Abstract
Background: Patients with irritable bowel syndrome (IBS) often feel they have some form of dietary intolerance and frequently try exclusion diets. Tests attempting to predict food sensitivity in IBS have been disappointing but none has utilised IgG antibodies.
Aims: To assess the therapeutic potential of dietary elimination based on the presence of IgG antibodies to food.
Patients: A total of 150 outpatients with IBS were randomised to receive, for three months, either a diet excluding all foods to which they had raised IgG antibodies (enzyme linked immunosorbant assay test) or a sham diet excluding the same number of foods but not those to which they had antibodies.
Methods: Primary outcome measures were change in IBS symptom severity and global rating scores. Non-colonic symptomatology, quality of life, and anxiety/depression were secondary outcomes. Intention to treat analysis was undertaken using a generalised linear model.
Results: After 12 weeks, the true diet resulted in a 10% greater reduction in symptom score than the sham diet (mean difference 39 (95% confidence intervals (CI) 5–72); p = 0.024) with this value increasing to 26% in fully compliant patients (difference 98 (95% CI 52–144); p<0.001). Global rating also significantly improved in the true diet group as a whole (p = 0.048, NNT = 9) and even more in compliant patients (p = 0.006, NNT = 2.5). All other outcomes showed trends favouring the true diet. Relaxing the diet led to a 24% greater deterioration in symptoms in those on the true diet (difference 52 (95% CI 18–88); p = 0.003).
Conclusion: Food elimination based on IgG antibodies may be effective in reducing IBS symptoms and is worthy of further biomedical research.
Keywords: irritable bowel syndrome, IgG, food sensitivity, food elimination
Irritable bowel syndrome (IBS) is a common disorder which causes abdominal pain, abdominal distension, and bowel dysfunction, characterised by loose bowels, constipation, or a fluctuation between these two extremes.1 This condition significantly impairs quality of life and places a large burden on health care resources.2 Treatment of IBS is largely based on the use of antispasmodics, antidepressants, and medications that modify bowel habit, depending on whether constipation or diarrhoea is the predominant problem.1 The notorious inadequacies of current drug therapy lead to much patient dissatisfaction and a tendency for patients to seek a variety of alternative remedies, especially of a dietary nature.
IBS is likely to be a multifactorial condition involving a number of different mechanisms although the prominence of any particular factor may vary from patient to patient.1,3 However, patients often strongly believe that dietary intolerance significantly contributes to their symptomatology and some sufferers seem to benefit from eliminating certain foods from their diet. Detection of food intolerance is often difficult due to its uncertain aetiology, non-specific symptomatology, and relative inaccessibility of the affected organ. Thus most previous studies have relied on the use of exclusion diets, which are extremely labour intensive and time consuming.4,5 Attempts to “test” for food intolerance in IBS have largely focused on “classic” food allergy based on the presence of IgE mediated antibody responses, although it appears that these “immediate type” reactions are probably quite rare in this condition.6–10 It is therefore possible that adverse reactions to food in patients with IBS might be due to some other form of immunological mechanism, rather than dietary allergy. Such reactions could be mediated by IgG antibodies, which characteristically give a more delayed response following exposure to a particular antigen11 and have been implicated in some cases of food hypersensitivity.12–14 However, this mechanism is controversial and is considered by some to be physiological15–17 especially as IgG food antibodies can be present in apparently healthy individuals.18–20 It has previously been suggested that IgG food antibodies may have a role in IBS21 and it was therefore the purpose of this study to formally evaluate, in a randomised controlled trial, the therapeutic potential of an elimination diet based on the presence of IgG antibodies to food in patients with IBS.
PATIENTS AND METHODS
Patients
All patients with uncomplicated IBS (all bowel habit subtypes) attending the Gastroenterology Department at the University Hospital of South Manchester were considered eligible for the study, and those aged between 18 and 75 years, who satisfied the Rome II criteria,22 were invited to participate. Tertiary care patients were excluded from the study. All patients had normal haematology, biochemistry, and endoscopic examination when indicated. Coeliac disease was excluded using the tissue transglutaminase test and a hydrogen breath test was used for excluding lactose intolerance. Patients were also excluded from participating in the study if they had any significant coexisting disease or a history of gastrointestinal surgery, excluding appendicectomy, cholecystectomy, and hiatus hernia repair. The study was approved by the local ethics committee and all patients provided written informed consent.
Methods
The study used a double blind, randomised, controlled, parallel design in which patients were randomised to either a “true” diet or a “sham” diet control group. At screening, blood was taken and sent, with only a numerical identifier, to YorkTest Laboratories Ltd (York, UK) where an enzyme linked immunosorbant assay (ELISA) test was performed to detect the presence of IgG antibodies specific to a panel of 29 different food antigens. This test has been described in detail elsewhere23 and involves specimens being diluted 1/50, 1/150, and 1/450 with each dilution applied to an allergen panel. Each test was calibrated using 0 arbitrary unit (AU) and 25 AU standards prepared from a serum with a high IgG titre to a cow’s milk allergen extract. A positive control serum at 45 AU was applied to each test. The test results were obtained from the 1/150 dilution of the specimen. Where a high specimen background was observed, the test results were obtained from the 1/450 dilution. The threshold for a positive (reactive) result was selected as three times the background signal obtained by the same sample against a no food allergen coated control well equivalent to 3 AU. Test results were scored as positive or negative only, relative to this cut off.
