granulation tissue, was visible. Myofibroblasts were connected by fibronexus to the newly generated basement membrane. Adjacent sections with disordered myofibroblasts were visible. They showed no gap junctions and the underlining basement membrane was fragmented. In the control group, the basement membrane was more continuous, not only in sections, as shown in CD fistulae. Details are shown in fig 3 ▶.
Figure 3.
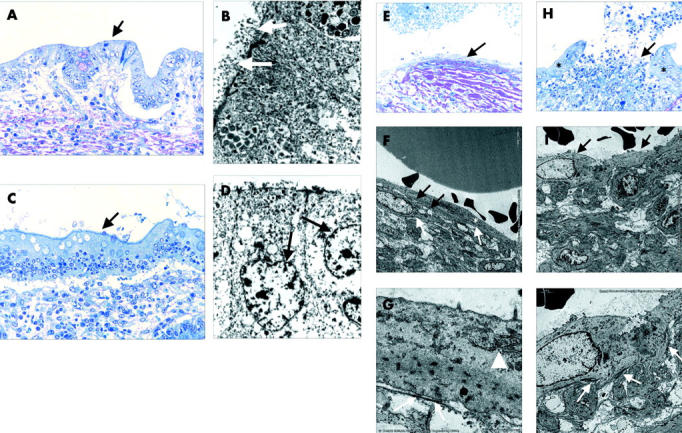
Electron micrographs of epithelialised and non-epithelialised fistulae of Crohn’s disease patients and controls. (A–D) Re-epithelialised fistulae of the small intestine (A: semithin section, toluidine blue, ×200; B: electron micrograph) and colon (C: semithin section, toluidine blue, ×200; D: electron micrograph). Histomorphologically, there is no similarity to the original lining epithelium of the small intestine (A: arrow) or colon (C: arrow) but by electron micrography there are typical microvilli (B: white arrows) and goblet cells (D: arrows). (E–G) Central fissure of a fistula lined by myofibroblasts (E: arrow). There is the typical appearance of myofibroblasts (F: asterisks): a cell membrane with multiple caveolae (F: white arrows), a rough endoplasmic reticulum and Golgi complex, and the cytoplasm containing bundles of microfilaments with focal densities (G: arrowhead). Myofibroblasts are connected to each other by adherens and gap junctions (F: black arrows) and are in contact with a newly formed thin basement membrane by fibronexus (G: white arrows). (H–J) Central fissure of a fistula lined by epithelium (H: asterisks) and myofibroblasts (H: arrow). The myofibroblasts rest irregularly on the surface of the fissure of the fistula (I: arrows), there are no generated adherens or gap junctions, and the underlining newly formed basement membrane (J: white arrows) is not continuous.
Immunohistochemistry
Immunohistochemically, there was a different cellular composition within the groups. In patients with CD, the interior wall of the fistulae was infiltrated by CD45RO positive T cells, followed by a small band of CD68 positive macrophages (in 19/21 fistulae (90.5%)). At the outer fistulae wall, there was a dense infiltrate of CD20 positive B cells (in 16/21 fistulae (76.2%)) (fig 4A ▶, C, E, G). In fistulae of the control group, there was intense infiltration by CD68 positive macrophages throughout the whole wall (in 10/13 fistulae (76.9%)). Only a few CD20 positive B lymphocytes could be identified (in 8/13 fistulae (61.5%)). CD45RO positive T lymphocytes were present in the outer two thirds of fistulae wall (fig 4B ▶, D, F, H). This distribution was independent of fistulae localisation and deepness of infiltration. Some (4/13), and mostly perianal fistulae of the control group, had an isolated infiltrate of only monocytes, surrounded by fibrosis, thereby resembling advanced chronified fistulae.
Figure 4.
