Abstract
Background: Mutations in NOD2, a putative intracellular receptor for bacterial peptidoglycans, are associated with a subset of Crohn’s disease but the molecular mechanism linking this protein with the disease pathogenesis remains unclear. Human α defensins (HD-5 and HD-6) are antibiotic effector molecules predominantly expressed in Paneth cells of the ileum. Paneth cells also express NOD2. To address the hypothesis that the function of NOD2 may affect expression of Paneth cell defensins, we compared their expression levels with respect to NOD2 mutations in Crohn’s disease.
Methods: Forty five Crohn’s disease patients (24 with NOD2 mutations, 21 with wild-type NOD2) and 12 controls were studied. Real time reverse transcription-polymerase chain reaction was performed with mucosal mRNA for HD-5, HD-6, lysozyme, secretory phospholipase A2 (sPLA2), tumour necrosis factor α, interleukin 8, and human hypoxanthine phosphoribosyltransferase (housekeeping gene). Immunohistochemistry with anti-HD-5 and histological Paneth cell staining were performed in 10 patients with NOD2 mutations or wild-type genotypes.
Results: Ileal expression of HD-5 and HD-6, but not sPLA2 or lysozyme, were diminished in affected ileum, and the decrease was significantly more pronounced in patients with NOD2 mutations. In the colon, HD-5, HD-6, and sPLA2 were increased during inflammation in wild-type but not in NOD2 mutated patients. In both the colon and ileum, proinflammatory cytokines and lysozyme were unaffected by NOD2 status. Immunohistochemistry identified Paneth cells as the sole source of HD-5.
Conclusion: As alpha defensins are important in the mucosal antibacterial barrier, their diminished expression may explain, in part, the bacterial induced mucosal inflammation and ileal involvement of Crohn’s disease, especially in the case of NOD2 mutations.
Keywords: Crohn’s disease, NOD2, Paneth cells, defensins, antimicrobial peptides
Although the aetiology of Crohn’s disease is still enigmatic, the recent finding of a loss of function mutation in the putative intracellular peptidoglycan receptor NOD2 in approximately one third of Crohn’s disease patients represents a major advance.1–3 The pathophysiology of NOD2 in Crohn’s disease was initially proposed as being linked to immunological dysregulation in monocytes.4,5 However, intestinal epithelial cells,6,7 including Paneth cells,8 have also been demonstrated to express this putative receptor. It has been demonstrated that colonic epithelial cells with NOD2 mutations display a disturbed response against salmonella, supporting the hypothesis of a deficient antimicrobial response of epithelial cells.9 Nevertheless, the molecular mechanisms leading to diminished antibacterial responses in intestinal mucosal cells carrying functionally defective mutations of the NOD2 gene are not understood.
It is well known that epithelia are equipped with various antimicrobial peptides that act rapidly to kill a broad range of microorganisms.10 One important class of antimicrobial peptides are defensins. Defensins are small cationic arginine rich peptides with a molecular weight of 3–5 kDa.11,12 Based on the position of three intramolecular disulphide bridges between cysteine residues, defensins are classified as α- and β-defensins. The known α-defensins include the human neutrophil peptides 1–4 as well as the epithelial human defensin 5 (HD-5) and human defensin 6 (HD-6). The α-defensins have a broad spectrum of antimicrobial activity against bacteria, fungi, and some enveloped viruses by perforating the cell membrane through formation of multimeric pores.13,14 They may also act as chemokines.15 HD-5 and HD-6 are Paneth cell products and major antimicrobial peptides of the small intestine.16 Expression of both HD-5 and HD-6 are increased in the colonic mucosa of inflammatory bowel disease patients through the appearance of metaplastic Paneth cells.17–19 Their functional importance is illustrated by the protection against salmonella in HD-5 transgenic mice20 and also by the increased susceptibility towards infection of matrilysin knockout mice, a protease which is responsible for the processing of murine prodefensins to defensins.20 Several investigations indicate that defensins are differentially expressed in Crohn’s disease and ulcerative colitis.21–23 Based on these data, as well as the recent finding of NOD2 expression in Paneth cells,8 we hypothesised that NOD2 may in part regulate or influence Paneth cell defensin expression. Another possible hypothesis is that NOD2 mutations could affect the number of Paneth cells. We reasoned that decreased expression of antimicrobial peptides may in principle lead to susceptibility to bacterial invasion which could trigger inflammation and loss of tolerance against the luminal flora. Furthermore, decreased Paneth cell defensin expression could potentially explain the ileal preference of Crohn’s disease patients with positive NOD2 mutations.5,24 To address these issues, we compared mucosal levels of human α defensins in Crohn’s disease patients with respect to NOD2 (CARD15) genotypes.
