The function of the oesophagus is relatively straightforward—to transport the swallowed food into the stomach. In order to meet this functional need, the design of the oesophagus is simple; a relatively straight muscular tube that is guarded at it two ends by the upper and lower oesophageal sphincters. Following a voluntary act of a swallow, the two sphincters relax and open and a contraction wave or peristalsis sweeps behind the bolus autonomously. The contraction wave sweeps through the entire length of the oesophagus followed by closure of the two sphincters. Neuromuscular control mechanisms that bring about normal functioning of the two sphincters and oesophageal peristalsis are complex and require fine coordination of the muscles and nerves at the level of the central and peripheral nervous system. Disturbance of sphincters and peristalsis causes symptoms of dysphagia and oesophageal pain. The latter may manifest either as chest pain (pressure-like sensation) or heartburn (retrosternal burning). The nature of dysfunction in oesophageal motor disorders has been the subject of intense investigation for several decades. In this paper, we will review briefly the physiology of oesophageal peristalsis and lower oesophageal sphincter and then attempt to understand what may be wrong in motor disorders of the oesophagus. Novel pharmacological approaches to treat oesophageal motor disorders are also discussed.
PHYSIOLOGY OF OESOPHAGEAL PERISTALSIS AND LOWER OESOPHAGEAL SPHINCTER
The anatomy of the oesophagus is unique; it is made up of skeletal muscle in the upper one third, a mixture of skeletal and smooth muscle in the middle one third, and smooth muscle only in the distal one third in humans. The upper oesophageal sphincter is composed of all skeletal muscle and the lower oesophageal sphincter of all smooth muscle. The muscularis propria of the oesophagus, similar to the rest of the gastrointestinal tract, is made of two distinct muscle layers that are arranged in a circular and longitudinal fashion. Topographical studies by Clouse and colleagues demonstrate that during peristalsis a segment, rather than a focal point in the oesophagus, is contracted at any given time.1,2 The length of this contracted segment may extend over 15±0.7 cm and the segment traverses through the length of the oesophagus in a peristaltic fashion. Pressure in the contracted segment is distributed in the shape of a bell-shaped curve with peak pressure in the middle of the contracted segment. Peak pressure in the contracted segment is located several centimetres behind the tail end of the bolus. Transition between skeletal and smooth muscle oesophagus is not seamless, as revealed by a trough in the amplitude of contraction in the transition zone. Similarly, the transition zone between the proximal and distal smooth muscle oesophagus shows lower contraction amplitude. It is suggested that the distal transition zone may be related to the type of neural innervation of the two segments of the oesophagus. The proximal smooth muscle oesophagus is under greater cholinergic control and the distal smooth muscle oesophagus under greater inhibitory control3 but hard evidence to support this hypothesis is lacking.
Peristalsis by definition means sequential contraction of the muscles along the length of the oesophagus. It is clear that similar to circular muscles, peristalsis also occurs in longitudinal muscle layers.4 Studies by several investigators suggest that longitudinal muscle contracts earlier than circular muscle by several seconds.5–7 The difference in the timing of contraction of the two muscle layers observed in the reported studies may be related to the techniques that have been used to measure circular and longitudinal muscle contraction in these experiments. For example, circular muscle contraction recorded by manometery is delayed because manometery can only record contraction when the lumen of the oesophagus is totally collapsed on the manometery catheter. Our own observations suggest that there is perfect synchrony between the two muscle layers.8 Furthermore, a stronger circular muscle contraction is associated with a stronger longitudinal muscle contraction in normal subjects.7,9 Fine coordination between the two muscle layers provides significant biomechanical advantage to circular muscle contraction; longitudinal muscle contraction brings together the rings of circular muscle fibres, increasing the thickness of circular muscle layers at the point of contraction which, in turn, increases the force generated by circular muscle. Furthermore, the increase in muscle thickness caused by longitudinal muscle contraction reduces the stress on the wall of the oesophagus at the site of contraction in accordance with Laplace’s law.10,11
Peristalsis in skeletal muscle oesophagus is the result of sequential activation of neurones at the level of the vagal nucleus (nucleus ambiguous). On the other hand, peristalsis in smooth muscle oesophagus is mediated at the level of the dorsomotor nucleus of the vagus nerve and at the level of the myenteric plexus. The peripheral mechanism of peristalsis resides in the latency of muscle contraction.12,13 Following electrical stimulation of circular muscle, the latency of contraction is greater in the distal compared with the proximal oesophagus. In other words, there is a gradient of latency of contraction along the length of the oesophagus. During this latency period the muscle cell membrane shows hyperpolarisation. The latency gradient seems to be related to nitric oxide mediated inhibitory innervation.14 The distal oesophagus shows greater inhibitory innervation compared with the proximal oesophagus.3 Blockade of nitric oxide reduces the latency gradients and converts a peristaltic contraction into a simultaneous contraction. The proximal oesophagus is under greater cholinergic control than the distal oesophagus and an increase in cholinergic stimulation delays the latency of contraction in the proximal oesophagus, again resulting in loss of peristalsis.15 Peristalsis in longitudinal muscle layers seems to be mediated at the level of the dorsomotor nucleus of the vagus nerve.16
Swallowing at short intervals elicits the phenomenon of initial inhibition and refractory period in circular muscles of the oesophagus. If a subject swallows twice during a period of 10 seconds, the second swallow inhibits the first, a phenomenon referred to as initial inhibition. On the other hand, the amplitude of the second swallow induced contraction can be affected by the first swallow if the former falls within the refractory period of the muscle.17 The initial inhibition is neurally mediated through the inhibitory neurotransmitter nitric oxide. Refractoriness, on the other hand, is most likely related to the property of the muscle itself. Initial inhibition and refractoriness form the basis for the practice of spacing swallows at least 30 seconds apart during clinical oesophageal motility studies. Recent studies suggest that, similar to circular muscles, longitudinal muscle also demonstrates the phenomenon of initial inhibition. Initial inhibition in the longitudinal muscle layers can be observed during two closely spaced swallows.18 A balloon placed in the distal oesophagus induces contraction of the circular and longitudinal muscle layers proximal to the site of distension. Oesophageal distension induced in response to a second balloon, placed proximal to the first balloon, causes inhibition in circular as well as longitudinal muscle contraction induced by the first balloon.19 Based on these observations it is clear that, similar to circular muscle, mechanisms of inhibition must exist in longitudinal muscle.
