Hirschsprung’s disease is a congenital disorder in which enteric neurones are absent from variable lengths of the terminal region of the bowel. The condition presents as failure to pass meconium, severe constipation, colonic distension, and sometimes enterocolitis. Treatment for Hirschsprung’s disease requires the surgical removal of the aganglionic segment. Mutant mice lacking enteric neurones in all or a major part of the gastrointestinal tract die soon after birth.1 Thus, after birth, the enteric nervous system is crucial for normal intestinal motility in both mice and humans.
During fetal development, amniotic fluid is swallowed, while epithelial cells, mucous, and bile are discharged into the intestine, and progress in an anal direction.2 Little is known about the mechanisms controlling gastrointestinal motility in fetal life. Some infants with aganglionosis extending into the ileum present with features of meconium ileus,3 suggesting that enteric neurones are required for the propulsion of meconium prior to birth.
In this study, we first examined the progression of intestinal contents in fetal wild-type (C57Bl/6) mice. As the wall of the fetal mouse gut is transparent and bile pigment is yellow, location of bile can be readily observed. Bile was first detected at embryonic day (E) 16.5 in the duodenum but there was no evidence of bile in the distal small intestine or colon. At E17.5, bile was present throughout the small intestine and extended into the proximal colon. Little or no bile was observed in the proximal duodenum (close to the stomach), indicating that lumenal contents move predominantly or exclusively in an anal direction. At E18.5 (one day prior to birth), bile (meconium) was present in the distal hindgut. Thus, as in humans,2 intestinal contents move anally during mouse fetal development.
In mice lacking the receptor tyrosine kinase, Ret, the enteric nervous system fails to develop in the small and large intestine4 but mice heterozygous for the Ret mutation have normal numbers of enteric neurones.5 To determine whether propulsion of intestinal contents in fetal mice requires enteric neurones, we examined the location of meconium in E18.5 mice lacking Ret (RetTGM/RetTGM mice6). The post-caecal hindgut of wild-type (+/+), heterozygous (+/−), and Ret null (−/−) littermates was photographed. The fetuses were genotyped using polymerase chain reaction,6 and NADPH diaphorase histochemistry was also performed on samples of proximal duodenum.7 All fetuses lacking NADPH diaphorase stained neurones in the duodenum were confirmed by polymerase chain reaction to be Ret null mice. There was no significant difference between Ret null, wild-type, and heterozygous mice in the length of the hindgut (data not shown), number of meconium boluses in the post-caecal hindgut, length of the hindgut occupied by meconium, or the distance from the caecum to meconium (fig 1 ▶). Thus progression of contents through the fetal mouse gut does not require enteric neurones. Slow waves do not appear in the murine colon until after birth,8 so it is unlikely that propulsion of gut contents during fetal life depends on slow waves and their propagation.
Figure 1.
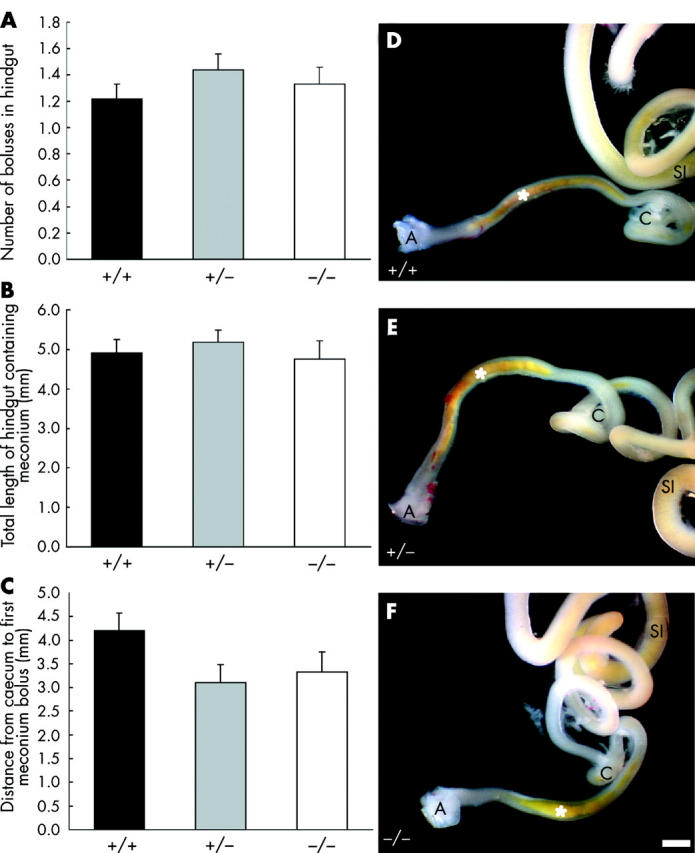
(A–C) Analysis of the presence and location of meconium in the hindgut of embryonic day 18.5 (E18.5) wild-type (+/+; n = 25), heterozygous (+/−; n = 39), and Ret null (−/−; n = 24) mice. There was no significant difference between the three groups in the number of meconium boluses in the post-caecal hindgut (A), length of the hindgut occupied by meconium (B), or distance from the caecum to the meconium (C) (one way ANOVAs; p>0.05). (D–F) Hindgut from E18.5 wild-type (D), heterozygous (E), and Ret null (F) mice. Meconium containing bile pigment is present in the caudal hindgut (asterisk) of mice of all three genotypes. A, anus; C, caecum; SI, small intestine. Scale bar = 1 mm.
Our data show that enteric neurones are not required for the anally directed propulsion of gut contents in fetal mice. Although there are some data from infants with long segment Hirschsprung’s disease suggesting that neurones contribute to the propulsion of gut contents in the fetus,3 humans are born at a developmentally later stage than mice, and there may be neurone independent propulsive motor patterns in fetal humans that are replaced by neurone dependent motor patterns prior to birth.
References
- 1.Newgreen D , Young HM. Enteric nervous system: development and developmental disturbances—part 1. Pediatr Dev Pathol 2002;5:224–47. [DOI] [PubMed] [Google Scholar]
- 2.McLain CR jr.Amniography studies of the gastrointestinal motility of the human fetus. Am J Obstet Gynecol 1963;86:1079–87. [DOI] [PubMed] [Google Scholar]
- 3.Stringer MD, Brereton RJ, Drake DP, et al. Meconium ileus due to extensive intestinal aganglionosis. J Pediatr Surg 1994;29:501–3. [DOI] [PubMed] [Google Scholar]
- 4.Schuchardt A , D’Agati V, Larsson-Blomberg L, et al. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 1994;367:380–3. [DOI] [PubMed] [Google Scholar]
- 5.Gianino S , Grider JR, Cresswell J, et al. GDNF availability determines enteric neuron number by controlling precursor proliferation. Development 2003;130:2187–98. [DOI] [PubMed] [Google Scholar]
- 6.Enomoto H , Crawford PA, Gorodinsky A, et al. RET signaling is essential for migration, axonal growth and axon guidance of developing sympathetic neurons. Development 2001;128:3963–74. [DOI] [PubMed] [Google Scholar]
- 7.Ward SM, Ordog T, Bayguinov JR, et al. Development of interstitial cells of Cajal and pacemaking in mice lacking enteric nerves. Gastroenterology 1999;117:584–94. [DOI] [PubMed] [Google Scholar]
- 8.Ward SM, Harney SC, Bayguinov JR, et al. Development of electrical rhythmicity in the murine gastrointestinal tract is specifically encoded in the tunica muscularis. J Physiol (Lond) 1997;505:241–58. [DOI] [PMC free article] [PubMed] [Google Scholar]