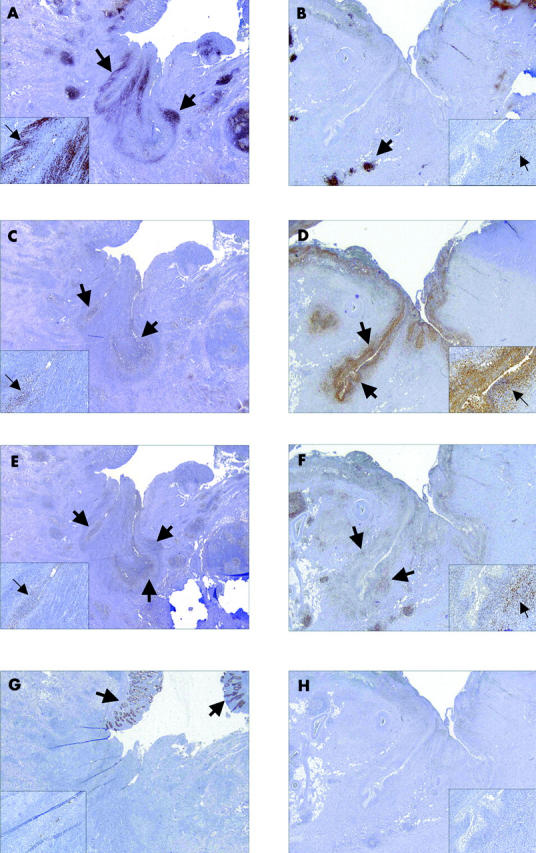
Immunohistochemical picture of Crohn’s disease (CD) (A, C, E, G) and control (B, D, F, H) fistula. Presented are representative pictures of the distribution of CD20 positive B cells (A and B: arrows), CD68 positive macrophages (C and D: arrows), CD45R0 positive T cells (E and F: arrows), and panCytokeratin positive epithelial cells (G: arrows) (overview ×16, inlay × 400).
Clinical and serological parameters
There was no significant association between histopathological findings and sex (p = 0.650), age (p = 0.942), fistulae location (p = 0.902), medical treatment at the time of surgery (p = 0.738), CRP level (p = 0.407), or white blood cell count (p = 0.918) prior to surgical intervention.
DISCUSSION
Fistulae are a complication of CD which is feared by patients and physicians because of their associated morbidity and difficult therapy. The specific pathogenesis of fistulae in CD, and also in non-CD, is not known. For perianal fistulae, two alternative hypotheses have been developed. The first suggests that fistulae begin as deep penetrating ulcers in the anus or rectum.22 Over time, faeces accumulate in these ulcers, and the intraluminal pressure during defecation forces the faecal matter into subcutaneous tissue, extending the ulcer and creating a fistula. The second differs in that the fistula results from an anal gland abscess that serves as the point of origin for a fistula tract.22
The natural history of CD fistulae is one of exacerbations and long episodes of active drainage.1,7 Despite the obvious clinical importance of this complication, little is known about its pathogenesis and histopathological composition.
Epithelialisation of fistulae was found in approximately one third of CD and control patients. Oberhuber et al described herniated mucosa within the muscularis propria or the subserosal fatty tissue in seven of 27 cases of fistulising CD.23 In our series, there was no herniated mucosa but the epithelium was similar to that of the surface, most likely mimicking a process of regeneration. Dysplasias were not detectable. Whether fistulae trigger malignant transformation and cancer is controversial. Early onset of disease, disease duration, chronic (pan-)colitis with high inflammatory activity, and the persistence of chronic fistulae and stenosis have been reported as risk factors for the development of malignancy.24 In contrast, Wong et al suggested that fistula associated adenocarcinomas represent anal gland carcinomas.25 Whether epithelialisation of fistula tracks with introduction of epithelial cells into deeper layers of the intestinal wall is associated with a higher risk of cancer is an intriguing question which cannot yet be answered. In addition, we do not know whether epithelialisation occurs in every fistula at the same stage or if epithelialised and non-epithelialised fistulae need different therapeutic approaches. It may be speculated that epithelialised fistulae represent a form of failed but stable restitution which is difficult to treat solely with medical therapy. Furthermore, if epithelialisation implies an increased cancer risk and there is a sequence from non-epithelialised to epithelialised fistulae, early aggressive medical therapy aimed at healing the fistula (for example, use of antibiotics, azathioprine,26 or infliximab27) may reduce this risk. Certainly, this is speculative at present and more data are needed.
