INTRODUCTION
Biologic therapies include:(1) naturally occurring or modified biologic compounds such as vaccines (live, live attenuated, or killed microorganisms), hormone extracts, and blood products;(2) recombinant proteins or peptides—for example, granulocyte macrophage colony stimulating factor and growth hormone;(3) monoclonal antibodies and fusion proteins; and (4) antisense oligonucleotides to nucleic acids. These biologic therapies, which are targeted towards specific disease mechanisms, have the potential to provide more effective and safe treatments for human diseases. Clinical trials have demonstrated that inhibition of the cytokine tumour necrosis factor α(TNF) and inhibition of the selective adhesion molecules α4 integrin and α4β7 integrin are effective in the treatment of various forms of the inflammatory bowel diseases (IBD) ulcerative colitis (UC) and Crohn’s disease (CD). It is also clear that drug toxicity related to biologic therapy, including hypersensitivity, serum sickness, autoimmunity, infection, and immunogenicity may occur. This article reviews the progress made to date in the treatment of IBD with biologic therapies, concentrating primarily on the TNF inhibitors infliximab, etanercept, adalimumab, CDP870, CDP571, and onercept, and on the selective adhesion molecule inhibitors natalizumab and MLN-02, but also reviewing other potentially promising therapies targeted towards other mechanisms of action (table 1 ▶).
Table 1.
Biotechnology compounds that have been or are being evaluated for the treatment of patients with inflammatory bowel disease
| Drug | Manufacturer | Indication | Phase of investigation |
| Infliximab (chimeric IgG1 monoclonal antibody against TNF) | Centocor and Schering Plough | Crohn’s disease/&;ulcerative colitis | Phase 4/phase 3 |
| CDP571 (humanised IgG4 monoclonal antibody against TNF) | Celltech | Crohn’s disease/&;ulcerative colitis | Failed phase 3/failed phase 2a |
| CDP870 (humanised Fab antibody fragment against TNF linked to PEG) | Celltech | Crohn’s disease | Phase 3 |
| Etanercept (recombinant human fusion protein comprised of IgG1 Fc antibody fragment linked to soluble p75 receptor to TNF) | Amgen (previously Immunex) | Crohn’s disease | Failed phase 2 |
| Onercept (recombinant human p55 soluble receptor to TNF) | Serono | Crohn’s disease | Phase 2, failed |
| Adalimumab (human IgG1 monoclonal antibody against TNF | Abbott | Crohn’s disease | Phase 3 |
| Natalizumab (humanised IgG 4 monoclonal antibody to α4 integrin) | Elan Pharmaceuticals and Biogen | Crohn’s disease, ulcerative colitis | Phase 3/phase 2a |
| MLN-02, LDP-02 (humanised IgG1 monoclonal antibody to α4β7 integrin) | Millennium Pharmaceuticals (previously Leukocyte Pharmaceuticals) | Crohn’s disease/&;ulcerative colitis | Phase 2/phase 2 |
| Alicaforsen, Isis 2302 (antisense nucleic acids against ICAM) | Isis Pharmaceuticals | Crohn’s disease/&;ulcerative colitis/&;pouchitis | Failed phase 3/phase 2/phase 2a |
| Fontolizumab (humanised anti-interferon γ antibody) | Protein Design Labs | Crohn’s disease | Phase 2 |
| J695, ABT-874 (human IgG1 monoclonal antibody to interleukin 12 p40) | Abbott Laboratories (previously Wyeth/Genetics Institute) | Crohn’s disease | Phase 2 |
| Interleukin 10 | Schering Plough | Crohn’s disease/&;ulcerative colitis | Failed phase 3/failed phase 2 |
| Interleukin 11 | Genetics Institute | Crohn’s disease | SQ delivery phase 2, discontinued oral delivery phase 2 |
| CNI-1493 (MAP-kinase inhibitor) | Cytokine PharmaSciences | Crohn’s disease | Phase 2 |
| BIRB-796 (MAP-kinase inhibitor) | Boehringer-Ingelheim | Crohn’s disease | Failed phase 2 |
| RDP58 (peptide consisting of D-amino acids and glycine which blocks the p38 and JNK MAP kinase pathways and inhibits the synthesis of TNF-α, γ interferon, and interleukin 12) | Proctor & Gamble (previously Genzyme and Sangstat) | Crohn’s disease/&;ulcerative colitis | Phase 2/phase 2 |
| MRA (humanised anti-interleukin 6 receptor antibody) | Roche (previously Chugai Pharmaceutical Company) | Crohn’s disease | Phase 2 |
| Somatropin (recombinant human growth hormone) | Eli Lily | Crohn’s disease | Phase 2 |
| Filgrastim (recombinant human granulocyte colony-stimulating factor) | Amgen | Crohn’s disease | Phase 2a |
| Sargramostim (recombinant human granulocyte-macrophage colony stimulating factor) | Berlex (previously Immunex) | Crohn’s disease | Phase 3 |
| Daclizumab humanised (anti-interleukin 2 receptor antibody) | Protein Design Labs | Ulcerative colitis | Phase 2 |
| Basiliximab (chimeric anti-interleukin 2 receptor antibody) | Novartis | Ulcerative colitis | Phase 2a |
| Visilizumab (anti-CD3 antibody) | Protein Design Labs | Ulcerative colitis | Phase 1/2a |
| Epidermal growth factor | Heber Biotec, a commercial subsidiary of the Center for Genetic Engineering and Biotechnology, Havana, Cuba | Ulcerative colitis | Phase 2 |
| Keratinocyte growth factor 2 (repifermin) | Human Genome Sciences | Ulcerative colitis | Failed phase 2 |
TNF, tumour necrosis factor; PEG, polyethylene glycol; ICAM, intercellular adhesion molecule 1
INHIBITION OF TNF
Comparative mechanisms of action for various anti-TNF agents
TNF is elevated in the mucosa of patients with CD,1 and inhibition of TNF has been an effective treatment strategy.2 Comparison of various anti-TNF agents with respect to biologic construction, ability to bind soluble and membrane bound TNF, ability to fix complement, ability to mediate antibody dependent cytotoxicity, ability to cause T cell apoptosis, and efficacy in unselected patients versus efficacy primarily in patients with elevated concentrations of C reactive protein (CRP) is shown in table 2 ▶. The efficacy of the anti-TNF agent infliximab in unselected patients with CD appears to be linked to the ability of the molecule to induce T cell apoptosis.3 Other agents with more pure anti-TNF effects appear to be effective primarily in a selected subset of patients with elevated CRP concentrations.