LOWER OESOPHAGEAL SPHINCTER
The lower end of the oesophagus is guarded by two sphincters, a smooth muscle or intrinsic lower oesophageal sphincter (LOS) and a skeletal muscle or extrinsic LOS. The latter is formed by the crus of the diaphragm.20 The role of the extrinsic LOS as an antireflux barrier has received significant attention during last decade but its role in the pathogenesis of oesophageal motor disorders in not known. Smooth muscle LOS is specialised muscle, 2–4 cm in length, that maintains a constant tone. Part of the tone is due to the unique properties of the muscles itself and the remainder is due to excitatory cholinergic activity of the myenteric neurone that may be either self driven or driven through the vagus nerve. LOS muscle fibres are arranged as clasp and sling. Clasp and the sling fibres have different properties; clasp fibres have higher resting tone and lesser responsiveness to cholinergic stimulation compared with sling fibres.21,22 It may be that the differences in contractile properties of clasp and sling fibres contribute to the asymmetric effects of atropine on circumferential LOS pressure. It may also be responsible for the asymmetry in the shape of the LOS which shows more circularity on the left compared with the right side.23 The asymmetry in shape is likely to be responsible for the circumferential asymmetry of LOS pressure. Nitric oxide is the major, but not necessarily the only, inhibitory neurotransmitter released from myenteric neurones that induce relaxation of the LOS.24 Nitric oxide knockout mice show lack of swallow induced LOS relaxation and an increase in baseline LOS pressure. The role of interstitial cells of Cajal (ICC) in inhibitory transmission has been proposed25; however, W/Wv mutant mice that lack ICC have lower LOS pressure than wild-type mice but normal swallow induced LOS relaxation, arguing against the role of ICC in inhibitory transmission.26
Details of brainstem control of LOS and oesophageal peristalsis are complex and beyond the scope of this review. Studies reveal a topographical representation of neurones representing the LOS in motor neurones of the dorsal motor nucleus (DMV).27,28 Rostral cells are involved in excitatory and caudal cells control inhibitory innervation of the LOS. Afferent information from the sensory nucleus of tractus solitarius (NTS) is relayed to the DMV motor neurones via interneurones. Glutamate is the neurotransmitter of sensory afferents. Motor neurones of the DMV contain acetylcholine, nitric oxide, dopamine, and adrenaline. Inhibition of transient LOS relaxation by GABA b agonist29 and cannabinnoid 1 (CB1) receptors30 is mediated at the level of both the NTS and motor DMV. Swallow pattern generator located in the NTS is also inhibited by GABA b agonists resulting in reduced swallow frequency.
OESOPHAGEAL MOTOR DISORDERS: CLASSIFICATION
Oesophageal motor disorders are classified as primary and secondary. The latter are due to systemic diseases such as diabetes, connective tissue disorders, dermatomyositis, scleroderma, amyloidosis, alcoholism, Chagas disease, and neoplasms of various sorts (most commonly adenocarcinoma of the stomach). The usual abnormalities in LOS and oesophageal peristalsis, seen in secondary motor disorders, are listed in table 1 ▶. As the pathophysiological processes in secondary motor disorders are relatively well defined, it is easy to understand the cause of the abnormality affecting oesophageal motor function. For example, in scleroderma oesophagus, there is replacement of muscle fibres with connective tissue in the LOS and oesophagus thereby causing a decrease in LOS pressure and low amplitude contractions.31 Autonomic neuropathy in diabetes mellitus is responsible for low amplitude and bipeaked oesophageal contractions. Infiltration of the myenteric plexus of the LOS in neoplastic diseases and Chagas disease is the cause of secondary achalasia of the oesophagus. On the other hand, in the absence of an obvious aetiology, classification of primary motor disorders is based on abnormalities of the LOS and oesophageal peristalsis, as recorded by manometery. These abnormalities however are not specific and overlap considerably with secondary motor disorders of the oesophagus. Recently, Spechler and Castell proposed classification of primary motor disorder based on abnormalities of the LOS and oesophageal peristalsis, as shown in table 2 ▶ and fig 1 ▶.32 Normal values for these parameters are shown in table 3 ▶. Motor abnormalities of the LOS and oesophageal peristalsis are also seen in reflux disease and it is not clear whether they are primary or secondary to reflux disease. Injury to the muscles of the LOS and oesophagus by acid in the gastro-oesophageal reflux can induce LOS hypotension and low amplitude peristalsis in the oesophagus.33
Table 1.
Secondary oesophageal motor disorders and manometric findings
| Disorder | Manometric findings |
| Diabetes | Low amplitude bipeaked contractions |
| Chronic idiopathic pseudo-obstruction | Repetitive contractions, segmental loss of peristalsis |
| Scleroderma, mixed connective tissue disease, rheumatoid arthritis, and systemic lupus erythematosus | Low LOS pressure and low amplitude simultaneous contractions in the distal two thirds of the oesophagus; proximal oesophagus may have normal contractions |
| Secondary achalasia, Chagas disease | Evidence of systemic disease, neoplasm cardiomyopathy, megacolon, and megaureter |
| Amyloidosis, alcoholism, myxoedma, and multiple sclerosis | Low amplitude contractions in the distal oesophagus |
LOS, lower oesophageal sphincter.
Table 2.
Classification of oesophageal motility abnormalities. Adapted from Spechler and Castell32
| Manometric findings | Motor disorders |
| Inadequate LOS relaxation | Classic achalasia |
| Atypical disorders of LOS relaxation | |
| Uncoordinated contraction | Diffuse oesophageal spasm |
| Hypercontraction | Nutcracker oesophagus |
| Isolated hypertensive LOS | |
| Hypocontraction | Ineffective oesophageal motility |
LOS, lower oesophageal sphincter.
Figure 1.