Staff based at the YorkTest Laboratories produced a true and sham diet sheet for each patient. The sham diet eliminated the same number of foods to which a patient exhibited IgG antibodies but not those particular foods. The goal was to try and include in the sham diet an equally difficult to eliminate staple food for every staple food in the true diet. Thus cow’s milk was (generally) replaced with potato, wheat with rice, and yeast with whole egg, where this was possible. Nut reactivities were replaced with other nuts in the sham diet, and legumes with other legumes, but this was not systematised.
The true and sham diet sheets for each patient were sent to the University of York, again with only a number for identification. Patients were allocated to one of the two diet sheets based on a randomisation schedule developed using a random computer number generator. Thus patients would receive either an elimination diet based on their true sensitivity results (true diet) or a sham diet. All patients and clinical staff in the Gastroenterology Research Department and YorkTest Laboratory were blinded to the group assignment of all patients for the duration of the study.
Patients were given their allocated diet sheet by staff at the Gastroenterology Research Department and asked to eliminate the indicated foods from their diet for a period of 12 weeks. They also received a booklet with advice on eliminating the different foods and the telephone contact details of a free nutritional advisor whom they were able to contact for further advice if necessary.
Symptoms were assessed using a questionnaire scoring system validated for use in IBS, including the IBS symptom severity score (range 0–500).24 This is a system for scoring pain, distension, bowel dysfunction, and general well being, with mild, moderate, and severe cases indicated by scores of 75–175, 175–300, and >300, respectively. A reduction in score of 50 or over is regarded as a clinically significant improvement.24 Non-colonic symptomatology,25 such as lethargy, backache, nausea, and urinary symptoms, was assessed and scored using visual analogue scales (range 0–500). Quality of life (QOL) was measured using an instrument proven to be sensitive to change in IBS (range 0–500).26–28 Anxiety and depression were evaluated using the hospital anxiety and depression scale (HAD).29 This instrument scores anxiety and depression up to a maximum score of 21 for each parameter, with a score above 9 indicating significant psychopathology. Data on these measures were recorded at baseline and after 4, 8, and 12 weeks of the dietary intervention period. In addition, at 4, 8, and 12 weeks, patients were asked to give a global rating of their IBS using the question, “Compared with your IBS before you started the food elimination diet, are you now: terrible, worse, slightly worse, no change, slightly better, better, or excellent?” The atopic status of all patients entering the study was also assessed.
During the treatment phase, patients were allowed to take concomitant medication provided it had been constant for six months prior to the start of the study. They were encouraged not to alter medication use during the course of the trial but any changes were recorded. Any patient withdrawing from the study was encouraged to complete a final symptom questionnaire at week 12 and their reasons for withdrawal were recorded. At the end of 12 weeks, patients were asked to resume consumption of the foods they had been advised to eliminate in order to assess the effect of their reintroduction. Patients were then reassessed after four weeks using the same measures and the result compared with their scores at the end of the elimination phase.
Data analysis
Questionnaires were scored by an assessor blinded to the randomisation. The primary outcome measures were changes in IBS symptom severity score and global impact score at 12 weeks. Changes in non-colonic symptoms, QOL, and HAD scores were regarded as secondary outcome measures. Two sample t tests were used to establish whether there was an overall difference in the change in continuous outcome measures between the two groups of patients. Patients were analysed according to the group to which they were randomised, independent of their adherence to the diet. The global impact score, an ordered categorical variable, was analysed using a Wilcoxon Mann-Whitney test to compare the numbers in the active and sham groups showing significant improvement (“better” or “excellent”), no significant change (“slightly worse”, “no change”, or “slightly better”), and significant deterioration (“worse” or “terrible”). The number needed to treat (NNT) was calculated from the global impact score by calculating the reciprocal of the difference in probability of a significant improvement between the treatment and control groups. General linear modelling in SPSS was used to explore whether there was a relationship between the change in symptoms from baseline and treatment group, patient characteristics (for example, IBS subtype, history of atopy, number of foods to which sensitive, and concomitant medication) and adherence to the diet.30
Sample size calculation
It was estimated that approximately 40% of the placebo arm would report a significant improvement in symptoms. It was calculated that a sample size of 55 patients would be required in each group to detect, with 90% power, a difference of 30% points in the proportion reporting such an improvement (that is, 70% in the treatment arm) as statistically significant at the 5% level. Assuming a 20% dropout rate, a minimum of 138 patients would need to be entered into the trial. Thus we aimed to recruit a total of 150 patients into the study.
RESULTS
Recruitment of patients and their flow through each stage of the study is illustrated in fig 1 ▶, as recommended by the CONSORT statement.31 In summary, between January 2001 and July 2002, 176 patients were eligible for the study, of which 26 (15%) were excluded from participation, leaving 150 patients who were all found to be sensitive to at least one food. Seventy five of these were randomised to receive an elimination diet based on their true food sensitivity results and 75 patients to a sham diet. Data from 131 (87%) patients who gave 12 week data were available for the intention to treat analysis: 65 and 66 patients from the true and sham groups, respectively.