Table 2.
Comparative mechanisms of action for various anti-tumour necrosis factor (TNF) agents
| Agent | Description of biologic construction | Binds soluble TNF | Binds membrane bound TNF | Fixes complement | Mediates &;ADC | Causes T cell apoptosis | Effective in unselected patients | Effective only in patients with elevated CRP |
| Infliximab | IgG1 chimeric monoclonal antibody | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Etanercept | Fusion protein comprised of a human IgG1 Fc antibody fragment linked to two human soluble p75 TNF receptors | Yes | Yes | No | No | No | No | ? |
| Adalimumab | Fully human IgG1 monoclonal antibody to TNF | Yes | Yes | Yes | Yes | Yes | ? | ? |
| CDP870 | Humanised Fab fragment linked to PEG | Yes | Yes | No | No | ? | No | Yes |
| CDP571 | Humanised IgG4 monoclonal antibody | Yes | Yes | No | No | ? | No | Yes |
| Onercept | Fully human soluble p55 TNF receptor monomer that binds soluble and membrane bound TNF but does not fix complement | Yes | Yes | No | No | No | No | ? |
ADC, antibody dependent cytotoxicity; CRP, C reactive protein; PEG, polyethylene glycol.
Infliximab
Inflixmab is a chimeric IgG1 monoclonal antibody against TNF-α that is administered intravenously. Infliximab is effective for induction of clinical response and remission in patients with active luminal inflammatory CD and in patients with draining enterocutaneous and perianal fistulas, and for subsequent maintenance of infliximab induced clinical response and remission in these patient groups. Clinical remission rates at week 4 in patients with active luminal inflammatory CD refractory to conventional therapy (aminosalicylates, prednisone, azathioprine, or 6-mercaptopurine) after a single infusion were 4% for placebo, 48% for infliximab 5 mg/kg, and 25% for infliximab 10 mg/kg and 20 mg/kg.2 The complete fistula closure rates in patients with CD and draining fistulas refractory to conventional therapy (antibiotics, aminosalicylates, prednisone, azathioprine, or 6-mercaptopurine) after three induction infusions at weeks 0, 2, and 6 were 13% for placebo, 55% for infliximab 5 mg/kg, and 38% for infliximab 10 mg/kg.4 There is a small increase in efficacy when patients with active CD receive three induction doses over six weeks rather than a single induction dose (clinical response rates at week 10 of 65% versus 52%),5 and multiple induction doses may confer immunological tolerance to the chimeric infliximab antibody (see below). For these reasons, a three dose induction regimen with 5 mg/kg at 0, 2, and 6 weeks is preferred over a single dose induction regimen with 5 mg/kg. Maintenance of clinical remission rates at one year in patients with inflammatory CD who have responded to induction therapy with infliximab were 9% for placebo every eight weeks, 24% for infliximab 5 mg/kg every eight weeks, and 32% for infliximab 10 mg/kg every eight weeks.6 Median duration of response in these patients was 19 weeks for placebo every eight weeks, 38 weeks for infliximab 5 mg/kg every eight weeks, and >54 weeks for infliximab 10 mg/kg every eight weeks.6 For patients who initially responded to infliximab and then lost their response during maintenance therapy, it was possible in many instances to regain response by escalating the infliximab dose up to 10 mg/kg7 or alternatively to shorten the dosing interval. Maintenance of complete fistula closure rates at one year in patients with fistulising CD who have responded to induction therapy with infliximab were 58% for infliximab 5 mg/kg every eight weeks and 38% for placebo every eight weeks.8 Median time to loss of fistula response was >40 weeks for maintenance infliximab 5 mg/kg every eight weeks and 14 weeks for placebo every eight weeks.
Two pilot controlled trials of infliximab for severe steroid refractory UC have been performed, one with a positive result9 and one with a negative result.10 Two large phase 3 trials of infliximab in patients with active UC are underway. Until the results of the ongoing trials are available, the use of infliximab in patients with UC should be considered investigational.
Although infliximab is well tolerated in the majority of patients, serious side effects may rarely occur, including: serious infections (see below); drug induced lupus11; acute infusion reactions12; delayed hypersensitivity reactions13; demyelination14; possibly an increased rate of lymphoma15; cardiac failure16; and death. In clinical trials, infections requiring treatment occurred in 32% of infliximab treated patients versus 22% of placebo treated patients.17 There was no significant increase in serious infections or sepsis, but pneumonia, sepsis, miliary tuberculosis, and disseminated coccidiomycosis were all observed. In post marketing surveillance, tuberculosis,18 histoplasmosis,19 listeriosis, aspergillosis, and pneumocystis pneumonia have all been observed, leading in some instances to death.20 Reactivation of latent tuberculosis is of particular concern.18 All patients treated with infliximab should undergo skin testing and chest x ray as well as a careful tuberculosis history prior to initiating infliximab therapy.17,21 A recent study reported the toxicity observed in 500 consecutive patients treated with infliximab at the Mayo Clinic (table 3 ▶).20
Table 3.