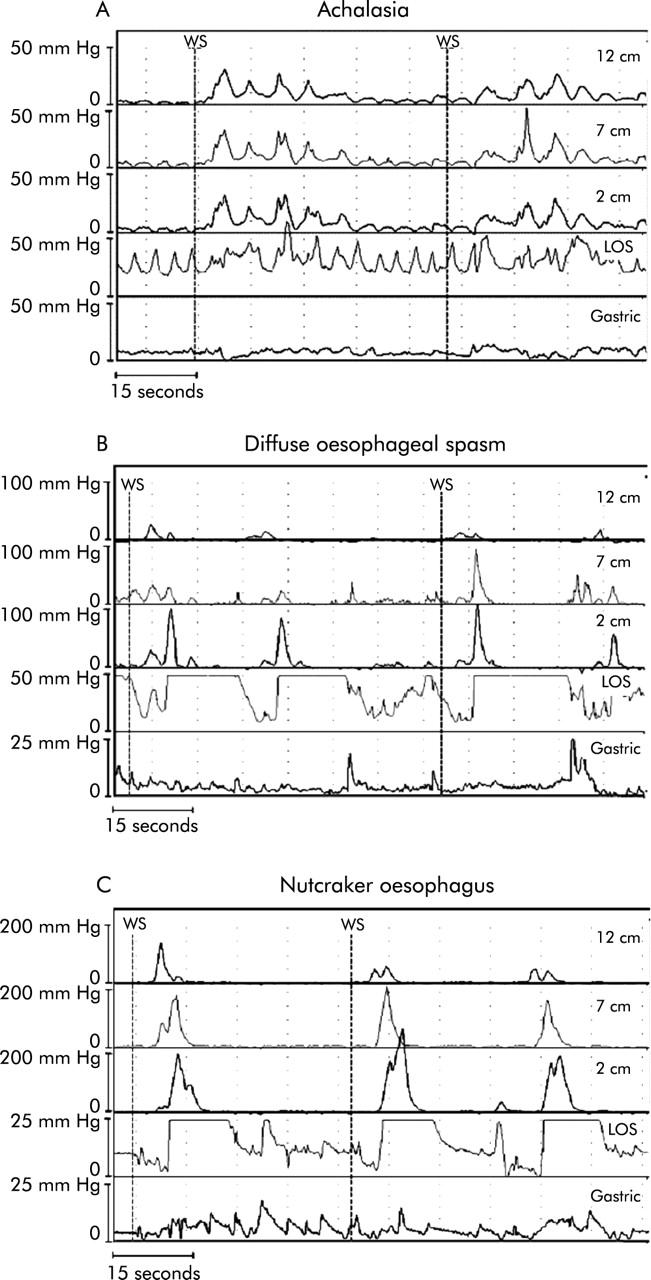
(A) Oesophageal manometry tracing from a patient with achalasia of the oesophagus. Recording sites are positioned 2, 7, and 12 cm above the lower oesophageal sphincter (LOS). Note the absence of LOS relaxation and loss of peristalsis to wet swallows (WS). (B) Oesophageal manometry tracing from a patient with diffuse oesophageal spasm. Recording sites are positioned 2, 7, and 12 cm above the LOS. Note that the WS are followed by simultaneous oesophageal contractions. (C) Oesophageal manometry tracing from a patient with nutcracker oesophagus. Recording sites are positioned 2, 7, and 12 cm above the LOS. Note the high amplitude peristaltic contractions initiated by WS.
Table 3.
Normal oesophageal manometric features. Adapted from Spechler and Castell32
| Basal LOS pressure | 10–45 mm Hg (mid respiratory pressure measured by station pull through technique) |
| LOS relaxation with swallow | Complete (to a level <8 mm Hg above gastric pressure) |
| Wave progression | Peristalsis progressing from UOS through LOS at a rate of 2–8 cm/s |
| Distal wave amplitude | 30–180 mm Hg (average of 10 swallows at two recording sites positioned 3 and 8 cm above the LOS) |
LOS, lower oesophageal sphincter; UOS, upper oesophageal sphincter.
PATHOGENESIS OF PRIMARY MOTOR DISORDERS
Primary motor disorders of the oesophagus are currently best explained on the basis of either defective inhibitory or defective excitatory innervation of the LOS and oesophagus. Achalasia of the oesophagus, the best characterised primary motor disorder, is associated with loss of nitric oxide or inhibitory innervation of the LOS.34,35 Unopposed excitatory innervation leads to high LOS pressure in achalasia of the oesophagus and high amplitude contraction of diffuse oesophageal spasm and nutcracker oesophagus.36 Degeneration of the myenteric plexus in the LOS and body of the oesophagus has been demonstrated on histopathological specimens of muscle samples obtained in patients with achalasia of the oesophagus.37 Furthermore, in achalasia patients there is loss of nitric oxide synthase from the myenteric plexus of the LOS. Similar observations are scant in patients with diffuse oesophageal spasm and nutcracker oesophagus because of the difficulty in obtaining tissue samples for histopathological examination. Physiological studies however suggest disorder of inhibitory innervation in patients with diffuse oesophageal spasm.38 Blockade of nitric oxide synthase, the enzyme responsible for nitric oxide synthesis, leads to loss of peristalsis and appearance of simultaneous contractions in the oesophagus.39 What causes degeneration of the myenteric plexus and specifically inhibitory nerves is not known. Antineuronal antibodies are commonly found in the serum of patients with achalasia but are not specific because they are also found in patients with reflux oesophagitis.40 Significant amounts of chronic inflammatory cells are seen around the myenteric ganglion in achalasia patients, the causative agent of which is not known.41 Defective excitatory innervation explains low amplitude or ineffective oesophageal peristaltic contraction in reflux disease and other non-specific motor disorders of the oesophagus.