Figure 1.
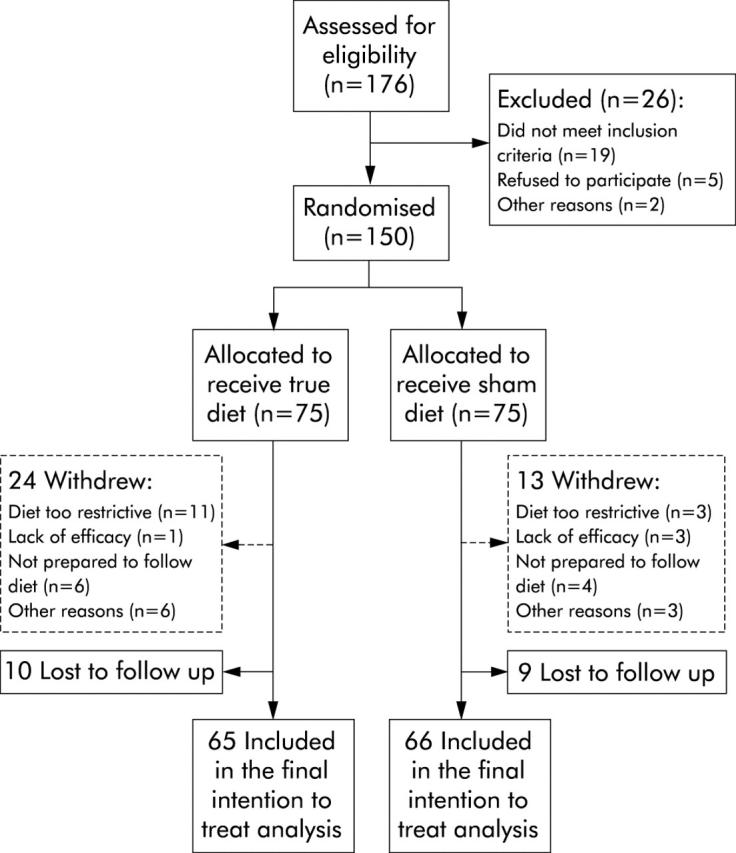
Study flow diagram.
Patient characteristics
The patients were typical of those with IBS in secondary care practice, the majority being women. Patients, on average, had experienced symptoms of IBS for over a decade and were found to be sensitive to approximately 6–7 foods (range 1–19). Baseline demographic and clinical characteristics of the two groups, including the use of concomitant medication, were found to be similar with the exception of the IBS symptom severity score which was slightly higher in the treatment group (table 1 ▶). Thirty per cent of patients were found to be atopic.
Table 1.
Baseline characteristics of the patients
| Group | True diet (n = 75) | Sham diet (n = 75) |
| Age (y) (range, SD) | 44 (17–72; 12.9) | 44 (19–74; 15.2) |
| No of males (%) | 7 (9.3%) | 13 (17.3%) |
| No of foods to which sensitive | 6.65 (3.66) | 6.63 (4.1) |
| Symptom duration (y) | 11.5 (9.9) | 10.1 (7.5) |
| IBS symptom severity score | 331.9 (70.8) | 309.0 (78.5) |
| Non-colonic features score | 459.1 (160.7) | 452.6 (170.1) |
| Quality of life score | 640.1 (252.6) | 639.3 (222.3) |
| HAD anxiety score | 9.5 (4.6) | 9.5 (4.5) |
| HAD depression score | 5.3 (3.4) | 6.0 (3.6) |
| No of diarrhoea predominant patients (%) | 37 (52.1%) | 41 (56.9%) |
| No of constipation predominant patients (%) | 19 (26.8%) | 16 (22.2%) |
| No of alternating predominant patients (%) | 15 (21.1%) | 15 (20.8%) |
Results are expressed as mean (SD).
HAD, hospital anxiety and depression scale.
The frequency of foods excluded from the diet is shown in table 2 ▶. Adherence was lower in those on the true diet although no specific adverse events were recorded in either group. Twenty four patients withdrew from the study in the true diet group (mainly because of difficulty in following the diet) and 13 from the sham diet group (for a variety of reasons). However, 12 week data were obtained from 14 of those who withdrew in the true diet group and four in the sham diet group. There were no significant differences between baseline characteristics of the 19 who were lost to follow up and those for whom 12 week data were obtained.
Table 2.