Adverse events occurring in 500 consecutive patients with Crohn’s disease treated with infliximab
| Adverse event | Frequency |
| Serious adverse event | 43 patients (8.6%) |
| Serious adverse event attributed to infliximab | 30 patients (6%) |
| Acute infusion reactions | 19 patients (3.8%) |
| Serum sickness-like disease | 19 patients (3.8%) |
| Serum-like disease attributed to infliximab | 14 patients (2.8%) |
| Drug induced lupus | 3 patients (0.6%) |
| New demyelination disorder | 1 patient (0.2%) |
| Any infectious event | 48 patients (9.6%) |
| Any infectious event attributed to infliximab | 41 patients (8.2%) |
| Serious infection | 20 patients (4%) |
| Fatal sepsis | 2 patients (0.4%) |
| Pneumonia | 8 patients (1.6%) &;(2 were fatal) |
| Viral infections | 6 patients (1.2%) |
| Abdominal abscess requiring surgery | 2 patients (0.4%) |
| Cellulitis of the arm | 1 patient (0.2%) |
| Histoplasmosis | 1 patient (0.2%) |
| Malignant disorder | 9 patients (1.8) |
| Malignant disorder possibly related to infliximab | 3 patients (0.6%) |
| Deaths | 10 patients (2.0%) |
| Deaths possibly related to infliximab | 5 patients (1.0%) |
Data from Colombel and colleagues.20
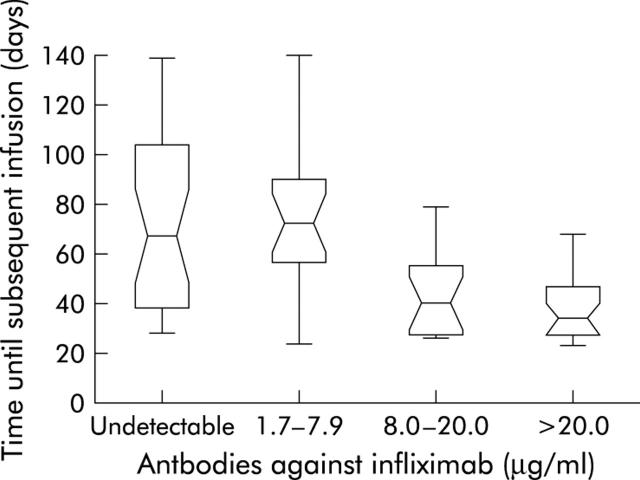
Infliximab is immunogenic and leads to the formation of human antichimeric antibodies (HACA).2,4,6,7,22–25 The presence of HACA antibodies is clinically important because they are associated with an increased frequency of infusion reactions and with loss of efficacy (fig 1 ▶).24,25 Premedication with 200 mg intravenous hydrocortisone, concomitant treatment with immunosuppressive therapy (azathioprine, 6-mercaptopurine, methotrexate), and administration of three induction doses over six weeks followed by systematic maintenance dosing every eight weeks all appear to be associated with a reduction in the rate of HACA formation.6,7,24,25
Figure 1.
Duration of response according to the concentration of antibodies against infliximab before infusion. Box plots show median values and first and third quartiles in each group. The T bars represent the rest of the data with a maximum of 1.5 times the interquartile range. The four categories can be divided in two groups: the first two categories have concentrations of antibodies against infliximab of <8.0 µg/ml and the second two have concentrations ⩾8.0 µg/ml. Median duration of response in the first two groups differed significantly (p<0.001) from the median duration of response in the groups with titres of ⩾8.0 µg/ml. Reprinted with permission from Baert and colleagues.24
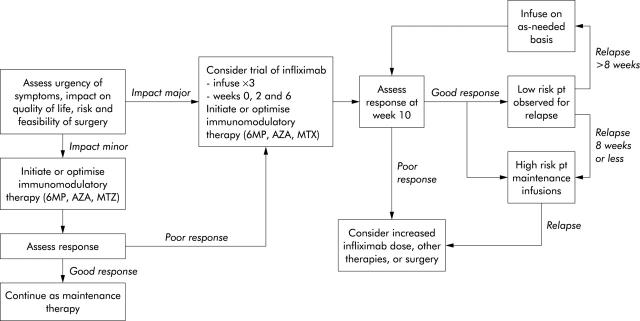
Regulatory approval for infliximab is limited to patients with moderate to severe CD unresponsive to conventional therapy (USA) or a full and adequate course of corticosteroids and immunosuppressive therapy (Europe) and to patients with actively draining fistulas (fig 2 ▶). Patients should routinely receive a concomitant immunosuppressive agent to minimise immunogenicity, and patients who respond to infliximab should generally receive maintenance dosing every eight weeks to minimise both immunogenicity and risk of relapse.
Figure 2.
Suggested treatment algorithm for managing patients with refractory Crohn’s disease. 6MP, 6-mercaptopurine; AZA, azathioprine; MTX, methotrexate. Modified with permission from: Sands BE. Therapy of inflammatory bowel disease. Gastroenterology 2000;118:S68–82.
CDP571
CDP571 is a humanised IgG4 monoclonal antibody against TNF-α that is administered intravenously. A phase 2 dose finding trial demonstrated the short term benefit of CD571 10 mg/kg for inducing a clinical response at two weeks in patients with active CD.26 A phase 3 trial of CDP571 10 mg/kg again showed short term benefit for inducing clinical response at two weeks in patients with active CD but no significant difference at 28 weeks with every eight week maintenance dosing.27 A post hoc exploratory analysis of a subgroup of patients with CRP concentrations ⩾10 mg/l demonstrated significantly increased response rates for CDP571 10 mg/kg at both two weeks and 28 weeks.27 Two controlled trials failed to demonstrate a steroid sparing benefit of CDP571 in patients with steroid dependent CD.28,29 CDP571 was well tolerated in patients with CD who developed infusion reactions or delayed-type hypersensitivity reactions to infliximab.30 Further clinical development of CDP571 for the treatment of CD has been discontinued.
CDP870
CDP870 is a humanised TNF-α Fab monoclonal antibody fragment linked to polyethylene glycol that is administered subcutaneously. A phase 2 study of subcutaneous CDP870 at doses of 100, 200, and 400 mg showed significant short term benefits at two weeks for CDP870 in patients with active CD but the difference was not sustained at 12 weeks in patients undergoing four weekly maintenance therapy.31 A post hoc exploratory analysis of a subgroup of patients with elevated CRP concentrations ⩾10 mg/l demonstrated a significant effect at both two weeks and 12 weeks for all of the CDP870 doses compared with placebo.31 Another smaller phase 2 study of intravenous CDP870 in patients with active CD failed to demonstrate efficacy.32 Two large phase 3 studies in patients with CD, primarily targeted towards patients with elevated CRP, are currently underway.
Etanercept
Etanercept is a fully human fusion protein comprised of two soluble TNF p75 receptors linked to an IgG1 Fc monoclonal antibody fragment that is administered subcutaneously. A phase 2 study of etanercept at a dose of 25 mg twice weekly in patients with active CD failed to demonstrate efficacy.33 Another unpublished phase 2 controlled trial in patients with active CD was also negative (Amgen, data on file).