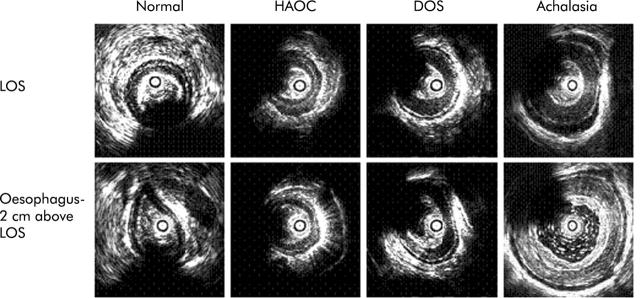
Even though loss of inhibitory innervation is widely accepted as the major dysfunction in primary oesophageal motor disorders; hypertrophy of oesophageal muscles has been observed in autopsy specimens of oesophagus in patients with achalasia of the oesophagus and diffuse oesophageal spasm.42,43 More recently, ultrasound imaging of the oesophagus using endoscope and high frequency intraluminal ultrasound probes has clarified that the increase in thickness of the muscularis propria is a common finding in patients with primary motor disorders (fig 2 ▶).44–47 We studied patients with achalasia of the oesophagus; diffuse oesophageal spasm (with high amplitude contractions) and nutcracker oesophagus using simultaneous manometery and HFIUS probes.44 The muscularis propria of the LOS was thicker in approximately half of patients with achalasia but a more consistent finding was an increase in muscle thickness of the body of the oesophagus. In achalasia patients with a markedly dilated oesophagus, muscle thickness is decreased due to distension but muscle mass, as measured by muscle cross sectional area, is significantly increased compared with normal subjects. Patients with diffuse oesophageal spasm and nutcracker oesophagus also show thickening of the muscularis propria. Muscle mass is greater in the distal (2 cm above the LOS) compared with the proximal (10 cm above the LOS) oesophagus in all of these patients. It is interesting that there are differences with regard to the degree of muscle mass in different groups: achalasia >diffuse oesophageal spasm >nutcracker oesophagus >normal subjects.44 Oesophageal muscle hypertrophy is a prominent response to oesophageal obstruction.48 It may be that the primary abnormality in patients with primary motor disorders is impairment of LOS relaxation/opening and changes in oesophageal musculature are secondary to LOS dysfunction. The major question that remains to be answered is the cause of LOS dysfunction in primary motor disorders. Based on the commonality of findings of impaired inhibitory and muscle hypertrophy in all primary oesophageal motor disorders, it is possible that these disorders represent a spectrum of the same, currently unknown, disease process.
Figure 2.
Ultrasound images of the lower oesophageal sphincter (LOS) and oesophageal body in normal subjects, patients with high amplitude oesophageal contractions (HAOC), diffuse oesophageal spasm (DOS), and achalasia of the oesophagus. The LOS image is from the centre of the LOS. Note the differences in muscle thickness in the four subjects, with the thickest muscle in patients with achalasia of the oesophagus (Mittal and colleagues44).
GENESIS OF SYMPTOMS IN OESOPHAGEAL MOTOR DISORDERS
Dysphagia, chest pain, and heartburn are symptoms of oesophageal motor disorders whether primary or secondary. The unique aspect of dysphagia in motor disorders of the oesophagus is that the swallow difficulty is for both solids and liquids. Delayed oesophageal transit is thought to be the cause of dysphagia but clearly there can be disassociation between the two. Dysphagia is usually severe in achalasia, mild to moderate in diffuse oesophageal spasm, and mild in nutcracker oesophagus and other non-specific motor disorders. Dysphagia in achalasia is mostly caused by resistance to the outflow caused by a dysfunctional LOS. Loss of peristalsis contributes to oesophageal stasis but the later is minimised by gravity which favours transit in the upright posture. Patients with ineffective oesophageal peristalsis usually do not present with dysphagia; the major symptom in these patients is related to poor clearance of gastro-oesophageal reflux that may cause heartburn and erosive oesophagitis.49 Oesophageal impedance measurement is a novel method to detect oesophageal clearance of a bolus and ineffective oesophageal peristalsis results in impaired bolus clearance.50 Ineffective oesophageal peristalsis is also common in patient with respiratory disorders such as cough and asthma.51
Acid reflux is one of the causes of angina chest pain even though the incidence with which acid reflux is responsible for oesophageal “angina-like pain” is debatable. It is clear that the majority of oesophageal pain events do not correlate with abnormal motor events or acid reflux events, as recorded by intraluminal pressure and pH monitoring techniques.52,53 Some of these patients demonstrate mechanical sensitivity to oesophageal distension.54 The reason for hypersensitivity may be located at the peripheral (within the wall of the oesophagus) or central (spinal cord or brain) level. Elegant studies by Sarkar et al in normal subjects and patients with symptoms indicate that acid in the oesophagus induces heightened sensitivity that is mediated either at the level of the spinal cord or at a higher level.55,56N-methyl d-aspartate (NMDA) receptor antagonists can reduce central sensitivity induced by acid infusion into the oesophagus.57
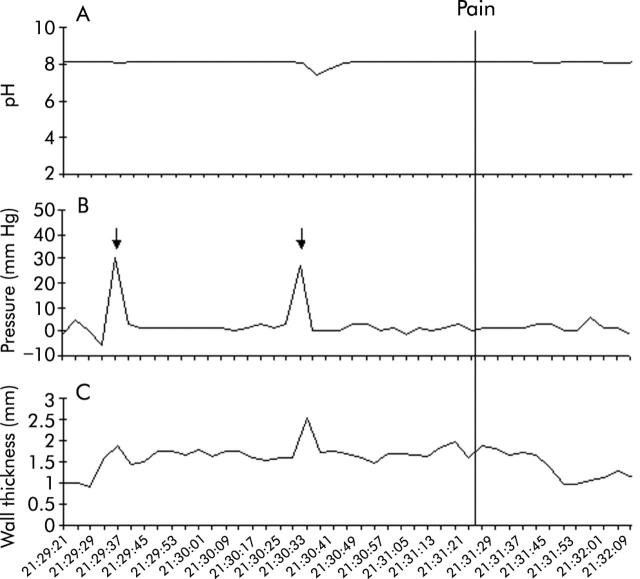
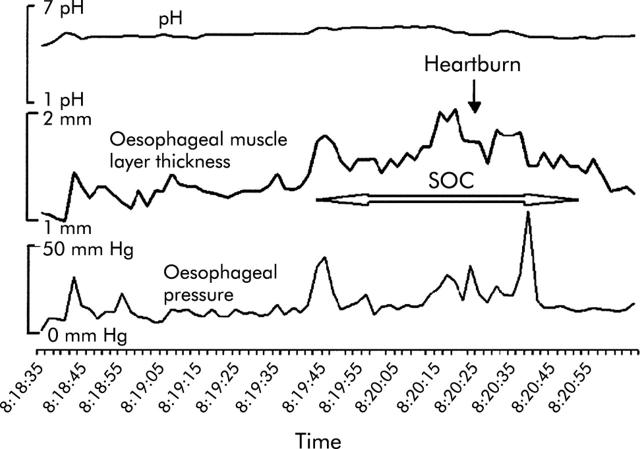
Several studies indicate that heartburn and “angina-like” oesophageal pain can be caused by many stimuli. Besides acid, distension of the oesophagus with a balloon can cause both heartburn and chest pain.58,59 Whether the symptom is heartburn or chest pain may depend on the degree of distension. Wall stretch rather than contraction appears to be the stimulus for distension induced oesophageal sensation.60,61 Using simultaneous pressure, pH, and ultrasound imaging, we have identified sustained oesophageal contraction (SOC) that is temporally related to the spontaneous chest pain event (fig 3 ▶).62 SOC is not recorded by intraluminal pressure measurement and actually represents sustained contraction of the longitudinal muscle of the oesophagus. SOC is also temporally related to the heartburn symptom that may or may not be associated with acid reflux (fig 4 ▶).63 SOC associated with chest pain is much longer in duration (68 seconds) than that associated with heartburn (45 seconds). Further work is required to prove the cause and effect relationship between SOC and oesophageal symptoms.