Frequency of foods excluded from the diet (% of patients)
| Food | Treatment group | Sham group |
| Barley | 26.7 | 9.3 |
| Corn | 22.7 | 14.7 |
| Rice | 8 | 54.7 |
| Rye | 8 | 25.3 |
| Wheat | 49.3 | 8 |
| Milk | 84.3 | 1.3 |
| Beef | 24 | 9.3 |
| Chicken | 21.3 | 13.3 |
| Pork | 5.3 | 36 |
| Cabbage | 12 | 24 |
| Celery | 5.3 | 21.3 |
| Haricot bean | 17.3 | 14.7 |
| Pea | 38.6 | 1.3 |
| Potato | 9.3 | 61.3 |
| Soy bean | 22.7 | 10.7 |
| Tomato | 4 | 44 |
| Apple | 1.3 | 33 |
| Orange | 6.7 | 29.3 |
| Strawberry | 0 | 20 |
| Almond | 28 | 12 |
| Brazil nut | 22.7 | 17.3 |
| Cashew nut | 49.3 | 8 |
| Peanut | 10.7 | 20 |
| Walnut | 2.7 | 29.3 |
| Cocoa bean | 1.3 | 21.3 |
| Shellfish | 21.3 | 10.7 |
| Fish mix | 17.3 | 28 |
| Whole egg | 57.3 | 26.7 |
| Yeast | 86.7 | 0 |
Primary outcomes
IBS symptom severity
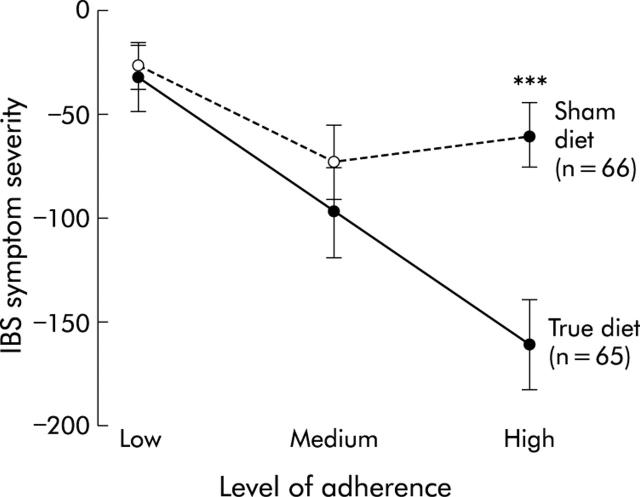
Patients in the true diet group experienced a 10% greater reduction in symptom severity than those allocated to the sham diet, with change in scores of 100 and 61.5, respectively (mean difference 39 (95% confidence interval (CI) 5.2, 72.3); p = 0.024): a standardised effect size of 0.52 (see fig 3A ▶). There were no differences in the response to the diet in terms of age, sex, IBS bowel habit subtype, or IBS duration. In addition, there was no difference in response to the diet between atopic and non-atopic patients. There was however a statistically significant interaction between treatment group and both adherence to the diet and number of foods to which patients were sensitive. For patients sensitive to the average number of foods who fully adhered to their allocated diet, a 26% difference in reduction in symptom severity score was observed in favour of the true diet (a difference in score of 98 (95% CI 52, 144), p<0.001: a standardised effect size of 1.3). This benefit increased by a further 39 points (12%) (95% CI 7, 70; p = 0.016) for each food to which they were sensitive over and above the average number. These results were not materially altered by carrying out an ANCOVA analysis (in which the final score is the dependent variable and the baseline score is included as a covariate) instead of modelling change in scores.30 The interaction between treatment group and adherence is demonstrated in fig 2 ▶ which shows a greater reduction in symptoms with full adherence in the true diet but not in the sham diet group. Figure 3A and 3B ▶ ▶ show the average change in symptom severity score over 12 weeks for the group as a whole and for those who fully adhered, respectively. This reveals that most improvements in symptoms are fully achieved within two months.