METHODS
Patients
Twelve patients with a routine colonoscopy lacking significant pathology were randomly selected as controls. A cohort of 68 Crohn’s disease patients from two inflammatory bowel disease centres (Stuttgart and Homburg) were screened for NOD2 mutations. Ileal biopsies were available from 26 of 68 patients with Crohn’s disease which were stratified into those with (n = 18) and without (n = 8) macroscopic ileal disease. Colonic biopsies were available from 39 of 68 patients. In the case of Crohn’s disease, samples were taken from macroscopically inflamed and not inflamed mucosa, if possible. The diagnosis was based on standard criteria using radiological and endoscopic findings in every case.25 Patients were treated mainly with aminosalicyates, corticosteroids, or azathioprine according to their clinical requirements without any systematic differences between patients with NOD2 mutated or wild-type genotypes. Patient characteristics are shown in table 1 ▶. All patients gave written informed consent before colonoscopy was performed. Samples were immediately snap frozen in liquid nitrogen. The study was approved by the local ethics committee.
Table 1.
Characteristics of Crohn’s disease patients with the NOD2 wild-type genotype or NOD2 mutations, and controls. In the case of non-inflamed controls, colonoscopy was performed for polyps, anaemia, post surgical follow up, or tumour
| Crohn’s disease wild-type | Crohn’s disease NOD2 mutation | Controls | |
| n | 44 | 24 (SNP13: 15, SNP8: 4, SNP12: 4) | 12 |
| Age (y) (median (range)) | 32 (17–67) | 41 (16–70) | 30 (20–65) |
| Disease duration (y) (median (range)) | 7 (0–36) | 10 (0–41) | |
| Ileal involvement | 58% | 88% | |
| Three most common treatments | |||
| Steroids | 42% | 44% | |
| 5-ASA | 57% | 66% | |
| Azathioprine | 28% | 22% |
Ribonucleic acid preparation and reverse transcription
Frozen biopsies were disrupted mechanically and total RNA was isolated using TRIzol reagent (Invitrogen, San Diego, California, USA) according to the supplier’s protocol. RNA quality and quantity were determined by gel electrophoresis and photometry. Subsequently, 1 μg of total RNA was reverse transcribed with oligo dT primers and 200 U of Superscript (Invitrogen) according to routine procedures. These RNA preparations from each biopsy were used for assay of all defensins and cytokines tested.
Mutation analysis
Genotyping of genomic and/or cDNA for the functionally relevant NOD2 mutations (SNP8, SNP12, and SNP13) was performed using TaqMan technology (Applied Biosystems, Foster City, California, USA), as described previously.26 In brief, amplification reactions (25 µl) were carried out with 20 ng of template DNA, 1× TaqMan Universal Master Mix buffer (Applied Biosystems), 900 nM of each primer, and 200 nM of each fluorogenic probe. Detection of fluorescence signals was performed using the ABI PRISM 7700 detection system and the results were analysed as described previously by use of the Sequence Detection System (SDS) Software Version 1.7 (Applied Biosystems).
Real time polymerase chain reaction
We studied 24 patients displaying a NOD2 mutation, a demographically comparable Crohn’s disease group of 21 patients randomly selected from those with a NOD2 wild-type genotype, and 12 non-IBD patients without significant pathology serving as controls. After isolation of mucosal RNA, real time reverse transcription-polymerase chain reaction (RT-PCR) was performed with specific primer pairs for HD-5, HD-6, lysozyme, secretory phospholipase A2 (sPLA2), proinflammatory cytokines tumour necrosis factor α (TNF-α) and interleukin 8 (IL-8), as well as human hypoxanthine phosphoribosyltransferase (HPRT) as a control, in a fluorescence temperature cycler (LightCycler, Roche Diagnostics GMBH, Mannheim, Germany) and normalised to the amount of total RNA, as recommended27 and described previously.22
This technique continuously monitors cycle by cycle accumulation of fluorescently labelled PCR product. Briefly, cDNA corresponding to 20 ng of RNA served as a template in a 20 µl reaction containing 4 mM MgCl2, 0.5 µM of each primer, and 1× LightCycler-FastStart DNA Master SYBR Green I mix (Roche Diagnostics). Samples were loaded into capillary tubes and incubated in the fluorescence thermocycler (LightCycler) for initial denaturation at 95°C for 10 minutes followed by 45 cycles, each cycle consisting of 95°C for 15 seconds, “touchdown” of −1°C/cycle from the primer specific start to end annealing temperature (table 2 ▶) for five seconds, and 72°C for 10 seconds. At the end of each run melting curve profiles were produced by cooling the sample to 65°C for 15 seconds and then heating slowly at 0.20°C/s up to 95°C with continuous measurement of fluorescence to confirm amplification of specific transcripts. Cycle to cycle fluorescence emission readings were monitored and analysed using LightCycler Software (Roche Diagnostics). Lysozyme and sPLA2 control plasmids were used for standard curves. Lysozyme primers were used as described previously.23 Specificity of the amplification products was further verified by subjecting the amplification products to electrophoresis on a 2% agarose gel. Fragments were visualised by ethidium bromide staining and the specificity of PCR products was verified by sequencing of representative samples. Standard curves were obtained for each primer set with serial dilutions of DNA. Primer sequences and specific temperatures were used as shown in table 2 ▶.