Figure 3.
Sustained oesophageal contraction associated with chest pain. Oesophageal pH (top), distal oesophageal pressure (middle), and oesophageal muscle thickness (bottom) are shown during a 2.5 minute recording interval. Onset of chest pain is depicted by the vertical line (time = 0). Onset of sustained oesophageal contraction (SOC) occurs approximately 120 seconds before the onset of pain. The pressure record shows two small contractions that are accompanied by brief increases in oesophageal muscle thickness during SOC (arrows) (Balaban and colleagues62).
Figure 4.
Heartburn without acid reflux associated with sustained oesophageal contraction (SOC). Onset of SOC occurs approximately 54 seconds before the onset of heartburn (Pehlivanov and colleagues63).
TREATMENT STRATEGIES FOR OESOPHAGEAL MOTOR DISORDERS
The mainstay of therapy in achalasia of the oesophagus is to reduce the outflow resistance caused by LOS dysfunction. There are three approaches to do so: botox injection into the LOS, pneumatic dilation, and surgical or Heller’s myotomy. Botox injection is effective in 32% of patients for a mean period of 1.1 years. Pneumatic dilation is more effective and in a larger number of patients (72%) for extended periods (five years) but carries a 3% risk of perforation at the time of dilation. Heller’s myotomy is effective in more than 84% of patients for five years and a partial fundoplication prevents the risk of reflux after surgery.64 Relief of dysphagia following dilation/surgery does not parallel relief of chest pain; the latter usually gets better with time.65
With the availability of potent acid inhibition therapy it is fairly simple to treat acid reflux related oesophageal pain extremely effectively. Fass et al found that patients with objective evidence of reflux had significant improvement in oesophageal pain during a two week course of high dose omeprazole.66 Achem et al also found significant but partial relief of chest pain in patients with evidence of reflux on pH monitoring.67 A two week course of double dose proton pump inhibitors as a therapeutic test to determine if reflux is the cause of chest pain should be standard practice.
Sublingual nitroglycerine and other longer acting oral nitrates (nitric oxide donors) have been used for the treatment of oesophageal pain; however, their efficacy has not been confirmed in controlled clinical trials. Sildenafil, a phosphodiesterase 5 inhibitor, the enzyme responsible for degrading nitric oxide, is a potent smooth muscle relaxant. It relaxes LOS and reduces oesophageal contraction amplitude in normal subjects and patients with achalasia of the oesophagus.68,69 There are no studies on the efficacy of sildenafil in achalasia of the oesophagus. A recent double blind study in patients with hypercontractile oesophageal motility disorders showed that sildenafil decreased contraction amplitude by more than 70% in normal subject and patients, and the effects lasted for more than eight hours.70 However, improvement in symptoms was only noticed in four of 11 subjects and two of these four subjects experienced significant side effects. Similarly, calcium channel blockers can reduce contraction amplitude in normal subjects and patients with high amplitude contractions but are not efficacious for relief of symptoms and produced significant side effects in controlled clinical trials.71,72 Therefore, it is clear that therapies other than smooth muscle relaxants are required in the treatment of oesophageal motor disorders. The antianxiety medication trazadone, at a dose of 100–150 mg once a day, was the only therapy that showed benefit in a controlled clinical trial.73
Botulinum toxin (botox), 100 units, injected into the LOS of patients with various types of primary motor disorders has been found to be useful in 72% of patients with a 50% reduction in symptoms in an uncontrolled study.74 Achalasia patients were not included in this study. Mean duration of follow up was 7.3 months and repeat injection was successful in some patients. A similar response to botox therapy was observed by Storr et al in their uncontrolled study of nine patients with diffuse oesophagus spasm.75 They injected botox along the distal 10–15 cm length of the oesophagus. Placebo controlled studies are needed to determine the true efficacy of botox in the treatment of primary motor disorders of the oesophagus.
In the absence of a clear understanding of the mechanism of oesophageal pain, blockade of sensory receptor of pain could be used to treat pain. The precise nature of the receptors at the nociceptive afferent nerve terminal is not known but adenosine, one of the candidates in myocardial ischaemic pain, may also be involved in oesophageal pain. Theophylline, an adenosine antagonist, inhibits adenosine induced pain in patients with stable angina.76 Theophylline increases the sensory threshold of distension induced oesophageal pain.77 Furthermore, a three month uncontrolled study found significant relief of symptoms in the majority of patients. Another receptor mediated approach is through NMDA antagonists which may be involved in oesophageal hypersensitivity at the spinal level. Ketamine, an NMDA receptor antagonist, decreases acid induced oesophageal sensitivity; however, the problem with medications in this category is their side effect profile.57
SUMMARY
Primary motor disorders of the oesophagus affect neural as well as muscular elements of the oesophagus and LOS. It is tempting to speculate that these disorders represent a hypertrophic myopathic state of the oesophagus secondary to LOS dysfunction, and neural dysfunction may be secondary. The relationship between pain and muscle hypertrophy in primary motor disorders is worthy of investigation. Dysphagia of primary oesophageal motor disorders is easier to treat than pain; the latter could be debilitating. Muscle relaxants acting at the peripheral level do not appear be the answer for treatment of dysphagia and pain of primary motor disorders. On the other hand, blockade of either primary sensory nociceptor at the peripheral level or receptors involved in oesophageal hypersensitivity at the peripheral/central level in the management of oesophageal pain deserves exploration.