Figure 3.
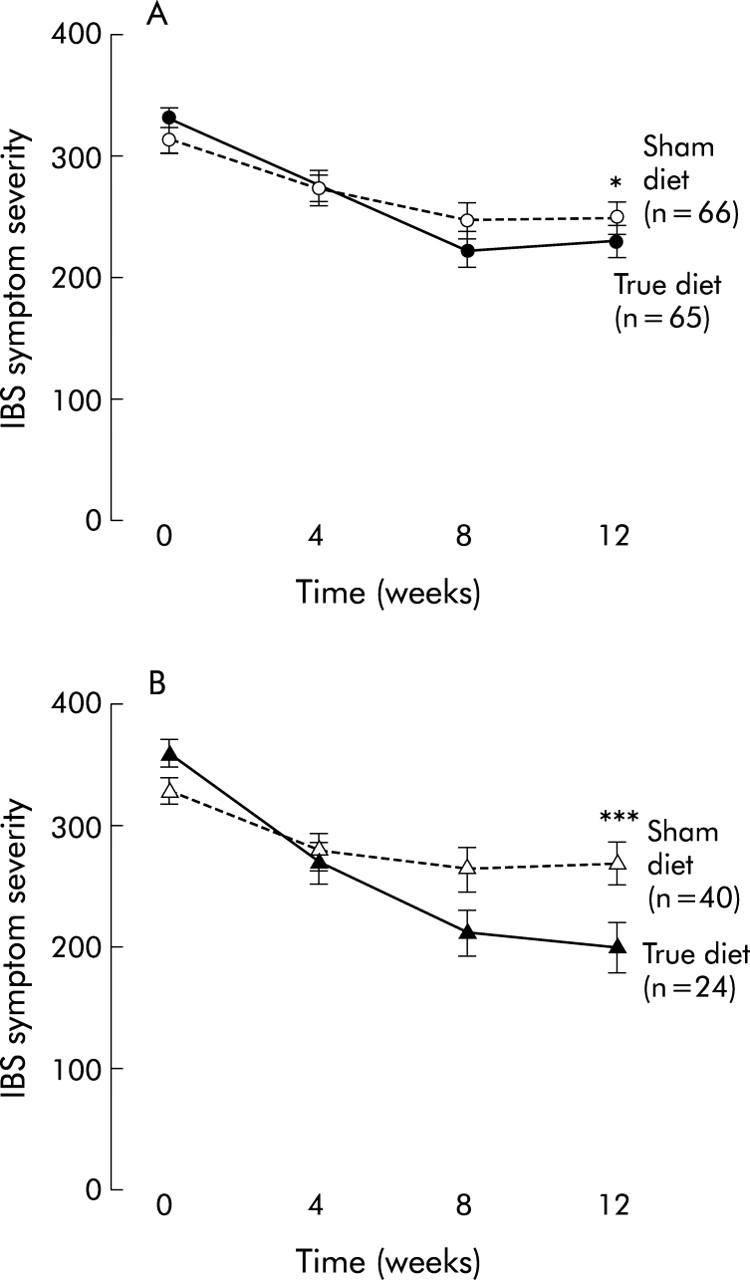
(A) Average symptom severity scores over time for the group as a whole. Difference in mean change from baseline at 12 weeks: true versus sham 39 (95% confidence interval 5, 72); *p = 0.024. (B) Average symptom severity scores over time for the full adherence group. Difference in mean change from baseline at 12 weeks: true versus sham 98 (95% confidence interval 52, 144); ***p<0.001.
Figure 2.
Mean change in symptom severity scores at 12 weeks according to degree of adherence. Difference between the groups with high adherence: 101 (95% confidence interval 54, 147); ***p<0.001.
Global impact score
The reported global rating of change by treatment group is shown in table 3 ▶. The difference in mean ranking (70.9 v 60.3) was statistically significant (p = 0.048). When this was repeated including only patients who fully adhered to their diets (table 3 ▶), a greater percentage difference favouring the true diet was found (p = 0.001). The NNT was 9 in the group as a whole and 2.5 in patients fully adherent to the diet.
Table 3.
Global impact score at 12 weeks
| Treatment group | |||
| True diet (n (%)) | Sham diet (n (%)) | ||
| All patients | |||
| Significantly worse | 3 (4.7) | 8 (12.1) | |
| No significant change | 44 (67.2) | 47 (71.2) | |
| Significantly improved | 18 (28.1) | 11 (16.7) | |
| Total | 65 | 66 | NNT = 9 |
| Patients fully adhering to the diet | |||
| Significantly worse | 1 (4.2) | 5 (12.5) | |
| No significant change | 10 (41.7) | 29 (72.5) | |
| Significantly improved | 13 (54.1) | 6 (15) | |
| Total | 24 | 40 | NNT = 2.5 |
Secondary outcome measures
As can be seen from fig 4A and 4B ▶ ▶, all data show changes favouring the true diet group and are consistent with the results for the primary outcomes. These trends were further strengthened after adjustment for adherence and number of food sensitivities but only reached statistical significance for non-colonic symptomatology (p = 0.05). There were no significant changes in medication use during the course of the trial.
Figure 4.
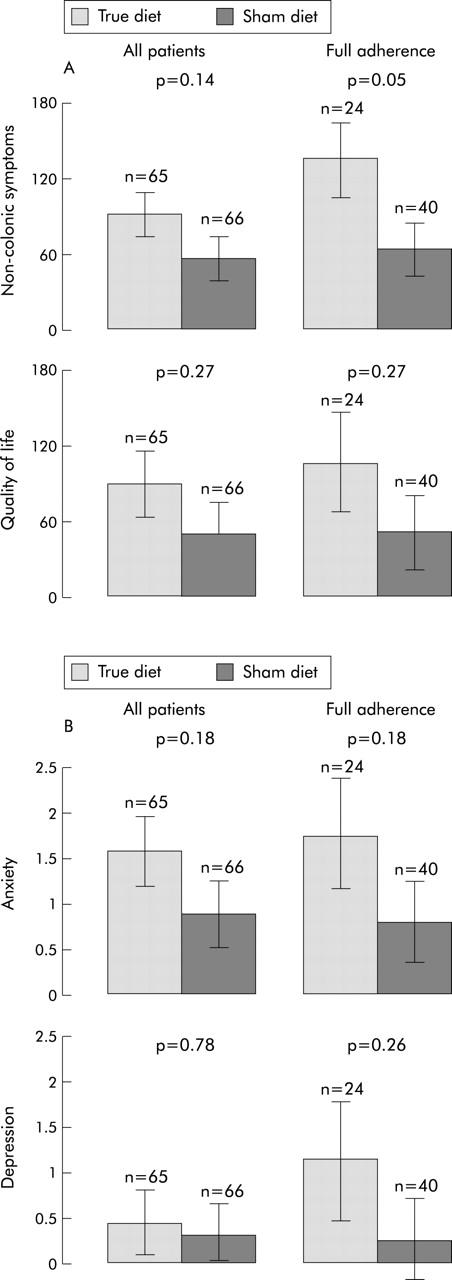
(A) Mean change in the secondary outcome measures of non-colonic symptoms and quality of life for the group as a whole and the full adherence group. (B) Mean change in the secondary outcome measures of anxiety and depression for the group as a whole and the full adherence group.