Table 2.
Primer sequences and temperatures for touchdown protocols
| Sense | Antisense | T | 2° target T | Step | |
| TNF-α | 5′TCT GGC CCA GGC AGT CAG ATC 3′ | 5′TCA GCT TGA GGG TTT GCT ACA A 3′ | 65°C | 59°C | 1°C |
| IL-8 | 5′ATG ACT TCC AAG CTG GCC GTG GC 3′ | 5′TCT CAG CCC TCT TCA AAA ACT TC 3′ | 66°C | 60°C | 1°C |
| HD-5 | 5′ GCC ATC CTT GCT GCC ATT C 3′ | 5′ AGA TTT CAC ACA CCC CGG AGA 3′ | 66°C | 60°C | 1°C |
| HD-6 | 5′ CCT CAC CAT CCT CAC TGC TGT TC 3′ | 5′ CCA TGA CAG TGC AGG TCC CAT A 3′ | 66°C | 60°C | 1°C |
| Lysozyme | 5′AAA ACC CCA GGA GCA GTT AAT 3′ | 5′CAA CCC TCT TTG CAC AAG CT 3′ | 60°C | ||
| sPLA2 | 5′TGA CGA CAG GAA AGG AAG CCG CAC 3′ | 5′AGG GAA GAG GGG ACT CAG CAA CGA G 3′ | 60°C | ||
| HPRT | 5′ATC AGA CTG AAG AGC TAT TGT AAT GAC CA 3′ | 5′ TGG CTT ATA TCC AAC ACT TCG TG 3′ | 66°C | 60°C | 1°C |
T, temperature; TNF-α, tumour necrosis factor α; IL-8, interleukin 8; HD-5, human defensin 5; HD-6, human defensin 6; sPLA2, secretory phospholipase A2; HPRT, human hypoxanthine phosphoribosyltransferase.
Immunohistochemistry
Immunohistochemistry on colonic as well as ileal biopsies was performed with anti-HD-5 from 10 patients with Crohn’s disease (SNP8, 12, and 13 wild-type, n = 5; SNP13 heterozygotic, n = 4; SNP13 homozygotic, n = 1). The polyclonal anti-HD-5 was donated as a gift from T Ganz and E Porter (UCLA, USA) and immunohistochemistry was performed as described previously.18
Paneth cell staining
In order to identify Paneth cells, we used phloxine tartrazine histological staining, as described previously.16 HD-5 immunohistochemistry and histological staining were performed in parallel sections from all patients with available tissue.
Statistics
Mean expression values were obtained from duplicate or triplicate real time PCR measurements. Mean (SEM) values are presented. The U test of Wilcoxon, Mann-Whitney was used for statistical comparison of grouped data.
RESULTS
NOD2 mutation analyses
Fifteen of 68 patients were positive for SNP13 (12 heterozygotes and three homozygotes). Five were positive for SNP8 (four heterozygotes, one homozygote) and four patients displayed a mutation for SNP12 (heterozygotes only). Two of the 12 SNP13 heterozygote patients also displayed a SNP12 mutation. Three patients with a SNP13 mutation were also positive for SNP8 (compound heterozygote).
Gene expression of HD-5 and HD-6 was decreased in Crohn’s disease patients carrying a NOD2 mutation
Ileal expression
Of the Crohn’s disease patients with a NOD2 mutation, only one had a normal unaffected ileum. The group with ileal disease had diminished HD-5 expression compared with either controls or those with non-ileal Crohn’s disease (fig 1A ▶). The level of expression was lowest in those with both the mutation and ileal disease. The expression pattern of HD6 was comparable (fig 1B ▶) but did not attain statistical significance. The group with ileal disease and NOD2 mutation had diminished HD-6 expression compared with either controls or those with non-ileal Crohn’s disease. One patient with a NOD2 mutation and a normal ileum displayed low HD-5 and HD-6 expression.
Figure 1.