Acknowledgments
Dr Mittal is supported by a PHS grant, NIH RO-1 DK60733.
REFERENCES
- 1.Clouse RE, Staiano A, Bickston SJ, et al. Characteristics of the propagating pressure wave in the oesophagus. Dig Dis Sci 1996;41:2369–76. [DOI] [PubMed] [Google Scholar]
- 2.Clouse RE, Staiano A. Topography of the oesophageal peristaltic pressure wave. Am J of Physiol 1991;261:G677–84. [DOI] [PubMed] [Google Scholar]
- 3.Crist J , Gidda JS, Goyal RK. Intramural mechanism of oesophageal peristalsis: roles of cholinergic and noncholinergic nerves. Proc Natl Acad Sci U S A 1984;81:3595–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dodds WJ, Stewart ET, Hodges D, et al. Movement of the feline oesophagus associated with respiration and peristalsis. An evaluation using tantalum markers. J Clin Invest 1973;52:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugarbaker DJ, Rattan S, Goyal RK. Swallowing induces sequential activation of oesophageal longitudinal smooth muscle. Am J Physiol 1984;247:G515–19. [DOI] [PubMed] [Google Scholar]
- 6.Pouderoux P , Lin S, Kahrilas PJ. Timing, propagation, coordination, and effect of oesophageal shortening during peristalsis. Gastroenterology 1997;112:1147–54. [DOI] [PubMed] [Google Scholar]
- 7.Nicosia MA, Brasseur JG, Liu JB, et al. Local longitudinal muscle shortening of the human oesophagus from high-frequency ultrasonography. Am J Physiol 2000;281:G1022–33. [DOI] [PubMed] [Google Scholar]
- 8.Bhalla V , Padda B, Puckett J, et al. Longitudinal and circular muscle contract synchronously in the esophagus during peristalsis: a new way to look at the contraction of two muscle layers. Gastroenterology 2004;126 (Suppl 2) :A–637#W1444. [Google Scholar]
- 9.Pehlivanov N , Liu J, Kassab G, et al. Relationship between oesophageal muscle thickness and intraluminal pressure: An ultrasonographic study. Am J Physiol 2001;280:G1093–8. [DOI] [PubMed] [Google Scholar]
- 10.Pal A , Brasseur JG. The mechanical advantage of local longitudinal shortening on peristaltic transport. J Biomech Eng 2002;124:94–100. [DOI] [PubMed] [Google Scholar]
- 11.Puckett J , Bhalla V, Liu J, et al. Esophageal wall stress: potential mechanism of increased muscle thickness in patients with high amplitude esophageal contractions. Gastroenterology 2004;126 (Suppl 2).
- 12.Weisbrodt NW, Christensen J. Gradients of contractions in the opossum oesophagus. Gastroenterology 1972;62:1159–66. [PubMed] [Google Scholar]
- 13.Gidda JS, Goyal RK. Regional gradient of initial inhibition and refractoriness in oesophageal smooth muscle. Gastroenterology 1985;89:843–51. [DOI] [PubMed] [Google Scholar]
- 14.Conklin JL. Nitric oxide: a mediator of oesophageal motor function. J Lab Clin Med 1998;131:10–20. [DOI] [PubMed] [Google Scholar]
- 15.Gidda JS, Buyniski JP. Swallow-evoked peristalsis in opossum oesophagus: role of cholinergic mechanisms. Am J Physiol 1986;251:G779–85. [DOI] [PubMed] [Google Scholar]
- 16.Sugarbaker DJ, Rattan S, Goyal RK. Mechanical and electrical activity of oesophageal smooth muscle during peristalsis. Am J Physiol 1984;246:G145–50. [DOI] [PubMed] [Google Scholar]
- 17.Meyer GW, Gerhardt DC, Castell DO. Human oesophageal response to rapid swallowing: muscle refractory period or neural inhibition? Am J Physiol 1981;241:G129–36. [DOI] [PubMed] [Google Scholar]
- 18.Shi G , Pandolfino JE, Zhang Q, et al. Deglutitive inhibition affects both oesophageal peristaltic amplitude and shortening. Am J Physiol 2003;284:G575–82. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto Y , Liu J, Smith TK, et al. Distension related responses in the circular and longitudinal muscles of the oesophagus: An ultrasonographic study. Am J Physiol 1998;38:G805–11. [DOI] [PubMed] [Google Scholar]
- 20.Mittal RK, Balaban D. Oesophagogastric junction: physiology and pathophysiology. N Engl J Med 1997;336:924–32. [DOI] [PubMed] [Google Scholar]
- 21.Preiksaitis HG, Diamant NE. Regional differences in cholinergic activity of muscle fibers from the human gastroesophageal junction. Am J Physiol 1997;272:G1321–7. [DOI] [PubMed] [Google Scholar]
- 22.Muinuddin A , Xue S, Diamant NE. Regional differences in the response of feline oesophageal smooth muscle to stretch and cholinergic stimulation. Am J Physiol 2001;281:G1460–7. [DOI] [PubMed] [Google Scholar]
- 23.Liu J , Parashar VK, Mittal RK. Asymmetry of the lower oesophageal sphincter pressure: Is it related to the shape or the thickness of the muscle? Am J Physiol 1997;272:G1509–17. [DOI] [PubMed] [Google Scholar]
- 24.Mashimo H , He XD, Huang PL, et al. Neuronal constitutive nitric oxide synthase is involved in murine enteric inhibitory neurotransmission. J Clin Invest 1996;98:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward SM, Morris G, Reese L, et al. Interstial cells of Cajal mediate enteric inhibitory neurotransmission in the lower oesophageal sphincter and pyloric sphincters. Gastroenterology 1998;115:314–29. [DOI] [PubMed] [Google Scholar]
- 26.Sivarao DV, Mashimo HL, Thatte HS, et al. Lower oesophageal sphincter is achalasic in nNOS(−/−) and hypotensive in W/W(v) mutant mice. Gastroenterology 2001;121:34–42. [DOI] [PubMed] [Google Scholar]