Reintroduction of eliminated foods
Of the 131 patients who gave 12 week data, 93 (41 in the true and 52 in the sham diet groups) agreed to attempt reintroduction of foods they had been asked to eliminate and provided further follow up data on the primary outcomes measures. Of these, 62% reported full adherence and 37% moderate adherence to the previous elimination diet. Mean IBS symptom severity score increased (that is, worsening of symptoms) by 83.3 in the true group and by 31 in the sham group, a statistically significant difference of 52 (24%) (95% CI 18, 86; p = 0.003). The change in global score following reintroduction of foods is shown in table 4 ▶. This indicates a reversal of the pattern observed during the active treatment phase, with more patients in the true diet group showing worsening of health compared with the sham diet group (p = 0.047).
Table 4.
Global rating following reintroduction of foods relative to the end of the elimination phase
| Treatment group | ||
| True diet group (n (%)) | Sham diet group (n (%)) | |
| Significantly worse | 17 (41.5) | 13 (25) |
| No significant change | 23 (56.1) | 35 (67.3) |
| Significantly improved | 1 (2.4) | 4 (7.7) |
| Total | 41 (100) | 52 (100) |
DISCUSSION
A clinically significant improvement in IBS symptomatology was observed in patients eliminating foods to which they were found to exhibit sensitivity, as identified by an ELISA test for the presence of IgG antibodies to these foods. The number needed to treat of 9 for the group as a whole and 2.5 for patients closely adhering to the diet are both considerably better than the value of 17 achieved after three months of treatment with tegaserod,32 a drug that has been recently licensed in the USA for use in IBS. IBS symptom severity and global rating scores were chosen as primary outcome measures in this study as they represented the most direct measure of clinical improvement in this condition based on patient self assessment. Rather than using the traditional method of classifying global improvement as any value exceeding adequate relief of symptoms, we used a much stricter definition requiring patients to report symptoms as being either “better” or “excellent” compared with pretreatment levels. Despite this, the diet still achieved a significant improvement. However, as might be expected, the placebo response using this end point was somewhat lower than that usually reported in IBS treatment trials which have used less demanding criteria. The observation that patients on the sham diet also improved, although to a lesser extent, emphasises the importance of conducting double blind randomised controlled trials of such non-drug interventions in order to avoid overestimating their potential.
Most patients with IBS have attempted at least some form of dietary modification, which in some cases can be very extreme. Conflicting results have been reported using exclusion diets4,5,33–36 and this approach also suffers from the limitation that it has to be empirical. Thus potentially offending foods can only be identified after their elimination and subsequent reintroduction. This time consuming process would be much reduced if the offending foods could be identified beforehand. Attempts to do this using IgE antibodies have been disappointing8–10 but the results of this study suggest that measuring IgG antibodies may be much more rewarding. The response to the IgG based diet in our trial did not correlate with atopic status, the prevalence of which was found to be no greater than that occurring in the general population.37
The observation that adherence to the diet is critical in determining a good outcome in the “true” diet group but not the “sham” group is indicative of the fact that the diet is an “active treatment” which if not adhered to, does not seem to have an effect. This notion is further supported by the observation that a significantly greater deterioration was observed in subjects in the true diet group compared with those in the sham group when they reintroduced eliminated foods at the end of the diet phase of the trial. Furthermore, the improvement of 98 in the symptom severity score in those fully adherent in the true diet group is well above the value of 50, which is regarded as being of clinical significance both in validation studies24 and clinical practice.26–28 It was interesting to note that patients exhibiting a greater number of sensitivities, as determined by the IgG test, experienced a greater symptom reduction if they adhered to the true but not the sham diet.
There is currently considerable interest in the concept that at least in some patients, IBS may have an inflammatory component.38–42 Most of the work in this area has centred on post dysenteric IBS, with gut pathogens being viewed as the initiators of this process which can be identified by subtle changes on histology.38 However, if, as indicated in this study, IgG antibodies to food are important in the pathogenesis of IBS in some patients, they too may be of relevance. Not all patients exhibiting histological features consistent with post dysenteric IBS give a history of a previous dysenteric illness. This is usually assumed to be due to the fact that this has been forgotten by the patient but our results may suggest an alternative mechanism for immune activation and inflammation without the need for prior infection.