Onercept
Onercept is a fully human recombinant soluble TNF p55 receptor administered subcutaneously. A pilot study of onercept in patients with active CD showed a benefit at a higher dose.34 However, a subsequent phase 2 trial of onercept in patients with active CD was negative (Serono, data on file).
Adalimumab
Adalimumab is a fully human IgG1 monoclonal antibody to TNF-α that is administered subcutaneously. An uncontrolled pilot study demonstrated that adalimumab was well tolerated in patients with CD who lost response, or developed infusion reactions or delayed-type hypersensitivity reactions to infliximab.35 Three phase 2 and phase 3 trials in patients with CD are currently underway.
INHIBITION OF CELL ADHESION
Mechanisms of action for various antiselective adhesion molecule agents
Lymphocyte trafficking to the gut is an important step in the initiation and perpetuation of intestinal inflammation in patients with IBD,36,37 and inhibition of lymphocyte trafficking has been an effective treatment strategy.38 A variety of therapeutic approaches have been used to inhibit lymphocyte trafficking in patients with IBD, including monoclonal antibodies to α4 integrin (natalizumab) and α4β7 integrin (MLN-02, LDP-02), and antisense to intercellular adhesion molecule 1 (ICAM-1)(alicaforsen, Isis 2303). Alpha 4 integrin is expressed at a moderate or high level on almost all lymphocytes and usually exists in combination with either a β1 subunit (that interacts predominantly with the endothelial ligands vascular cellular adhesion molecule 1) or a β7 subunit (that interacts predominantly with the mucosal addressin cellular adhesion molecule (Mad-CAM-1)].39 The interaction between α4β7 integrin and Mad-CAM-1 is important in mediating leucocyte homing to gut mucosa.40
Natalizumab
Natalizumab is a humanised IgG4 monoclonal antibody to α4 integrin. Two phase 2 studies of intravenous natalizumab at doses of 3 mg/kg, 3 mg/kg every four weeks ×2 doses, and 6 mg/kg every 4 weeks ×2 doses showed significant short term benefit for natalizumab in patients with active CD.38,41 A large phase 3 study in patients with active CD failed to show a benefit for natalizumab 300 mg every four weeks ×3 doses, primarily due to an unexpectedly high placebo response rate.42 A post hoc exploratory analysis of a subgroup of patients with CRP concentrations elevated above the normal range demonstrated a significant effect for natalizumab compared with placebo.42 Patients who responded to natalizumab in the phase 3 induction study were re-randomised to maintenance therapy with nazalizumab 300 mg or placebo every four weeks through six months (natalizumab withdrawal study). This maintenance study demonstrated a highly significant maintenance benefit, with the difference between the treatment groups at six months exceeding 30%.43 An additional phase 3 induction study in patients with active CD is currently being initiated. A pilot study of natalizumab in patients with active UC suggested clinical benefit.44
MLN-02 (LDP-02)
MLN-02 is a humanised IgG1 monoclonal antibody to α4β7 integrin that selectively inhibits leucocyte adhesion in the gastrointestinal mucosa. Fc receptor recognition and binding has been deleted thus eliminating complement fixation and cytokine release. A phase 2 trial of intravenous MLN-02 in patients with active CD failed to achieve the primary end point of clinical improvement but did show efficacy for remission at the highest dose studied.45 A phase 2 trial of intravenous MLN-02 for active UC demonstrated efficacy for both clinical and endoscopic end points.46
Alicaforsen (Isis 2302)
Alicaforsen (Isis 2302) is a 20 base phosphorothioate oligodeoxy nucleotide designed to hybridise to a sequence in the 3′ untranslated region of the human ICAM-1 message. The oligonucleotide-RNA heterodimer so formed serves as a substrate for the ubiquitious nuclease RNase-H with subsequent cleavage and reduction in cellular specific message content and consequent reduction in ICAM-1 expression. A phase 2 trial indicated that intravenous alicaforsen had a beneficial effect in active CD47 but a phase 3 trial failed to demonstrate efficacy.48 Another phase 2 trial failed to show efficacy of subcutaneous alicaforsen for active CD.49 Subgroup analysis of the phase 3 trial suggested that patients with high blood levels of alicaforsen responded better48 and a dose ranging pilot study identified a higher dose of intravenous alicaforsen that could more consistently achieve high blood levels.50 Two phase 3 trials of high dose intravenous alicaforsen in patients with active CD are underway (Isis Pharmaceuticals press releases, 2002). A phase 2 study of alicaforsen enemas suggested a beneficial effect at the highest dose in patients with active distal UCs. A phase 3 study in active UC is underway.51 A phase 2a study suggested a possible benefit of alicaforsen enemas for chronic pouchitis.52
MISCELLANEOUS AGENTS
Fontolizumab (anti-interferon γ)
Increased production of interferon γ by Th1 cells is part of the process of polarisation towards a Th1 immunological response seen in patients with CD. Fontolizumab is a humanised monoclonal antibody to interferon γ. A small phase 2a study of fontolizumab in patients with active CD did not show a clear benefit.53 A larger phase 2 study of fontolizumab at subcutaneous doses of 1.0 mg/kg or 4.0 mg/kg in 196 patients with active CD did not demonstrate efficacy.54 A second phase 2 study using larger intravenous doses of 4.0 mg/kg and 10 mg/kg failed to demonstrate efficacy in 133 patients with active CD at week 4 but a post hoc exploratory analysis of 96 patients who received a second 4.0 mg/kg or 10 mg/kg dose of fontolizumab did demonstrate efficacy.54 Additional studies of fontolizumab for induction and maintenance of remission in patients with CD are anticipated.