- 27.Rossiter CD, Norman WP, Jain M, et al. Control of lower oesophageal sphincter pressure by two sites in dorsal motor nucleus of the vagus. Am J Physiol 1990;259:G899–906. [DOI] [PubMed] [Google Scholar]
- 28.Hyland NP, Abrahams TP, Fuchs K, et al. Organization and neurochemistry of vagal preganglionic neurons innervating the lower oesophageal sphincter in ferrets. J Comp Neurol 2001;430:222–34. [DOI] [PubMed] [Google Scholar]
- 29.McDermott CM, Abrahams TP, Partosoedarso E, et al. Site of action of GABA(B) receptor for vagal motor control of the lower oesophageal sphincter in ferrets and rats. Gastroenterology 2001;120:1749–62. [DOI] [PubMed] [Google Scholar]
- 30.Partosoedarso ER, Abrahams TP, Scullion RT, et al. Cannabinoid1 receptor in the dorsal vagal complex modulates lower oesophageal sphincter relaxation in ferrets. J Physiol 2003;550:149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller LS, Liu JB, Klenn PJ, et al. Endoluminal ultrasonography of the distal oesophagus in systemic sclerosis. Gastroenterology 1993;105:31–9. [DOI] [PubMed] [Google Scholar]
- 32.Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut 2001;49:145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rich H , Sohn UD, Behar J, et al. Experimental oesophagitis affects intracellular calcium stores in the cat lower oesophageal sphincter. Am J Physiol 1997;272:G1523–9. [DOI] [PubMed] [Google Scholar]
- 34.Mearin F , Mourelle M, Guarner F, et al. Patients with achalasia lack nitric oxide synthase in the gastro-oesophageal junction. Eur J Clin Invest 1993;23:724–8. [DOI] [PubMed] [Google Scholar]
- 35.Hirano I , Tatum RP, Shi G, et al. Manometric heterogeneity in patients with idiopathic achalasia. Gastroenterology 2001;120:789–98. [DOI] [PubMed] [Google Scholar]
- 36.Murray JA, Ledlow A, Launspach J, et al. The effects of recombinant human hemoglobin on oesophageal motor function in human. Gastroenterology 1995;109:1241–8. [DOI] [PubMed] [Google Scholar]
- 37.Goldblum JR, Rice TW, Richter JE. Histopathologic features in oesophagomyotomy specimens from patients with achalasia. Gastroenterology 1996;111:648–54. [DOI] [PubMed] [Google Scholar]
- 38.Behar J , Biancani P. Pathogenesis of simultaneous oesophageal contractions in patients with motility disorders. Gastroenterology 1993;105:111–18. [DOI] [PubMed] [Google Scholar]
- 39.Konturek JW, Gillessen A, Domschke W. Diffuse oesophageal spasm: a malfunction that involves nitric oxide? Scand J Gastroenterology 1995;30:1041–5. [DOI] [PubMed] [Google Scholar]
- 40.Moses PL, Ellis LM, Anees MR, et al. Antineuronal antibodies in idiopathic achalasia and gastro-oesophageal reflux disease. Gut 2003;52:629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raymond L , Lach B, Shamji FM. Inflammatory aetiology of primary oesophageal achalasia: an immunohistochemical and ultrastructural study of Auerbach’s plexus. Histopathology 1999;35:445–53. [DOI] [PubMed] [Google Scholar]
- 42.Gillies M , Nicks R, Skyring A. Clinical manometric and pathologic studies in diffuse oesophageal spasm. Br Med J 1967;2:527–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferguson TB, Woodbury JD, Roper CL, et al. Giant muscular hypertrophy of the oesophagus. Ann Thorac Surg 1969;8:209. [DOI] [PubMed] [Google Scholar]
- 44.Mittal RK, Kassab G, Puckett JL, et al. Hypertrophy of the muscularis propria of the lower oesophageal sphincter and the body of the oesophagus in patients with primary motility disorders of the oesophagus. Am J Gastroenterol 2003;98:1705–12. [DOI] [PubMed] [Google Scholar]
- 45.Pehlivanov N , Liu J, Kassabe G, et al. Relationship between muscle thickness and pressure in patients with spastic motor disorders of the oesophagus. Am J Physiol 2002;282:G910–16. [Google Scholar]
- 46.Melzer E , Tiomny A, Coret A, et al. Nutcracker oesophagus: Severe muscular hypertrophy on endosonography. Gastrointest Endosc 1995;42:366–7. [DOI] [PubMed] [Google Scholar]
- 47.Kojima Y , Ikeda M, Nakamura T, et al. Nonspecific oesophageal motor disorder associated with thickened muscularis propria of the oesophagus. Gastroenterology 1992;103:333–5. [DOI] [PubMed] [Google Scholar]
- 48.Tung HN, Schulze-Delrieu K, Shirazi S, et al. Hypertrophic smooth muscle in the partially obstructed opossum oesophagus. The model: histological and ultrastructural observations, Gastroenterology 1991;100:853–64. [DOI] [PubMed] [Google Scholar]
- 49.Ineffective oesophageal motility (IEM). The primary finding in patients with nonspecific oesophageal motor disorders. Dig Dis Sci 1997;42:1859–65. [DOI] [PubMed] [Google Scholar]
- 50.Simren M , Silny J, Holloway R, et al. Relevance of ineffective oesophageal motility during oesophageal acid clearance. Gut 2003;52:784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foud YM, Katz P, Hatlebakk JG, et al. Ineffective oesophageal peristalsis: The most common motility abnormality in patients with GERD-associated respiratory disorder. Am J Gastroenterol 1999;94:1464–76. [DOI] [PubMed] [Google Scholar]