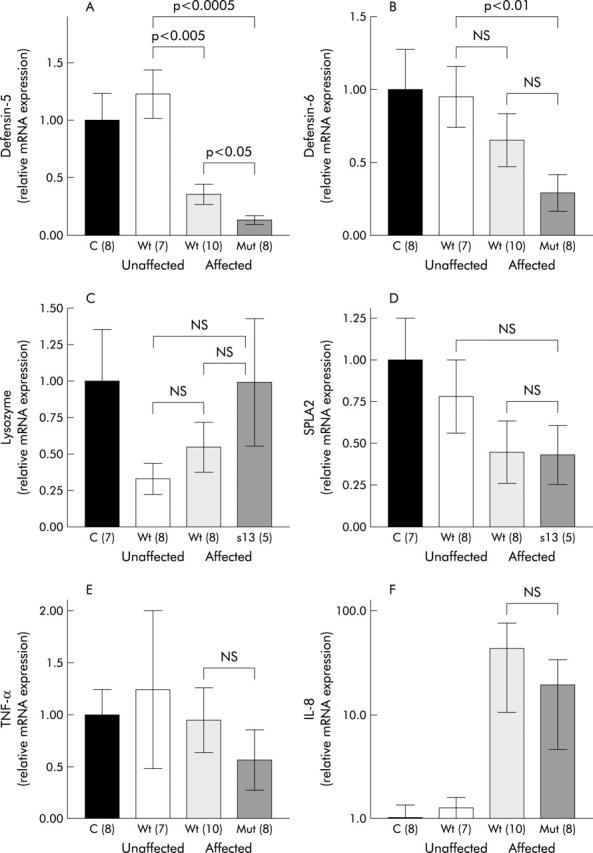
Ileal expression of Paneth cell defensins (A, B), lysozyme (C), secretory phospholipase A2 (SPLA2) (D), and the proinflammatory cytokines tumour necrosis factor α (TNF-α) (E) and interleukin 8 (IL-8) (F) with respect to involvement in Crohn’s disease and NOD2 genotype. Real time polymerase chain reaction was performed in controls and Crohn’s disease patients showing means of relative transcription levels. Values are mean (SEM). NS, not significant; C, controls; Wt, NOD2 wild-type; Mut, NOD2 mutation (number of patients).
Compared with HD-5 and HD-6, expression of other Paneth cell products was not diminished by NOD2 mutations. Even though the difference failed to achieve statistical significance, lysozyme showed a tendency towards higher expression in NOD2 mutated patients (fig 1C ▶). NOD2 mutated patients with ileal disease showed the highest levels of lysozyme (fig 1C ▶). Ileal expression of sPLA2 was not influenced by NOD2 genotype. In common with HD-5 and HD-6, there was a tendency towards weaker expression in patients with ileal disease but this difference did not reach statistical significance (fig 1D ▶).
Colonic expression
In non-inflamed colonic tissue, no difference was detected in expression of HD-5 or HD-6 in Crohn’s disease patients compared with controls. As expected, compared with ileal expression of Paneth cell defensins, expression levels in the colon were much lower. In samples with colonic inflammation, HD-5 was increased threefold in Crohn’s disease with the NOD2 wild-type genotype. However, Crohn’s disease patients with NOD2 mutations failed to show increased HD-5 or HD-6 expression in inflamed colonic tissue (fig 2A ▶, B). Moreover, analysis of inflamed specimens showed that levels of HD-5 and HD-6 expression were lower in NOD2 mutated compared with wild-type Crohn’s disease patients (fig 2A ▶, B). In both HD-5 and HD-6, the difference between wild-type NOD2 genotype and SNP13 mutated patients was even more pronounced (insert in fig 2A ▶, B).
Figure 2.
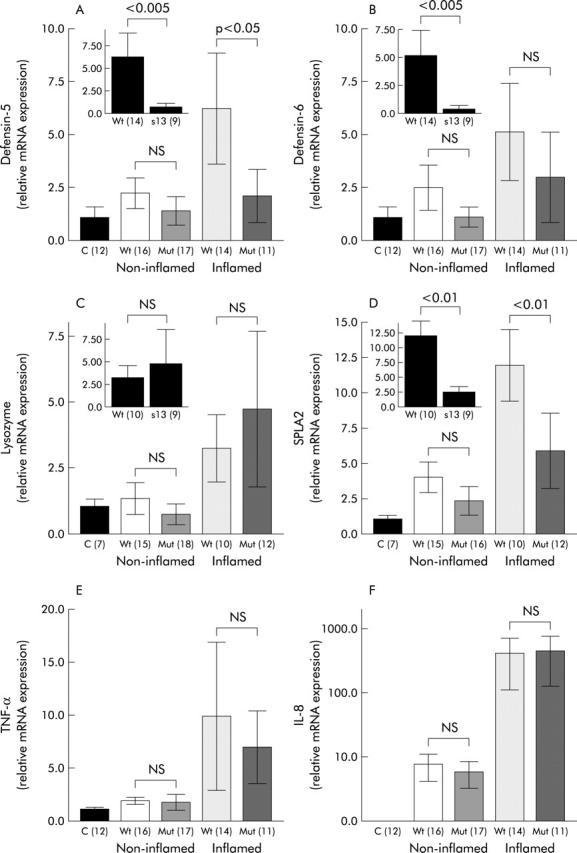
Colonic expression of Paneth cell defensins (A, B), lysozyme (C), secretory phospholipase A2 (SPLA2) (D), and the proinflammatory cytokines tumour necrosis factor α (TNF-α) (E) and interleukin 8 (IL-8) (F) with respect to NOD2 genotype and presence of macroscopic inflammation. Expression of HD-5, HD-6, lysozyme, and SPLA2 is shown separately for SNP13 mutations as inserts in cases of inflamed specimens. Real time polymerase chain reaction was performed in controls and Crohn’s disease patients showing means of relative transcription levels. Values are mean (SEM). NS, not significant; C, controls; Wt, NOD2 wild-type; Mut, NOD2 mutation (number of patients).