Whereas on histology, fistulae of both patient groups shared some similarities, they differed markedly immunohistochemically. The most conspicuous finding was accumulation of CD45R0 positive T cells in the inner layer and dense infiltration of CD20 positive B cells surrounding the fistulae in patients with CD, as opposed to predominance of CD68 positive histiocytes in the granulation tissue of control fistulae. Similar to inflammation in CD itself, the role of various cell types is likely to differ in the development and evolution of fistulae. Activation of T cells and monocytes/macrophages is regarded as an important factor in the pathogenesis of IBD.28–31 Memory T cells play a role in initiating or perpetuating the inflammatory process. They may be primed to foreign micro-organisms or targeted to self antigens.32,33 Under normal circumstances, regulatory mucosal T cells maintain a state of controlled inflammation and suppress pathological inflammation.34
Treatment of CD has focused on neutralisation of proinflammatory mediators or administration of anti-inflammatory molecules but also on reduction of proinflammatory cells within the intestine.35 Medical treatment of CD reduces the mucosal content of proinflammatory cytokine producing T cells. All of our CD patients had received medication. This may have influenced the cellular composition of CD fistulae and may explain why there were less histiocytic cells compared with control fistulae. Nevertheless, we did not observe any association between the type of medication and the histological picture of CD fistulae. Therefore, it seems possible that the mechanisms involved in the pathogenesis of CD and non-CD fistulae are different and that the various cell types contribute differently.
It is likely that epithelial restitution in vivo involves a complex interaction between epithelial cells and the underlying lamina propria cells, which occurs via the basement membrane or pores within it.36,37 Intestinal myofibroblasts are located adjacent to the basement membrane and in close proximity to the basal surface of epithelial cells.38,39 In this position, myofibroblasts are capable of regulating a number of epithelial functions such as epithelial restitution,40 barrier function,41 and electrolyte transport.42 In contrast with the control group, CD fistulae had sections with disordered myofibroblasts and fragmented underlining basement membrane.
Myofibroblasts appear to be key cells in the events of tissue repair but CD myofibroblasts seem to differ from control myofibroblasts43 and this may account for induction of some dissimilarities observed in CD and control fistulae.
In the case of deep ulcers, subepithelial tissue containing interstitial substance, blood vessels, nerves, and mesenchymal cells must be reconstituted in addition to the epithelium. If the basement membrane has been destroyed by noxious stimuli (for example, inflammation), epithelial cells and mesenchymal elements form a new basement membrane.44 Our electron microscopy findings suggest that myofibroblasts also play a role in this repair process, being able to form a basement membrane even in the absence of epithelial cells.
Rapid migration of epithelial cells over a denuded basement membrane is an important response to injury. Whether this migration also occurs with time in non-epithelialised fistulae or whether these represent a different type of fistula cannot be determined from our data. However, based on our findings here, we hypothesise that the development and maturation of fistulae follows several successive steps: (1) initially, a stimulus in combination with a proinflammatory response leads to deep tissue destruction which may be perpetuated in the base of the fistula by intruding luminal antigens/bacteria; (2) myofibroblasts migrate or divide in the vicinity of the lesion; (3) they become organised by development of gap junctions and adherens and, trying to protect the body from the contents within the intestinal lumen, form a basement membrane; and (4) epithelial cells migrate from the surrounding of the inner lumen of the fistula onto the freshly formed basement membrane, therefore leading to an epithelialised fistula. According to this hypothesis, fistula formation would be caused by failure to close a tissue defect by a linear basement membrane. Perpetuation of the inflammation at the base of the fistula may prevent the migration and arrangement of myofibroblasts which might be a necessary step to achieve closure of the fistula. Differences in the cellular constitution of the inflammatory infiltrate between CD and normal fistulae suggest that either the response to a proinflammatory stimulus or the stimulus itself is different. However, it is necessary to further understand the molecular mechanisms and cellular interplay which are responsible for the formation of fistulae and differences between CD and non-CD fistulae.