Anti-interleukin 12
Interleukin 12 plays a central role in promoting Th1 responses and is critical in the regulation of differentiation and activation of helper T lymphocytes. J695 (ABT-874) is a human IgG1 monoclonal antibody to interleukin 12 p40 that has been genetically modified in its variable region so that it has a high affinity for human interleukin 12. A phase 2 dose finding trial with in patients with active CD demonstrated efficacy.55
Interleukin 10
Interleukin 10 is a T helper type 2 cytokine that suppresses the production of interleukin 2 and interferon γ by T helper type 1 cells and decreases interleukin 12 production. A phase 2a study of intravenous interleukin 10 in patients with active CD suggested benefit.56 Phase 2 trials of subcutaneous interleukin 10 in patients with mild to moderately active CD57 and postoperative remission,58 and phase 3 trials in patients with chronically active CD59,60 did not demonstrate efficacy. A phase 2 trial of interleukin 10 in patients with active UC did not demonstrate efficacy.61
Interleukin 11
Interleukin 11 is a cytokine produced by cells of mesenchymal origin whose biologic effects include thrombocytopoiesis and enhancement of the barrier function of intestinal mucosal. Two placebo controlled trials of subcutaneous interleukin 11 in patients with active CD did not demonstrate clearcut efficacy.62,63 Interleukin 11 is stable in the gastrointestinal lumen and an oral formulation has been developed.64 A phase 2 study of oral interleukin 11 in patients with active CD is underway.
CNI-1493
CNI-1493 is a guanylhydrazone small molecule that inhibits the mitogen activated protein kinases (MAP kinases) JNK and p38, resulting in indirect inhibition of TNF-α production. A small phase 2 study of intravenous CNI-1493 suggested a benefit for active CD.65 A larger phase 2 trial of intravenous CNI-1493 for active CD is underway.
BIRB-796
BIRB 796 is a small molecule inhibitor of the MAP kinase p38 that can be administered orally.66 A large phase 2 trial of BIRB-796 in patients with active CD failed to demonstrate efficacy.
RDP58
RDP58 is an anti-inflammatory peptide consisting of nine D-amino acids and glycine which was developed by computer aided rational design using artificial intelligence. RDP58 blocks the p38 and JNK MAP kinase pathways and inhibits the synthesis of TNF-α, interferon γ, and interleukin 12 in animal models. RDP58 is not systemically bioavailable. A phase 2 trial of RDP58 in patients with active CD failed to demonstrate efficacy.67 A phase 2 trial of RDP58 for active UC demonstrated efficacy for a clinical remission end point.68
MRA (anti-interleukin 6 receptor antibody)
Interleukin 6 is a cytokine with a central role in immune regulation and inflammation that correlates with CRP and is elevated in patients with CD. MRA is a humanised IgG1 monoclonal antibody to interleukin 6 receptor. A phase 2 study of MRA in patients with active CD demonstrated efficacy.69
Somatropin (growth hormone)
Growth hormone is a regulatory protein that increases amino acid and electrolyte absorption by the intestines, decreases intestinal permeability, induces expression of insulin-like growth factor I, and decreases intra-abdominal fat.70 The rationale for the use of growth hormone in CD is to reverse the catabolic process associated with inflammation and to reduce the intra-abdominal (mesenteric) fat associated with CD. A small placebo controlled trial of somatropin (recombinant human growth hormone) plus a high protein diet in patients with active CD demonstrated a greater decrease in mean Crohn’s disease activity index scores for somatropin treated patients than for placebo treated patients (remission rates were not reported).71
Sargramostim (granulocyte-macrophage colony stimulating factor) and filgrastim (granulocyte colony stimulating factor)
Genetic syndromes resulting in neutrophil dysfunction such as glycogen storage diseases, Chediak-Higashi syndrome, and chronic granulomatous disease, which commonly manifest intestinal inflammation that has a phenotype similar to CD, have successfully been treated with filgrastim (recombinant human granulocyte colony stimulating factor) and sargramostim (recombinant human granulocyte-macrophage colony stimulating factor).72,73 Based on these observations, phase 2a studies were conducted in patients with CD which suggested that filgrastim and sargramostim may be of benefit in patients with active and fistulising CD, possibly via an immunostimulant effect on neutrophils.74,75 A phase 2 trial with sargramostim in patients with active CD demonstrated efficacy.76
Daclizumab
Interleukin 2 is produced by Th1 cells after interleukin 12, interferon γ, and interleukin 18 induce differentiation of naïve T helper cells to Th1 cells. Daclizumab, a humanised monoclonal antibody to the interleukin 2 receptor, blocks the binding of interleukin 2 to the interleukin 2 receptor. A phase 2a study of daclizumab suggested benefit in patients with refractory UC.77 A large phase 2 dose finding trial in patients with active UC is underway.
Basiliximab
Basiliximab is a chimeric monoclonal antibody to the interleukin 2 receptor that blocks the binding of interleukin 2 to the interleukin 2 receptor. A phase 2a study of basiliximab suggested benefit in patients with steroid dependant UC.78
Visalizumab
Visilizumab is a humanised monoclonal antibody to CD3. A phase 1/2a dose finding study in hospitalised patients with UC failing intravenous corticosteroids has shown promising preliminary results, with no serious toxicity observed to date.79
Epidermal growth factor enemas
Human epidermal growth factor is a potent mitogenic peptide produced by salivary and duodenal Brunner’s glands which stimulates cell proliferation in the gastrointestinal tract. A phase 2 study of recombinant epidermal growth factor enemas demonstrated efficacy in patients with active distal UC.80
Repifermin
Keratinocyte growth factor 1 (also known as fibroblast growth factor 7) is a potent stimulant of intestinal epithelial cells. Repifermin (keratinocyte growth factor 2 also known as fibroblast growth factor 10) is a homologue of keratinocyte growth factor 1. A phase 2 study of recombinant intravenous repifermin in patients with active UC failed to demonstrate efficacy.81
CONCLUSION
Infliximab is effective for induction and maintenance of remission in patients with inflammatory and fistulising CD. The optimal treatment regimen appears to be three induction doses of 5 mg/kg at 0, 2, and 6 weeks, followed by systematic maintenance dosing every eight weeks. Infliximab should be coadministered with an immunosuppressive agent to minimise the formation of HACA. Adverse events observed with infliximab include drug induced lupus, acute infusion reactions, delayed-type hypersensitivity reactions, demyelination, serious infections including reactivation of latent tuberculosis, possibly non-Hodgkin’s lymphoma, and death. The role of the humanised and human anti-TNF therapeutic alternatives to infliximab (CDP870 and adalimumab) in patients with CD remains to be determined. The antiselective adhesion molecule agents natalizumab and MLN-02 appear to have beneficial effects for CD and UC. Other promising agents include anti-inteleukin 12, sargramostim, daclizumab, visalizumab, and epidermal growth factor enemas.