Similar to observations in the ileum, lysozyme showed higher expression in the colon during inflammation (fig 2C ▶). In comparison with control patients, expression of lysozyme was not altered in non-inflamed colonic tissue from Crohn’s disease patients. During inflammation, expression of lysozyme was higher but this increase was not statistically significant (fig 2C ▶). The NOD2 genotype did not influence expression of lysozyme.
Similar to other products in non-inflamed colonic tissue, no difference was detected in expression of sPLA2 in Crohn’s disease patients compared with controls. In samples with colonic inflammation, sPLA2 was increased in Crohn’s disease with the wild-type NOD2 genotype (fig 2D ▶). However, Crohn’s disease patients with NOD2 mutations failed to show increased sPLA2 expression in inflamed colonic tissue.
Ileal and colonic gene expression of TNF-α and IL-8 were not affected by NOD2 mutations
In contrast with HD-5 and HD-6, expression of the proinflammatory cytokines TNF-α and IL-8 were not significantly influenced by NOD2 genotype (fig 1E, 1F, 2E, 2F ▶ ▶ ▶ ▶). A weak non-significant decrease in TNF-α and IL-8 gene expression in Crohn’s disease patients carrying a NOD2 mutation could be detected in ileal biopsies (fig 1E, 1F ▶ ▶).
Normal number of ileal Paneth cells in patients carrying NOD2 mutations
Staining with phloxine tartrazine revealed a normal number of Paneth cells in ileal biopsies from four wild-type patients compared with three patients with a NOD2 mutation. Staining for a control and the SNP13 homozygote is shown in fig 3 ▶. A heterozygote patient exhibited metaplastic Paneth cells in the colon, similar in number to five NOD2 wild-type patients. However, the number of colonic biopsies with Paneth cell staining was too limited for a quantitative evaluation. Essentially, although with the same limitations, the number of HD-5 positive cells was comparable between the groups. In parallel sections, Paneth cells also stained positive for HD-5, even in the homozygous patient, but the technique excludes quantitation of staining intensity (fig 3 ▶).
Figure 3.
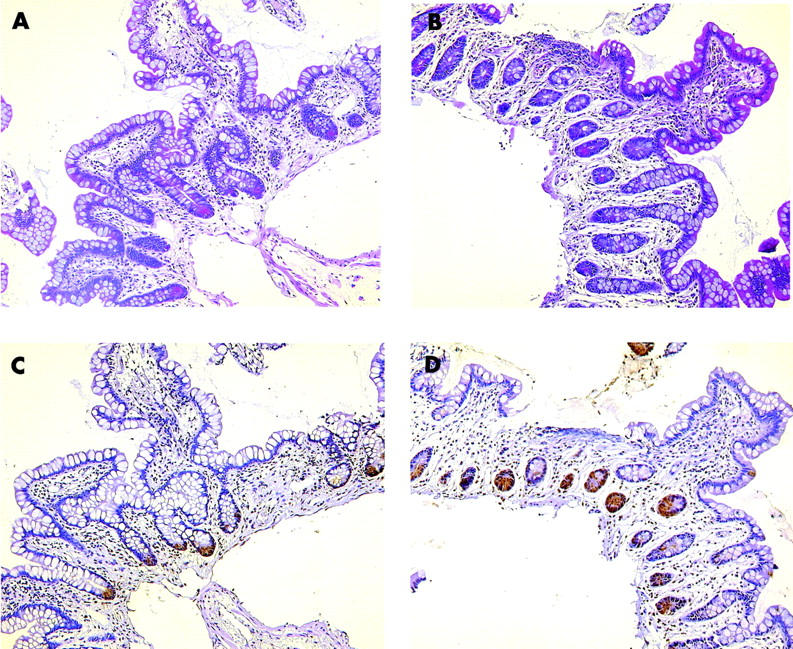
Paneth cell staining with phloxine tartrazine in a Crohn’s disease wild-type genotype patient (A) as well as a NOD2 SNP13 mutation homozygotic patient (B). Qualitative immunohistochemistry with anti-human defensin 5 is shown in a parallel tissue section for the wild-type (C) as well as the mutated patient (D).