Table 2.
Clinical data on Crohn’s disease fistulae. In Crohn’s disease, every fistula is counted as a single event
| No | Year of birth | Sex | Year of surgery | Location of fistula | Year of diagnosis of CD |
| 1 | 1959 | M | 1994 | Sigma | 1982 |
| 1994 | Sigma | ||||
| 1994 | Sigma | ||||
| 1997 | Colon | ||||
| 1998 | Colon | ||||
| 2 | 1973 | F | 1997 | Perianal | 1997 |
| 3 | 1964 | F | 1994 | Rectum | 1993 |
| 4 | 1970 | F | 2003 | Ileum | 1996 |
| 2003 | Ileum | ||||
| 5 | 1967 | M | 2000 | Ileum | 1998 |
| 6 | 1948 | M | 1999 | Colon | 1982 |
| 7 | 1963 | F | 1998 | Ileum | 1998 |
| 8 | 1972 | M | 1994 | Ileum | 1989 |
| 9 | 1967 | M | 1997 | Ileum | 1987 |
| 10 | 1954 | M | 2003 | Perianal | Not known |
| 11 | 1975 | M | 1997 | Ileum | 1997 |
| 12 | 1951 | M | 1999 | Ileum | 1988 |
| 13 | 1939 | M | 1994 | Ileum | 1994 |
| 14 | 1940 | F | 2003 | Ileum | Not known |
| 15 | 1953 | F | 2000 | Ileum | 1985 |
| 16 | 1974 | M | 1995 | Ileum | 1995 |
| 1998 | Ileum | ||||
| 1998 | Colon | ||||
| 17 | 1970 | F | 2002 | Colon | 1990 |
| 18 | 1964 | M | 2002 | Sigma | 2002 |
| 19 | 1972 | F | 1993 | Ileum | 1982 |
| 20 | 1956 | F | 1993 | Ileum | 1993 |
| 1993 | Perianal | ||||
| 21 | 1925 | M | 2000 | Ileum | 2000 |
| 22 | 1953 | F | 1995 | Rectum | 1980 |
| 23 | 1954 | M | 2001 | Ileum | 1992 |
| 24 | 1953 | M | 1997 | Ileum | 1978 |
| 25 | 1950 | F | 1997 | Colon | 1973 |
| 26 | 1969 | M | 2000 | Sigma | 1999 |
| 27 | 1947 | F | 2000 | Perianal | 1973 |
| 28 | 1984 | M | 2002 | Colon | 1998 |
| 29 | 1985 | M | 2002 | Colon | 2001 |
| 30 | 1962 | F | 1999 | Perianal | 1993 |
| 31 | 1972 | F | 2000 | Ileum | 1988 |
| 32 | 1957 | F | 1994 | Ileum | 1986 |
| 1996 | Ileum | ||||
| 33 | 1971 | M | 1993 | Ileum | 1988 |
| 34 | 1954 | F | 1998 | Colon | 1997 |
| 2003 | Ileum | ||||
| 35 | 1969 | M | 2002 | Ileum | 1995 |
| 36 | 1934 | F | 2002 | Ileum | 2002 |
| 37 | 1952 | F | 1998 | Colon | 1986 |
| 38 | 1951 | F | 1994 | Ileum | 1993 |
| 39 | 1937 | F | 1997 | Colon | 1994 |
| 40 | 1961 | F | 1998 | Ileum | 1998 |
| 41 | 1943 | F | 2001 | Ileum | 2001 |
| 42 | 1975 | F | 2002 | Colon | 1994 |
| 43 | 1968 | M | 1994 | Ileum | 1991 |
| 1994 | Colon | ||||
| 2003 | Colon | ||||
| 44 | 1963 | M | 1999 | Ileum | 1991 |
| 45 | 1934 | M | 1995 | Ileum | 1994 |
| 2002 | Ileum | ||||
| 46 | 1968 | M | 2001 | Ileum | 1995 |
| 2001 | Colon | ||||
| 47 | 1969 | M | 2000 | Ileum | 2000 |
| 48 | 1988 | M | 2001 | Ileum | 2001 |
| 49 | 1963 | M | 1994 | Ileum | 1994 |
| 50 | 1959 | M | 1999 | Perianal | 1991 |
| 51 | 1971 | M | 1996 | Ileum | 1989 |
| 52 | 1973 | F | 1999 | Ileum | 1999 |
| 53 | 1975 | F | 2002 | Ileum | 2001 |
| 54 | 1961 | M | 2001 | Ileum | 1997 |
| 55 | 1949 | F | 1993 | Perianal | 1993 |
| 56 | 1976 | F | 2002 | Perianal | 1996 |
| 57 | 1956 | M | 1999 | Ileum | 1999 |
| 58 | 1978 | M | 2003 | Ileum | 2003 |
| 59 | 1964 | F | 2001 | Ileum | 1987 |
| 60 | 1938 | M | 2001 | Ileum | 2001 |
| 61 | 1969 | F | 1993 | Ileum | 1993 |