It is now well recognised that up to 70% of patients with IBS have evidence of hypersensitivity of the rectum,43 which probably extends to involve most of the gut in many individuals.44 It is possible that this hypersensitivity renders patients more reactive to a low grade inflammatory process which would not necessarily cause symptoms in a normal individual. This would explain why excluding foods to which patients have IgG antibodies might be particularly beneficial in IBS despite the fact that these antibodies may also be present in the general population. Indeed, if this mechanism is particularly important in IBS, it might be anticipated that IgG food antibodies would be relatively common in this condition, as was the case in our study.
Many patients with IBS would prefer a dietary solution to their problem rather than having to take medication, and the economic benefits of this approach to health services are obvious. It is well known that patients expend large sums of money on a variety of unsubstantiated tests in a vain attempt to identify dietary intolerances. The results of this study suggest that assay of IgG antibodies to food may have a role in helping patients identify candidate foods for elimination and is an approach that is worthy of further biomedical and clinical research.
Abbreviations
IBS, irritable bowel syndrome
ELISA, enzyme linked immunosorbant assay
AU, arbitrary unit
HAD, hospital anxiety and depression scale
QOL, quality of life
NNT, number needed to treat
REFERENCES
- 1.Drossman DA, Camilleri M, Mayer EA, et al. American Gastroenterological Association Technical Review on Irritable Bowel Syndrome. Gastroenterology 2002;123:2108–31. [DOI] [PubMed] [Google Scholar]
- 2.Lea R , Whorwell PJ. Quality of life in irritable bowel syndrome. Pharmacoeconomics 2001;19:643–53. [DOI] [PubMed] [Google Scholar]
- 3.Talley NJ, Spiller R. Irritable bowel syndrome: a little understood organic bowel disease? Lancet 2002;360:555–64. [DOI] [PubMed] [Google Scholar]
- 4.Jones VA, McLaughlan P, Shorthouse M, et al. Food intolerance: a major factor in pathogenesis of irritable bowel syndrome. Lancet 1982;2:1115–17. [DOI] [PubMed] [Google Scholar]
- 5.Nanda R , James R, Smith H, et al. Food intolerance and the irritable bowel syndrome. Gut 1989;30:1099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zwetchkenbaum J , Burakoff R .The irritable bowel syndrome and food hypersensitivity. Ann Allerg 1988;61:47–9. [PubMed] [Google Scholar]
- 7.Zar S , Kumar D, Benson M. J. Review article: food hypersensitivity and irritable bowel syndrome, Aliment Pharm Ther 2001;15:439–43. [DOI] [PubMed] [Google Scholar]
- 8.Petitpierre M , Gumowski P, Girard JP. Irritable bowel syndrome and hypersensitivity to food. Ann Allergy 1985;54:538–40. [PubMed] [Google Scholar]
- 9.Barau E , Dupont C. Modifications of intestinal permeability during food provocation procedures in pediatric irritable bowel syndrome. J Pediatr Gastroenterol Nutr 1990;11:72–7. [DOI] [PubMed] [Google Scholar]
- 10.Roussos A , Koursarakos P, Patsopoulos D, et al. Increased prevalence of irritable bowel syndrome in patients with bronchial asthma. Respir Med 2003;97:75–9. [DOI] [PubMed] [Google Scholar]
- 11.Crowe SE, Perdue MH. Gastrointestinal food hypersensitivity: basic mechanisms of pathophysiology. Gastroenterol 1992;103:1075–95. [DOI] [PubMed] [Google Scholar]
- 12.el Rafei A , Peters SM, Harris N, et al. Diagnostic value of IgG4 measurements in patients with food allergy. Ann Allergy 1989;62:94–9. [PubMed] [Google Scholar]
- 13.Host A , Husby S, Gjesing B, et al. Prospective estimation of IgG, IgG subclass and IgE antibodies to dietary proteins in infants with cow’s milk allergy. Levels of antibodies to whole milk protein, BLG and ovalbumin in relation to repeated milk challenge and clinical course of cow’s milk allergy. Allergy 1992;47:218–29. [DOI] [PubMed] [Google Scholar]
- 14.Awazuhara H , Kawai H, Maruchi N. Major allergens in soybean and clinical significance of IgG4 antibodies investigated by IgE and IgG4 immunoblotting with sera from soybean-sensitive patients. Clin Exp Allergy 1997;27:325–32. [PubMed] [Google Scholar]
- 15.Barnes RMR, Johnson PM, Harvey MM, et al. Human serum antibodies reactive with dietary proteins: IgG subclass distribution. Int Arch Allergy Appl Immunol 1988;87:184–8. [DOI] [PubMed] [Google Scholar]
- 16.Lessof MH, Kemeny DM, Price JF. IgG antibodies to food in health and disease. Allergy Proc 1991;12:305–7. [DOI] [PubMed] [Google Scholar]
- 17.Husby S , Mestecky J, Moldoveanu Z, et al. Oral tolerance in humans. T cell but not B cell tolerance after antigen feeding. J Immunol 1994;152:4663–70. [PubMed] [Google Scholar]