REFERENCES
- 1.Van Deventer SJ. Tumour necrosis factor and Crohn’s disease. Gut 1997;40:443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med 1997;337:1029–35. [DOI] [PubMed] [Google Scholar]
- 3.van den Brande JMH, Braat H, van den Brink GR, et al. Infliximab but not etanercept induces apopotosis in lamina propria T-lymphocytes from patients with Crohn’s diseease. Gastroenterology 2003;124:1774–85. [DOI] [PubMed] [Google Scholar]
- 4.Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 1999;340:1398–405. [DOI] [PubMed] [Google Scholar]
- 5.Mayer L , Han C, Bala M, et al. Three dose induction regimen of infliximab (Remicade) is superior to a single dose in patients with Crohn’s disease (CD). Am J Gastroenterol 2001;96:S303. [Google Scholar]
- 6.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002;359:1541–9. [DOI] [PubMed] [Google Scholar]
- 7.Rutgeerts P , Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology 2004;126:402–13. [DOI] [PubMed] [Google Scholar]
- 8.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 2004;350:876–85. [DOI] [PubMed] [Google Scholar]
- 9.Sands BE, Tremaine WJ, Sandborn WJ, et al. Infliximab in the treatment of severe, steroid-refractory ulcerative colitis: a pilot study. Inflamm Bowel Dis 2001;7:83–8. [DOI] [PubMed] [Google Scholar]
- 10.Probert CS, Hearing SD, Schreiber S, et al. Infliximab in moderately severe glucocorticoid resistant ulcerative colitis: a randomised controlled trial. Gut 2003;52:998–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vermeire S , Noman M, Van Assche G, et al. Autoimmunity associated with anti-tumor necrosis factor alpha treatment in Crohn’s disease: A prospective cohort study. Gastroenterology 2003;125:32–9. [DOI] [PubMed] [Google Scholar]
- 12.Cheifetz A , Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenerol 2003;98:1315–24. [DOI] [PubMed] [Google Scholar]
- 13.Hanauer S , Rutgeerts P, Targan S, et al. Delayed hypersensitivity to infliximab (Remicade) re-infusion after a 2–4 year interval without treatment. Gastroenterology 1999;116:A731. [Google Scholar]
- 14.Mohan N , Edwards ET, Cupps TR, et al. Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheum 2001;44:2862–9. [DOI] [PubMed] [Google Scholar]
- 15.Brown SL, Greene MH, Gershon SK, et al. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum 2002;46:3151–8. [DOI] [PubMed] [Google Scholar]
- 16.Kwon HJ, Cote TR, Cuffe MS, et al. Case reports of heart failure after therapy with a tumor necrosis factor antagonist. Ann Intern Med 2003;138:807–11. [DOI] [PubMed] [Google Scholar]
- 17. Remicade (infliximab) for IV injection. Package Insert. Thompson PDR, Montuale, New Jersey, USA 2002.
- 18.Keane J , Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha- neutralizing agent. N Engl J Med 2001;345:1098–104. [DOI] [PubMed] [Google Scholar]
- 19.Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum 2002;46:2565–70. [DOI] [PubMed] [Google Scholar]
- 20.Colombel JF, Loftus EV jr, Tremaine WJ, et al. The safety profile of infliximab in patients with Crohn’s disease: the Mayo clinic experience in 500 patients. Gastroenterology 2004;126:19–31. [DOI] [PubMed] [Google Scholar]
- 21.Sandborn WJ, Hanauer SB. Infliximab in the treatment of Crohn’s disease: a user’s guide for clinicians. Am J Gastroenterol 2002;97:2962–72. [DOI] [PubMed] [Google Scholar]
- 22.Rutgeerts P , D’Haens G, Targan S, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology 1999;117:761–9. [DOI] [PubMed] [Google Scholar]
- 23. Remicade Infliximab for IV injection. Prescribing information. Thompson PDR, Montuale, New Jersey, USA 1998.
- 24.Baert F , Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med 2003;348:601–8. [DOI] [PubMed] [Google Scholar]
- 25.Farrell RJ, Alsahli M, Jeen YT, et al. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn’s disease: a randomized controlled trial. Gastroenterology 2003;124:917–24. [DOI] [PubMed] [Google Scholar]
- 26.Sandborn WJ, Feagan BG, Hanauer SB, et al. An engineered human antibody to TNF (CDP571) for active Crohn’s disease: a randomized double-blind placebo-controlled trial. Gastroenterology 2001;120:1330–8. [DOI] [PubMed] [Google Scholar]
- 27.Sandborn W , Feagan B, Radford-Smith G, et al. A randomized, placebo-controlled trial of CDP571, a humanized monoclonal antibody to TNF-a, in patients with moderate to severe Crohn’s disease. Gastroenterology 2003;124:A61. [Google Scholar]
- 28.Feagan BG, Sandborn WJ, Baker J, et al. A randomized, double-blind, placebo-controlled, multi-center trial of the engineered human antibody to TNF (CDP571) for steroid sparing and maintenance of remission in patients with steroid-dependent Crohn’s disease. Gastroenterology 2000;118:A655. [Google Scholar]
- 29. Celltech announces results from CDP 571 Phase III studies in Crohn’s disease. Celltech: Internet Press Release, 2002; (http://celltechgroup.com).