| 62 | 1957 | F | 1992 | Colon | 1992 |
| 63 | 1982 | M | 1997 | Colon | 1996 |
| 1999 | Ileum | ||||
| 64 | 1967 | M | 1998 | Ileum | 1997 |
| 65 | 1963 | F | 2000 | Ileum | 1996 |
| Colon | |||||
| 66 | 1967 | F | 2000 | Ileum | 1997 |
| Colon | |||||
| 67 | 1946 | F | 2002 | Ileum | 1985 |
Acknowledgments
This work was supported by the BMBF Competence network “Inflammatory Bowel Disease”. We thank Sabine Troppmann and Doris Gaag for outstanding technical assistance.
Abbreviations
CD, Crohn’s disease
IBD, inflammatory bowel disease
CRP, C reactive protein
H&E, haematoxylin-eosin
DAB, diaminobenzidine chromogen
REFERENCES
- 1.Hellers G , Bergstrand O, Ewerth S, et al. Occurrence and outcome after primary treatment of anal fistulae in Crohn’s disease. Gut 1980;21:525–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michelassi F , Stella M, Balestracci T, et al. Incidence, diagnosis, and treatment of enteric and colorectal fistulae in patients with Crohn’s disease. Ann Surg 1993;218:660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon MJ. Fistulae and abscesses in symptomatic perianal Crohn’s disease. Int J Colorectal Dis 1996;11:222–6. [DOI] [PubMed] [Google Scholar]
- 4.Allan A , Keighley MR. Management of perianal Crohn’s disease. World J Surg 1988;12:198–202. [DOI] [PubMed] [Google Scholar]
- 5.Bell SJ, Williams AB, Wiesel P, et al. The clinical course of fistulating Crohn’s disease. Aliment Pharmacol Ther 2003;17:1145–51. [DOI] [PubMed] [Google Scholar]
- 6.Loftus EV jr, Schoenfeld P, Sandborn WJ. The epidemiology and natural history of Crohn’s disease in population-based patient cohorts from North America: a systematic review. Aliment Pharmacol Ther 2002;16:51–60. [DOI] [PubMed] [Google Scholar]
- 7.Loftus EV jr, Silverstein MD, Sandborn WJ, et al. Crohn’s disease in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survival. Gastroenterology 1998;114:1161–8. [DOI] [PubMed] [Google Scholar]
- 8.Givel JC, Hawker P, Allan R, et al. Entero-enteric fistula complicating Crohn’s disease. J Clin Gastroenterol 1983;5:321–3. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz DA, Loftus EV jr, Tremaine WJ, et al. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology 2002;122:875–80. [DOI] [PubMed] [Google Scholar]
- 10.D’Haens G . Medical management of major internal fistulae in Crohn’s disease. Inflamm Bowel Dis 2000;6:244–5. [PubMed] [Google Scholar]
- 11.Present DH. Urinary tract fistulas in Crohn’s disease: surgery versus medical therapy. Am J Gastroenterol 2002;97:2165–7. [DOI] [PubMed] [Google Scholar]
- 12.Present DH. Crohn’s fistula: current concepts in management. Gastroenterology 2003;124:1629–35. [DOI] [PubMed] [Google Scholar]
- 13.O’Hanlon DM, O’Connel PR. Complex fistulae in Crohn’s disease. J Am Coll Surg 2002;194:87. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann JC, Zeitz M. Treatment of Crohn’s disease. Hepatogastroenterology 2000;47:90–100. [PubMed] [Google Scholar]