DISCUSSION
Crohn’s disease is a complex multifactorial disease whose pathogenesis is still not well understood. Recent research has focused on the role of luminal bacteria and their essential role in pathophysiology. A major advance for better understanding the disease is the discovery of mutations in the NOD2 gene in approximately one third of Crohn’s disease patients. NOD2 is a putative receptor of bacterial peptidoglycan which is also expressed by Paneth cells and crypt epithelial cells.7 Paneth cells are secretory cells expressing α defensins HD-5 and HD-6. These antibiotic peptides are predominantly expressed in the small intestine and by metaplastic Paneth cells during inflammation in the colon.17,18 The findings reported herein for the first time link a mutation in NOD2 to reduced α defensin expression in Crohn’s disease.
Mutations in the NOD2 gene are associated with ileal involvement in Crohn’s disease.24 As NOD27 and α-defensins are coexpressed in ileal Paneth cells, their relative deficiency in Crohn’s disease may be related to ileal preference, especially in patients with NOD2 mutations. Although the deficit in ileal α-defensins is particularly pronounced in NOD2 mutated patients, HD-5 is also reduced in NOD2 wild-type patients with ileal disease. This is compatible with the concept that other signalling peptides may be affected in NOD2 wild-type patients. Diminished expression of α defensins, especially in patients with the NOD2 mutation, contrasts with the comparably enhanced levels of ileal mucosal TNF and IL-8 in both the NOD2 wild-type and mutated group with ileal involvement. Previous studies showed that Paneth cell populations as well as the secretory granule area are preserved8 or even increased in ileal Crohn’s disease,28,29 suggesting that Paneth cell function rather than number may be compromised in this disease. In the colonic mucosa, expression of HD-5 and HD-6 also seem to be regulated or influenced by NOD2 because lack of function mutations, especially SNP13, lead to diminished induction during inflammation. In contrast with diminished HD-5 and HD6 levels in the ileum of patients with a NOD2 genotype, enhanced levels of lysozyme and unchanged levels of sPLA2 in these samples suggests a selective influence on HD-5 and HD-6 expression by NOD2. However, in the colon, the observation that sPLA2 levels are influenced by NOD2 status is compatible with a role for this receptor in Paneth cell function or number. Possibly, the higher levels of lysozyme are produced by inflammatory cells in this tissue and unaffected by NOD2 mutation status.
Thus stratification of the Crohn’s disease cohort according to NOD2 status confirms the induction of α defensins reported previously,17,18 and induction of sPLA230 only for those with intact NOD2 (that is, the majority of the patients).
In vitro data identify NOD2 as a putative intracellular peptidoglycan receptor molecule.9 We now suggest that it may be involved in the signalling cascade mediating α defensin expression. In both cases, the defensin deficiency secondary to NOD2 mutations could lead to a breakdown of the mucosal barrier with a secondary inflammation. Recently, it has become apparent that the mucosal immune response in inflammatory bowel disease appears to be directed towards a multitude of common luminal bacteria. The most convincing evidence for a break in mucosal tolerance in intestinal inflammation stems from the observation that knockout mice lacking several relevant genes, including interleukins 2 or 10, develop experimental colitis only when raised in conventional housing, but not under germ free conditions.31 This fits well with the consistent finding of a break in mucosal tolerance towards various luminal bacteria in inflammatory bowel diseases.32,33 The permeable mucosal barrier may also explain the development of anti-Saccharomyces cerevisiae antibodies, especially in familial Crohn’s disease,34 as well as the presence of pathogenic bacteria.35 A more relevant finding in this regard is the presence of adherent Escherichia coli in the terminal ileum of Crohn’s disease patients.36 Similarly, Swidsinski et al demonstrated that the diseased epithelium may display attaching and even invading bacteria whereas normal mucosa handled under the same conditions is virtually sterile.37 These findings are difficult to reconcile with an immunological dysregulation as the sole basis of intestinal inflammation in these diseases. Rather, there may be a primary defect in the chemical defensin barrier, possibly involving other intestinal antimicrobial peptides which protect the normal mucosa extremely efficiently against adherent or entering microbes. It is also possible that a change in expression or function of this chemical defence may in part explain the changes in bacterial flora in inflammatory bowel diseases, as reviewed by Linskens and colleagues.38
As some specific bacteria may downregulate defensin expression39 it cannot be definitely excluded that diminished defensins may be due to a specific bacterial flora associated with Crohn’s disease.36 However, this possibility would be less attractive to reconcile the difference between NOD2 mutation and wild-type Crohn’s disease patients. The observation that HD-5 and HD-6 are unchanged during pouchitis (Kiehne et al abstract DDW 2002, Fellermann et al submitted for publication) strongly argues against speculation that downregulation of HD-5 and HD-6 accompanies inflammation alone. Although the functional relevance of defensins has been shown in mice,20,40 it still has to be proven in Crohn’s disease. The fact that ileal and colonic expression of proinflammatory cytokines are not influenced by NOD2 mutations further supports the specificity of the partial lack of certain defensins. Therefore, these data support the hypothesis that Crohn’s disease may be a complex defensin deficiency syndrome.41
Acknowledgments
This work was supported by the Robert Bosch Foundation, Stuttgart, Germany. We thank Dr N Homann for data collection as well as Drs T Ganz and E Porter for supplying human defensin-5 antibody. We also thank P Miller, K Siegel, D Weller, D Biegger, as well as R Feathers for excellent technical assistance.