- 52.Janssens J , Vantrappen G, Ghillebert G. 24-hour recording of oesophageal pressure and pH in patients with noncardiac chest pain. Gastroenterology 1986;90:1978–84. [DOI] [PubMed] [Google Scholar]
- 53.Peters L , Maas L, Petty D, et al. Spontaneous noncardiac chest pain. Evaluation by 24-hour ambulatory oesophageal motility and pH monitoring. Gastroenterology 1988;94:878–86. [PubMed] [Google Scholar]
- 54.Richter JE, Barish CF, Castell DO. Abnormal sensory perception in patients with oesophageal chest pain. Gastroenterology 1986;91:845–52. [DOI] [PubMed] [Google Scholar]
- 55.Sarkar S , Aziz Q, Woolf CJ, et al. Contribution of central sensitisation to the development of non-cardiac chest pain. Lancet 2000;356:1154–9. [DOI] [PubMed] [Google Scholar]
- 56.Sarkar S , Hobson AR, Furlong PL, et al. Central neural mechanisms mediating human visceral hypersensitivity. Am J Physiol 2001;281:G1196–202. [DOI] [PubMed] [Google Scholar]
- 57.Willert RP, Woolf CJ, Hobson AR, et al. The development and maintenance of human visceral pain hypersensitivity is dependent on the N-methyl-D-asparate receptor. Gastroenterology 2004;126:683–92. [DOI] [PubMed] [Google Scholar]
- 58.Fass R , Naliboff B, Higa L, et al. Differential effect of long-term oesophageal acid exposure on mechanosensitivity and chemosensitivity in humans. Gastroenterology 1998;115:1363–73. [DOI] [PubMed] [Google Scholar]
- 59.Jones CM. Digestive tract pain: diagnosis and treatment, experimental observations. New York: MacMillan Co, 1938.
- 60.Barlow JD, Gregersen H, Thompson DG. Identification of the biomechanical factors associated with the perception of distension in the human oesophagus. Am J Physiol 2002;282:G683–9. [DOI] [PubMed] [Google Scholar]
- 61.Takida T , Liu J, Nabe T, et al. Oesophageal stretch the mechanim of distension induced oesophageal pain. Neurogastroenterol Motil 2004; (in press). [DOI] [PubMed]
- 62.Balaban DH, Yamamoto Y, Liu J, et al. Sustained oesophageal contraction: a marker of oesophageal chest pain identified by intraluminal ultrasonography. Gastroenterology 1999;116:29–37. [DOI] [PubMed] [Google Scholar]
- 63.Pehlivanov N , Liu J, Mittal RK. Sustained oesophageal contraction: a motor correlate of heartburn symptom. Am J Physiol 2001;281:G743–51. [DOI] [PubMed] [Google Scholar]
- 64.Spiess AE, Kahrilas PJ. Treating achalasia: from whalebone to laparoscope. JAMA. 1998;19 280:638–42. [DOI] [PubMed]
- 65.Eckardt VF, Stauf B, Bernhard G. Chest pain in achalasia: patient characteristics and clinical course. Gastroenterology 1999;116:1300–4. [DOI] [PubMed] [Google Scholar]
- 66.Fass R , Fennerty MB, Ofman JJ, et al. The clinical and economic value of a short course of omeprazole in patients with noncardiac chest pain. Gastroenterology 1998;115:42–9. [DOI] [PubMed] [Google Scholar]
- 67.Achem SR, Kolts BE, MacMath T, et al. Effects of omeprazole versus placebo in treatment of noncardiac chest pain and gastroesophageal reflux. Dig Dis Sci 1997;42:2138–45. [DOI] [PubMed] [Google Scholar]
- 68.Lee JI, Park H, Kim JH, et al. The effect of sildenafil on oesophageal motor function in healthy subjects and patients with nutcracker oesophagus. Neurogastroenterol Motil 2003;15:617–23. [DOI] [PubMed] [Google Scholar]
- 69.Bortolotti M , Mari C, Lopilato C, et al. Effects of sildenafil on oesophageal motility of patients with idiopathic achalasia. Gastroenterology 2000;118:253–7. [DOI] [PubMed] [Google Scholar]
- 70.Eherer AJ, Schwetz I, Hammer HF, et al. Effect of sildenafil on oesophageal motor function in healthy subjects and patients with oesophageal motor disorders. Gut 2002;50:758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Richter JE, Dalton CB, Bradley CA, et al. Oral nifedipine in the treatment of non cardiac chest pain in patients with nutcracker oesophagus. Gastroenterology 1987;93:21–8. [DOI] [PubMed] [Google Scholar]
- 72.Davies HA, Lewis MJ, Rhodes J, et al. Trial of nifedipine for prevention of oesophageal spasm. Digestion 1987;36:81–3. [DOI] [PubMed] [Google Scholar]
- 73.Clouse RE, Lustman PJ, Eckert TC, et al. Low-dose trazodone for symptomatic patients with oesophageal contraction abnormalities. A double-blind, placebo-controlled trial. Gastroenterology 1987;92:1027–36. [DOI] [PubMed] [Google Scholar]
- 74.Miller LS, Pullela SV, Parkman HP, et al. Treatment of chest pain in patients with noncardiac, nonreflux, nonachalasia spastic oesophageal motor disorders using botulinum toxin injection into the gastroesophageal. Am J Gastroenterol 2002;97:1640–6. [DOI] [PubMed] [Google Scholar]
- 75.Storr M , Allescher HD, Rosch T, et al. Treatment of symptomatic diffuse oesophageal spasm by endoscopic injection of botulinum toxin: a prospective study with long term follow-up. Gastrointest Endosc 2001;54:18. [PubMed] [Google Scholar]
- 76.Minton NA, Henry JA. Pharmacodynamic interaction between infused adenosine and oral theophylline. Hum Exp Toxicol 1991;10:411–18. [DOI] [PubMed] [Google Scholar]
- 77.Rao SS, Mudipalli RS, Mujica V, et al. An open-label trial of theophylline for functional chest pain. Dig Dis Sci 2002;47:2763–8. [DOI] [PubMed] [Google Scholar]