- 15.Francois Y , Vignal J, Descos L. Outcome of perianal fistulae in Crohn’s disease—value of Hughes’ pathogenic classification. Int J Colorectal Dis 1993;8:39–41. [DOI] [PubMed] [Google Scholar]
- 16.Takesue Y , Ohge H, Yokoyama T, et al. Long-term results of seton drainage on complex anal fistulae in patients with Crohn’s disease. J Gastroenterol 2002;37:912–15. [DOI] [PubMed] [Google Scholar]
- 17.Williams JG, MacLeod CA, Rothenberger DA, et al. Seton treatment of high anal fistulae. Br J Surg 1991;78:1159–61. [DOI] [PubMed] [Google Scholar]
- 18.Bell SJ, Kamm MA. Review article: the clinical role of anti-TNFalpha antibody treatment in Crohn’s disease. Aliment Pharmacol Ther 2000;14:501–14. [DOI] [PubMed] [Google Scholar]
- 19.Present DH. Review article: the efficacy of infliximab in Crohn’s disease—healing of fistulae. Aliment Pharmacol Ther 1999;13 (suppl 4) :23–8. [DOI] [PubMed] [Google Scholar]
- 20.Gasche C , Scholmerich J, Brynskov J, et al. A simple classification of Crohn’s disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis 2000;6:8–15. [DOI] [PubMed] [Google Scholar]
- 21.Modigliani R , Mary JY, Simon JF, et al. Clinical, biological, and endoscopic picture of attacks of Crohn’s disease. Evolution on prednisolone. Groupe d’Etude Therapeutique des Affections Inflammatoires Digestives. Gastroenterology 1990;98:811–18. [DOI] [PubMed] [Google Scholar]
- 22.Hughes LE. Surgical pathology and management of anorectal Crohn’s disease. J R Soc Med 1978;71:644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oberhuber G , Stangl PC, Vogelsang H, et al. Significant association of strictures and internal fistula formation in Crohn’s disease. Virchows Arch 2000;437:293–7. [DOI] [PubMed] [Google Scholar]
- 24.Winkler R , Wittmer A, Heusermann U. Cancer and Crohn’s disease. Z Gastroenterol 2002;40:569–76. [DOI] [PubMed] [Google Scholar]
- 25.Wong NA, Shirazi T, Hamer-Hodges DW, et al. Adenocarcinoma arising within a Crohn’s-related anorectal fistula: a form of anal gland carcinoma? Histopathology 2002;40:302–4. [DOI] [PubMed] [Google Scholar]
- 26.Dejaco C , Harrer M, Waldhoer T, et al. Antibiotics and azathioprine for the treatment of perianal fistulas in Crohn’s disease. Aliment Pharmacol Ther 2003;18:1113–20. [DOI] [PubMed] [Google Scholar]
- 27.Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 1999;340:1398–405. [DOI] [PubMed] [Google Scholar]
- 28.Saubermann LJ, Probert CS, Christ AD, et al. Evidence of T cell receptor beta-chain patterns in inflammatory and noninflammatory bowel disease states. Am J Physiol 1999;276:G613–21. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber S . Monocytes or T cells in Crohn’s disease: does IL-16 allow both to play at that game? Gut 2001;49:747–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zareie M , Singh PK, Irvine EJ, et al. Monocyte/macrophage activation by normal bacteria and bacterial products: implications for altered epithelial function in Crohn’s disease. Am J Pathol 2001;158:1101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boirivant M , Marini M, Di Felice G, et al. Lamina propria T cells in Crohn’s disease and other gastrointestinal inflammation show defective CD2 pathway-induced apoptosis. Gastroenterology 1999;116:557–65. [DOI] [PubMed] [Google Scholar]