Abbreviations
HD-5, human defensin 5
HD-6, human defensin 6
TNF-α, tumour necrosis factor α
HPRT, human hypoxanthine phosphoribosyltransferase
RT-PCR, reverse transcription-polymerase chain reaction
sPLA2, secretory phospholipase A2
IL-8, interleukin 8
REFERENCES
- 1.Hugot J-P, Chamaillard C, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001;411:599–603. [DOI] [PubMed] [Google Scholar]
- 2.Ogura Y , Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s diease. Nature 2001;411:603–6. [DOI] [PubMed] [Google Scholar]
- 3.Hampe J , Cuthbert A, Croucher PJ, et al. Association between insertion mutation in NOD2 gene and Crohn’s disease in German and British populations. Lancet 2001;357:1925–8. [DOI] [PubMed] [Google Scholar]
- 4.Ogura Y , Inohara N, Benito A, et al. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem 2001;276:4812–18. [DOI] [PubMed] [Google Scholar]
- 5.Bonen DK, Cho JH. The genetics of inflammatory bowel disease. Gastroenterology 2003;124:521–36. [DOI] [PubMed] [Google Scholar]
- 6.Berrebi D , Maudinas R, Hugot JP, et al. Card15 gene overexpression in mononuclear and epithelial cells of the inflamed Crohn’s disease colon. Gut 2003;52:840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenstiel P , Fantini M, Brautigam K, et al. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology 2003;124:1001–9. [DOI] [PubMed] [Google Scholar]
- 8.Lala S , Ogura Y, Osborne C, et al. Crohn’s disease and the NOD2 gene: a role for paneth cells. Gastroenterology 2003;125:47–57. [DOI] [PubMed] [Google Scholar]
- 9.Hisamatsu T , Suzuki M, Reinecker HC, et al. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology 2003;124:993–1000. [DOI] [PubMed] [Google Scholar]
- 10.Schroder JM. Epithelial antimicrobial peptides: innate local host response elements. Cell Mol Life Sci 1999;56:32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganz T , Lehrer RI. Defensins. Curr Opin Immunol 1994;6:584–9. [DOI] [PubMed] [Google Scholar]
- 12.Fellermann K , Stange EF. Defensins—Innate immunity at the epithelial fontier. Eur J Gastroenterol Hepatol 2001;13:771–6. [DOI] [PubMed] [Google Scholar]
- 13.Lehrer RI, Barton A, Daher KA, et al. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J Clin Invest 1989;84:553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kagan BL, Selsted ME, Ganz T, et al. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci U S A 1990;87:210–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chertov O , Michiel DF, Xu L, et al. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem 1996;271:2935–40. [DOI] [PubMed] [Google Scholar]
- 16.Jones DE, Bevins CL. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem 1992;267:23216–25. [PubMed] [Google Scholar]
- 17.Cunliffe RN, Rose FRAJ, Keyte J, et al. Human defensin 5 is stored in precursor form in normal Paneth cells and is expressed by some villous epithelial cells and by metaplastic Paneth cells in the colon in inflammatory bowel disease. Gut 2001;48:176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wehkamp J , Schwind B, Herrlinger KR, et al. Innate immunity and colonic inflammation: enhanced expression of epithelial alpha-defensins. Dig Dis Sci 2002;47:1349–55. [DOI] [PubMed] [Google Scholar]
- 19.Lawrance IC, Fiocchi C, Chakravarti S. Ulcerative colitis and Crohn’s disease: distinctive gene expression profiles and novel susceptibility candidate genes. Hum Mol Genet 2001;10:445–56. [DOI] [PubMed] [Google Scholar]
- 20.Wilson CL, Ouellette AJ, Satchell DP, et al. Regulation of intestinal α-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 1999;286:113–17. [DOI] [PubMed] [Google Scholar]
- 21.Wehkamp J , Fellermann K, Herrlinger KR, et al. Human beta-defensin 2 but not beta-defensin 1 is expressed preferentially in colonic mucosa of inflammatory bowel disease. Eur J Gastroenterol Hepatol 2002;14:745–52. [DOI] [PubMed] [Google Scholar]
- 22.Wehkamp J , Harder J, Weichenthal M, et al. Inducible and constitutive beta-defensins are differentially expressed in Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis 2003;9:215–23. [DOI] [PubMed] [Google Scholar]