- 18.Haddad ZH, Vetter M, Friedmann J, et al. Detection and kinetics of antigen-specific IgE and IgG immune complexes in food allergy. Ann Allergy 1983;51:255. [PubMed] [Google Scholar]
- 19.Husby S , Oxelius VA, Teisner B, et al. Humoral immunity to dietary antigens in healthy adults. Occurrence, isotype and IgG subclass distribution of serum antibodies to protein antigens. Int Arch Allergy Appl Immunol 1985;77:416–22. [DOI] [PubMed] [Google Scholar]
- 20.Kruszewski J , Raczka A, Klos M, et al. High serum levels of allergen specific IgG-4 (asIgG-4) for common food allergens in healthy blood donors. Arch Immunol Ther Exp 1994;42:259–61. [PubMed] [Google Scholar]
- 21.Finn R , Smith MA, Youngs GR, et al. Immunological hypersensitivity to environmental antigens in the irritable bowel syndrome. Br J Clin Pract 1987;41:1041–3. [PubMed] [Google Scholar]
- 22.Drossman DA, Corazziari E, Talley NJ, et al. Rome II: a multinational consensus document on functional gastrointestinal disorders. Gut 1999;45:1–81.10369691 [Google Scholar]
- 23.Foster AP, Knowles TG, Hotston Moore A, et al. Serum IgE and IgG responses to food antigens in normal and atopic dogs, and dogs with gastrointestinal disease. Vet Immunol Immunopathol 2003;92:113–24. [DOI] [PubMed] [Google Scholar]
- 24.Francis CY, Morris J, Whorwell PJ. The irritable bowel scoring system: A simple method of monitoring IBS and its progress. Aliment Pharmacol Therap 1997;11:395–402. [DOI] [PubMed] [Google Scholar]
- 25.Whorwell PJ, McCallum H, Creed FH, et al. Non-colonic features of irritable bowel syndrome. Gut 1986;27:452–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Houghton LA, Heyman DJ, Whorwell PJ. Symptomatology, quality of life and economic features of irritable bowel syndrome—the effect of hypnotherapy. Aliment Pharmacol Ther 1996;10:91–5. [DOI] [PubMed] [Google Scholar]
- 27.Gonsalkorale WM, Toner BB, Whorwell PJ. Cognitive change in patients undergoing hypnotherapy for irritable bowel syndrome. J Psychosom Res 2004;56:271–8. [DOI] [PubMed] [Google Scholar]
- 28.Gonsalkorale WM, Houghton LA, Whorwell PJ. Hypnotherapy in irritable bowel syndrome: a large scale audit of a clinical service with examination of factors influencing responsiveness. Am J Gastroenterol 2002;97:954–61. [DOI] [PubMed] [Google Scholar]
- 29.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- 30.Everitt BS, Pickles A. Statistical aspects of the design and analysis of clinical trials. London: Imperial College Press Publishers, 2003:108–42.
- 31.Altman DG, Schulz KF, Moher D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 2001;134:663–94. [DOI] [PubMed] [Google Scholar]
- 32.Novick J , Miner P, Krause R, et al. A randomised, double blind, placebo controlled trial of tegaserod in female patients suffering from irritable bowel syndrome with constipation. Aliment Pharmacol Ther 2002;16:1877–88. [DOI] [PubMed] [Google Scholar]
- 33.Niec AM, Frankum B, Talley NJ. Are adverse reactions to food linked to irritable bowel syndrome? Am J Gastroenterol 1998;93:2184–90. [DOI] [PubMed] [Google Scholar]
- 34.Burden S . Dietary treatment of irritable bowel syndrome: current evidence and guidelines for future practice. J Hum Nutr Diet 2001;14:231–41. [DOI] [PubMed] [Google Scholar]
- 35.Bentley SJ, Pearson DJ, Rix KJB. Food hypersensitivity in irritable bowel syndrome. Lancet 1983;2:295–7. [DOI] [PubMed] [Google Scholar]
- 36.McKee AM, Prior A, Whorwell PJ. Exclusion diets in irritable bowel syndrome: Are they worthwhile? J Clin Gastroenterol 1987;9:526–8. [DOI] [PubMed] [Google Scholar]
- 37.Durham SR, Church MK. Principles of allergy diagnosis. In: Holgate ST, Church MK, Lichtenstein LM, eds. Allergy, 2nd edn. London: Mosby, 2001:3–16.
- 38.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 2000;47:804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonsalkorale WM, Perrey C, Pravica V, et al. Interleukin 10 genotypes in irritable bowel syndrome: evidence for an inflammatory component? Gut 2003;52:91–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collins SM, Piche T, Rampal P. The putative role of inflammation in the irritable bowel syndrome. Gut 2001;49:743–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins SM. A case for an immunological basis for irritable bowel syndrome. Gastroenterology 2002;122:2078–80. [DOI] [PubMed] [Google Scholar]
- 42.Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002;122:1778–83. [DOI] [PubMed] [Google Scholar]
- 43.Mertz H . Review article: visceral hypersensitivity. Aliment Pharmacol Ther 2003;17:623–33. [DOI] [PubMed] [Google Scholar]
- 44.Francis CY, Houghton LA, Whorwell PJ. Enhanced sensitivity of the whole gut in patients with irritable bowel syndrome. Gastroenterology 1995;108:601abstract. [Google Scholar]