- 32.Picarella D , Hurlbut P, Rottman J, et al. Monoclonal antibodies specific for beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted with CD45RBhigh CD4+ T cells. J Immunol 1997;158:2099–106. [PubMed] [Google Scholar]
- 33.Ludviksson BR, Strober W, Nishikomori R, et al. Administration of mAb against alpha E beta 7 prevents and ameliorates immunization-induced colitis in IL-2−/− mice. J Immunol 1999;162:4975–82. [PubMed] [Google Scholar]
- 34.Shanahan F . Crohn’s disease. Lancet 2002;359:62–9. [DOI] [PubMed] [Google Scholar]
- 35.Caprilli R , Viscido A, Guagnozzi D. Review article: biological agents in the treatment of Crohn’s disease. Aliment Pharmacol Ther 2002;16:1579–90. [DOI] [PubMed] [Google Scholar]
- 36.Mahida YR, Galvin AM, Gray T, et al. Migration of human intestinal lamina propria lymphocytes, macrophages and eosinophils following the loss of surface epithelial cells. Clin Exp Immunol 1997;109:377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McAlindon ME, Gray T, Galvin A, et al. Differential lamina propria cell migration via basement membrane pores of inflammatory bowel disease mucosa. Gastroenterology 1998;115:841–8. [DOI] [PubMed] [Google Scholar]
- 38.Kaye GI, Pascal RR, Lane N. The colonic pericryptal fibroblast sheath: replication, migration, and cytodifferentiation of a mesenchymal cell system in adult tissue. 3. Replication and differentiation in human hyperplastic and adenomatous polyps. Gastroenterology 1971;60:515–36. [PubMed] [Google Scholar]
- 39.Powell DW, Mifflin RC, Valentich JD, et al. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol 1999;277:C183–201. [DOI] [PubMed] [Google Scholar]
- 40.McKaig BC, Makh SS, Hawkey CJ, et al. Normal human colonic subepithelial myofibroblasts enhance epithelial migration (restitution) via TGF-beta3. Am J Physiol 1999;276:G1087–93. [DOI] [PubMed] [Google Scholar]
- 41.Beltinger J , McKaig BC, Makh S, et al. Human colonic subepithelial myofibroblasts modulate transepithelial resistance and secretory response. Am J Physiol 1999;277:C271–9. [DOI] [PubMed] [Google Scholar]
- 42.Berschneider HM, Powell DW. Fibroblasts modulate intestinal secretory responses to inflammatory mediators. J Clin Invest 1992;89:484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKaig BC, Hughes K, Tighe PJ, et al. Differential expression of TGF-beta isoforms by normal and inflammatory bowel disease intestinal myofibroblasts. Am J Physiol Cell Physiol 2002;282:C172–82. [DOI] [PubMed] [Google Scholar]
- 44.Wallace JL, Granger DN. The cellular and molecular basis of gastric mucosal defense. Faseb J 1996;10:731–40. [DOI] [PubMed] [Google Scholar]