- 23.Fahlgren A , Hammarstrom S, Danielsson A, et al. Increased expression of antimicrobial peptides and lysozyme in colonic epithelial cells of patients with ulcerative colitis. Clin Exp Immunol 2003;131:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuthbert A , Fisher S, Mirza M, et al. The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease. Gastroenterology 2002;122:867–74. [DOI] [PubMed] [Google Scholar]
- 25.Kornbluth A , Sachar DB, Salomon P. Crohn’s disease. In: Feldman M, Scharschmidt BF, Sleisenger MH, eds. Sleisenger and Fordtran’s gastrointestinal and liver disease: Pathophysiology/diagnosis/management. Philadelphia: WB Saunders, 1998:1708–34.348557
- 26.Schwab M , Schaeffeler E, Marx C, et al. Association between the C3435T MDR1 gene polymorphism and susceptibility for ulcerative colitis. Gastroenterology 2003;124:26–33. [DOI] [PubMed] [Google Scholar]
- 27.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 2000;25:169–93. [DOI] [PubMed] [Google Scholar]
- 28.Dvorak AM, Dickersin GR. Crohn’s disease: transmission electron microscopic studies. I. Barrier function. Possible changes related to alterations of cell coat, mucous coat, epithelial cells, and Paneth cells. Hum Pathol 1980;11 (suppl 5) :561–71. [PubMed] [Google Scholar]
- 29.Elmes ME, Jones JG, Stanton MR. Changes in the Paneth cell population of human small intestine assessed by image analysis of the secretory granule area. J Clin Pathol 1983;36:867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minami T , Shinomura Y, Miyagawa J, et al. Immunohistochemical localization of group II phospholipase A2 in colonic mucosa of patients with inflammatory bowel disease. Am J Gastroenterol 1997;92:289–92. [PubMed] [Google Scholar]
- 31.Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun 1998;66:5224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duchmann R , May E, Heike M, et al. T cell specifity and cross reactivity towards enterobacteria, Bacteroides, Bifidobacterium, and antigens from resident intestinal flora in humans. Gut 1999;44:812–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutton CL, Kim J, Yamane A, et al. Identification of a novel bacterial sequence associated with Crohn disease. Gastroenterology 2000;119:23–31. [DOI] [PubMed] [Google Scholar]
- 34.Annese V , Andreoli A, Andriulli A, et al. Familial expression of anti-Saccharomyces cerevisiae Mannan antibodies in Crohn’s disease and ulcerative colitis: a GISC study. Am J Gastroenterol 2001;96:2407–12. [DOI] [PubMed] [Google Scholar]
- 35.Lisby G , Andersen J, Engbaek K, et al. Mycobacterium paratuberculosis in intestinal tissue from patients with Crohn’s disease demonstrated by a nested primer polymerase chain reaction. Scand J Gastroenterol 1994;29:923–9. [DOI] [PubMed] [Google Scholar]
- 36.Darfeuille-Michaud A , Neut C, Barnich N, et al. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn’s disease. Gastroenterology 1998;115:1405–13. [DOI] [PubMed] [Google Scholar]
- 37.Swidsinski A , Ladhoff A, Pernthaler A, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology 2002;122:44–54. [DOI] [PubMed] [Google Scholar]
- 38.Linskens RK, Huijsdens XW, Savelkoul PH, et al. The bacterial flora in inflammatory bowel disease: current insights in pathogenesis and the influence of antibiotics and probiotics. Scand J Gastroenterol Suppl 2001;234:29–40. [DOI] [PubMed] [Google Scholar]
- 39.Salzman NH, Chou MM, de Jong H, et al. Enteric salmonella infection inhibits Paneth cell antimicrobial peptide expression. Infect Immun 2003;71:1109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salzman NH, Ghosh D, Huttner KM, et al. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 2003;422:522–6. [DOI] [PubMed] [Google Scholar]
- 41.Fellermann K , Wehkamp J, Herrlinger KR, et al. Crohn’s disease: a defensin deficiency syndrome? Eur J Gastroenterol Hepatol 2003;15:627–34. [DOI] [PubMed] [Google Scholar]