- 30.Hanauer S , Present D, Targan SR, et al. CDP571, a humanized monoclonal antibody to TNF-a, is well tolerated in Crohn’s disease patients with previous hypersensitivity to infliximab. Gastroenterology 2003;124:A517. [Google Scholar]
- 31.Schreiber S , Rutgeerts P, Fedorak R, et al. CDP870, a humanized anti-TNF antibody fragment, induces clinical response with remission in patients with active Crohn’s disease (CD). Gastroenterology 2003;124:A61. [Google Scholar]
- 32.Winter T , Wright J, Ghosh S, et al. Intravenous CDP870, a humanized anti-TNF antibody fragment, in patients with active Crohn’s disease—an exploratory study. Gastroenterology 2003;124:A377. [Google Scholar]
- 33.Sandborn WJ, Hanauer SB, Katz S, et al. Etanercept for active Crohn’s disease: A randomized, double-blind, placebo-controlled trial. Gastroenterology 2001;121:1088–94. [DOI] [PubMed] [Google Scholar]
- 34.Rutgeerts P , Lemmens L, Van Assche G, et al. Treatment of active Crohn’s disease with onercept (recombinant human soluble p55 tumour necrosis factor receptor): results of a randomized, open-label, pilot study. Aliment Pharmacol Ther 2003;17:185–92. [DOI] [PubMed] [Google Scholar]
- 35.Sandborn WJ, Hanauer S, Loftus EV, et al. An open-label study of the human anti-TNF monoclonal antibody adalimumab in subjects with prior loss of response or intolerance to infliximab for Crohns disease. Gastroenterology 2004;126:A53–4. [DOI] [PubMed] [Google Scholar]
- 36.Bernstein CN, Sargent M, Gallatin WM. Beta2 integrin/ICAM expression in Crohn’s disease. Clin Immunol Immunopathol 1998;86:147–60. [DOI] [PubMed] [Google Scholar]
- 37.Binion DG, West GA, Volk EE, et al. Acquired increase in leucocyte binding by intestinal microvascular endothelium in inflammatory bowel disease. Lancet 1998;352:1742–6. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh S , Goldin E, Gordon FH, et al. Natalizumab for active Crohn’s disease. N Engl J Med 2003;348:24–32. [DOI] [PubMed] [Google Scholar]
- 39.Hemler ME, Huang C, Takada Y, et al. Characterization of the cell surface heterodimer VLA-4 and related peptides. J Biol Chem 1987;262:11478–85. [PubMed] [Google Scholar]
- 40.Farstad IN, Halstensen TS, Kvale D, et al. Topographic distribution of homing receptors on B and T cells in human gut-associated lymphoid tissue: relation of L-selectin and integrin alpha 4 beta 7 to naive and memory phenotypes. Am J Pathol 1997;150:187–99. [PMC free article] [PubMed] [Google Scholar]
- 41.Gordon FH, Lai CW, Hamilton MI, et al. A randomized placebo-controlled trial of a humanized monoclonal antibody to alpha4 integrin in active Crohn’s disease. Gastroenterology 2001;121:268–74. [DOI] [PubMed] [Google Scholar]
- 42.Rutgeerts P , Colombel J, Enns R, et al. Subanalyses from a phase 3 sudy on the evaluation of natalizumab in active Crohn’s disease tharapy-1 (ENACT-1). Gut 2003;52 (suppl VI) :A239. [Google Scholar]
- 43. Elan and Biogen Idec announce ANTEGREN-natalizumab-phase III maintenance trial in Crohn’s disease met its primary endpoint. Elan and Biogen Idec: Internet Press Release, 2004; (http://www.elan.com).
- 44.Gordon FH, Hamilton MI, Donoghue S, et al. A pilot study of treatment of active ulcerative colitis with natalizumab, a humanized monoclonal antibody to alpha-4 integrin. Aliment Pharmacol Ther 2002;16:699–705. [DOI] [PubMed] [Google Scholar]
- 45.Feagan BG, Greenberg G, Wild G, et al. Efficacy and safety of a humanized a4ß7 antibody in active Crohn’s disease (CD). Gastroenterology 2003;124:A25–6. [Google Scholar]
- 46.Feagan B , Greenberg G, Wild G, et al. A randomized trial of a humanized a4β7 antibody in ulcerative colitis. Gastroenterology 2003;125:606–7. [Google Scholar]
- 47.Yacyshyn BR, Bowen-Yacyshyn MB, et al. A placebo-controlled trial of ICAM-1 antisense oligonucleotide in the treatment of Crohn’s disease. Gastroenterology 1998;114:1133–42. [DOI] [PubMed] [Google Scholar]
- 48.Yacyshyn BR, Chey WY, Goff J, et al. Double blind, placebo controlled trial of the remission inducing and steroid sparing properties of an ICAM-1 antisense oligodeoxynucleotide, alicaforsen (ISIS 2302), in active steroid dependent Crohn’s disease. Gut 2002;51:30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schreiber S , Nikolaus S, Malchow H, et al. Absence of efficacy of subcutaneous antisense ICAM-1 treatment of chronic active Crohn’s disease. Gastroenterology 2001;120:1339–46. [DOI] [PubMed] [Google Scholar]
- 50.Yacyshyn BR, Barish C, Goff J, et al. Dose ranging pharmacokinetic trial of high-dose alicaforsen (intercellular adhesion molecule-1 antisense oligodeoxynucleotide)(ISIS 2302) in active Crohn’s disease. Aliment Pharmacol Ther 2002;16:1761–70. [DOI] [PubMed] [Google Scholar]
- 51. Phase II study of antisense drug ISIS 2302 demonstrates significant and long-lasting improvement of symptoms in patients with ulcerative colitis. Internet Press Release 2001; (http://www.isip.com).
- 52.Miner P , Wedel M, Bane B, et al. An enema formulation of alicaforsen, an antisense inhibitor of intercellular adhesion molecule-1, in the treatment of chronic, unremitting pouchitis. Aliment Pharmacol Ther 2004;19:281–6. [DOI] [PubMed] [Google Scholar]
- 53.Rutgeerts P , Reinisch W, Colombel JF, et al. Preliminary results of a phase I/II study of Huzaf, an anti-INF-gamma monoclonal antibody, in patients with moderate to severe active Crohn’ disease. Gastroenterology 2002;122:A61. [Google Scholar]
- 54. Protein Design Labs reports progress on two humanized antibodies at International Organization of Inflammatory Bowel Disease. Internet Press Release, 2004; (http://www.pdl.com).
- 55.Mannon P , Fuss I, Strober W. Anti-interleukin-12 treats active Crohn’s disease. Gastroenterology 2004;126:A22–3. [Google Scholar]
- 56.van Deventer SJ, Elson CO, Fedorak RN. Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn’s disease. Crohn’s Disease Study Group. Gastroenterology 1997;113:383–9. [DOI] [PubMed] [Google Scholar]
- 57.Fedorak RN, Gangl A, Elson CO, et al. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn’s disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology 2000;119:1473–82. [DOI] [PubMed] [Google Scholar]
- 58.Colombel JF, Rutgeerts P, Malchow H, et al. Interleukin 10 (Tenovil) in the prevention of postoperative recurrence of Crohn’s disease. Gut 2001;49:42–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schreiber S , Fedorak RN, Nielsen OH, et al. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn’s disease. Crohn’s Disease IL-10 Cooperative Study Group. Gastroenterology 2000;119:1461–72. [DOI] [PubMed] [Google Scholar]
- 60.Fedorak R , Nielsen O, Williams N, et al. Human recombinant interleukin-10 is safe and well tolerated but does not induce remission in steroid dependent Crohn’s disease. Gastroenterology 2001;120:A127. [Google Scholar]
- 61.Schreiber S , Fedorak R, Wild G, et al. Safety and tolerence of rHuIL-10 treatment in patients with mild/moderate active ulcerative colitis. Gastroenterology 1998;114:A1080–1. [Google Scholar]
- 62.Sands BE, Bank S, Sninsky CA, et al. Preliminary evaluation of safety and activity of recombinant human interleukin 11 in patients with active Crohn’s disease. Gastroenterology 1999;117:58–64. [DOI] [PubMed] [Google Scholar]
- 63.Sands BE, Winston BD, Salzberg B, et al. Randomized, controlled trial of recombinant human interleukin-11 in patients with active Crohn’s disease. Aliment Pharmacol Ther 2002;16:399–406. [DOI] [PubMed] [Google Scholar]
- 64.Cotreau MM, Stonis L, Schwertschlag US. A phase 1, randomized, double-blind, placebo-controlled, dose-escalating, safety, tolerability, pharmacokinetic, and pharmacodynamic study of oral recombinant human interleukin eleven (O-rhIL-11) in normal healthy subjects. Gastroenterology 2003;124:A377. [Google Scholar]
- 65.Hommes D , Van Den Blink B, Plasse T, et al. Inhibition of stress-activated MAP kinases induces clinical improvement in moderate to severe Crohn’s disease. Gastroenterology 2002;122:7–14. [DOI] [PubMed] [Google Scholar]
- 66.Pargellis C , Tong L, Churchill L, et al. Inhibition of p38 MAP kinase by utilizing a novel allosteric binding site. Nat Struct Biol 2002;9:268–72. [DOI] [PubMed] [Google Scholar]
- 67. Preliminary results of Sangstat’s phase 2 studies of RDP58 show peak response of 77% and a 71% remission rate in ulcerative colitis patients. Additional investigation needed to determine efficacy in Crohn’s disease. Internet Press Release 2003; (http://www.sangstat.com).
- 68.Travis SPL, RDP Investigators Study Group, Yap ML, et al. RDP-58: novel and effective therapy for ulcerative colitis. Results of parallel, prospective, placebo-controlled trials. Am J Gastroenterol 2003;98:S239 (abstract 721). [Google Scholar]
- 69.Ito H , Takazoe M, Fukuda Y, et al. A pilot randomized trial of a human anti-interleukin-6 monoclonal antibody in active Crohn’s disease. Gastroenterology 2004;126:989–96. [DOI] [PubMed] [Google Scholar]
- 70.Katznelson L , Fairfield WP, Zeizafoun N, et al. Effects of growth hormone secretion on body composition in patients with Crohn’s disease. J Clin Endocrinol Metab 2003;88:5468–72. [DOI] [PubMed] [Google Scholar]
- 71.Slonim AE, Bulone L, Damore MB, et al. A preliminary study of growth hormone therapy for Crohn’s disease. N Engl J Med 2000;342:1633–7. [DOI] [PubMed] [Google Scholar]
- 72.Korzenik JR, Dieckgraefe BK. Is Crohn’s disease an immunodeficiency? A hypothesis suggesting possible early events in the pathogenesis of Crohn’s disease. Dig Dis Sci 2000;45:1121–9. [DOI] [PubMed] [Google Scholar]
- 73.Dieckgraefe BK, Korzenik JR, Husain A, et al. Association of glycogen storage disease 1b and Crohn disease: results of a North American survey. Eur J Pediatr 2002;161 (suppl 1) :S88–92. [DOI] [PubMed] [Google Scholar]
- 74.Dieckgraefe BK, Korzenik JR. Treatment of active Crohn’s disease with recombinant human granulocyte- macrophage colony-stimulating factor. Lancet 2002;360:1478–80. [DOI] [PubMed] [Google Scholar]
- 75.Korzenik J , Dieckgraefe B. Immunostimulation in Crohn’s disease: results of a pilot study of G-CSF (R-Methug-CSF) in mucosal and fistulizing Crohn’s disease. Gastroenterology 2000;118:A874. [Google Scholar]
- 76. Berlex Laboratories announces results from randomized, placebo-controlled study of Leukine(R) for Crohn’s disease. Internet Press Release, 2003; (http://www.berlex.com).
- 77.Van Assche G , Dalle I, Noman M, et al. A pilot study on the use of the humanized anti-interleukin-2 receptor antibody daclizumab in active ulcerative colitis. Am J Gastroenterol 2003;98:369–76. [DOI] [PubMed] [Google Scholar]
- 78.Creed TJ, Norman MR, Probert CS, et al. Basiliximab (anti-CD25) in combination with steroids may be an effective new treatment for steroid-resistant ulcerative colitis. Aliment Pharmacol Ther 2003;18:65–75. [DOI] [PubMed] [Google Scholar]
- 79.Plevy S , Salzberg B, van Assche G, et al. A humanized anti-CD3 monoclonal antibody, visilizumab, for treatment of severe steroid-refractory ulcerative colitis: Results of a phase I study. Gastroenterology 2004;126:A75. [DOI] [PubMed] [Google Scholar]
- 80.Sinha A , Nightingale J, West KP, et al. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med 2003;349:350–7. [DOI] [PubMed] [Google Scholar]
- 81.Sandborn WJ, Sands BE, Wolf DC, et al. Repifermin (keratinocyte growth factor-2) for the treatment of active ulcerative colitis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Aliment Pharmacol Ther 2003;17:1355–64. [DOI] [PubMed] [Google Scholar]