Abstract
Background: Cyclooxygenase 2 (COX-2) and matrix metalloproteinases (MMPs) have been implicated in tissue injury and fibrogenesis in animal models but little is known regarding their role in hepatitis C virus (HCV) related liver disease in humans.
Aims: To characterise the intrahepatic expression pattern of COX-2 and MMPs in chronic HCV infection and determine whether HCV core and NS5A proteins could promote their expression in cultured hepatocyte derived cell lines.
Patients: Thirty two anti-HCV+ and 10 anti-HCV− patients were studied.
Methods: Western blot, reverse transcription-polymerase chain reaction (RT-PCR), enzyme immunoassay, and immunohistochemistry were used to assess the expression pattern of COX-2 and MMPs in liver biopsy samples from all patients. COX-2 gene expression and MMP-9 protein levels were also determined by immunoblot, RT-PCR, and luciferase assays in core and NS5A transfected hepatocyte derived cells.
Results: The intrahepatic expression level of COX-2, MMP-2, and MMP-9 was significantly higher in HCV+ than in HCV− patients, increasing with the fibrotic stage of liver disease. We further demonstrated that COX-2 mRNA, protein, and activity were induced in resting and activated core and NS5A transfectants. Both viral proteins induced transcriptional activity of the COX-2 gene promoter whereas core, but not NS5A, exerted an inducer effect on MMP-9 protein levels in cultured hepatocyte derived cells.
Conclusions: Intrahepatic COX-2, MMP-2, and MMP-9 overexpression is associated with progressive hepatic fibrosis in chronic HCV infection, suggesting their pathogenic role in fibrogenesis. HCV core and NS5A proteins were able to upregulate COX-2 and MMP-9 gene expression in hepatocyte derived cells, providing a potential mechanism for hepatic fibrosis during chronic HCV infection.
Keywords: cyclooxygenase 2, cirrhosis, hepatitis C virus, matrix metalloproteinases, prostaglandins
Cyclooxygenases catalyse the first step in the biosynthesis of various prostaglandins (PG) and thromboxanes.1,2 There are two cyclooxygenase (COX) isoforms: COX-1 is constitutively expressed in a number of cell types and is involved in the homeostatic functions of PG whereas COX-2 is induced by a variety of proinflammatory stimuli, such as cytokines and lipopolysaccharide (LPS).3–5 COX-2 has been implicated in inflammation, fibrogenesis, and carcinogenesis.6–8 In this regard, different authors have reported that increased COX-2 expression and PG production may contribute to liver damage and tumorigenesis in distinct animal models9–11 as well as to fibrosis and hepatocellular carcinoma (HCC) in humans.12,13 Moreover, in vitro pharmacological inhibition of COX-2 has been shown to be effective in modulating hepatic stellate cell activation14 and inhibiting proliferation of human hepatoma cells,15 suggesting a potential therapeutic role for this strategy in chronic liver diseases.
Hepatitis C virus (HCV) infection is the most frequent cause of chronic liver disease in Western countries16 and 20–30% of these patients will develop cirrhosis with the risk of HCC.17 As HCC occurs mainly in patients with cirrhosis,18 a common pathogenic basis at the molecular level between hepatic fibrogenesis and carcinogenesis must exist. A potential link may be activation of the proteolytic enzymes termed matrix metalloproteinases (MMPs) which play a central role in extracellular matrix remodelling and are involved in a variety of pathophysiological processes, such as fibrogenesis and carcinogenesis.19 The clinical relevance of these MMPs, in particular MMP-2 and MMP-9, has been recently highlighted by the fact that increased hepatic gelatinase levels were associated with the fibrotic index in patients with HCV induced liver disease and with tumour progression and recurrence in HCC patients.20,21 In this context, we have recently shown that COX-2 expression promotes the release of MMP-2 and MMP-9 in rat fetal hepatocytes,22 suggesting that COX-2 may play a pivotal role in the secretion of active MMPs by liver cells.
The aims of this work were to determine the expression pattern of COX-2 and MMPs in liver tissue from patients with HCV induced chronic liver disease, and to assess whether HCV proteins induce COX-2 and MMP-9 expression in cultured human hepatocyte derived cells.
METHODS
Patient characteristics
The study included 32 patients with biochemical, virological, and histological findings compatible, according to internationally accepted criteria,23 with HCV related chronic liver disease (27 with chronic hepatitis and five with end stage cirrhosis). Baseline characteristics of HCV patients are shown in table 1 ▶. In addition, six non-alcoholic patients with isolated steatosis and four patients with histologically normal liver were also studied and used as controls (CT). These CT subjects were seronegative for antibodies to HCV, and all HCV+ and HCV− patients included in this study were seronegative for hepatitis B surface antigen (HBsAg) and for antibodies to human immunodeficiency virus (HIV). Written informed consent was obtained from each patient, and approval for the study was granted by the institutional ethics committee.
Table 1.
Baseline characteristics of hepatitis C virus patients studied
| MCH | SCH | CIR | |
| No of patients | 22 | 5 | 5 |
| Age (y) | 43.8 (8.3) | 43 (10.8) | 55.3 (4.7) |
| Sex (M/F) | 16/6 | 5/0 | 4/1 |
| AST (U/l) | 43.2 (19.3) | 77.6 (28.6) | 87.6 (53.5) |
| ALT (U/l) | 83.3 (40.9) | 140 (48.3) | 87.5 (56.6) |
| Serum bilirubin (mg/dl) | 0.5 (0.3) | 0.7 (0.2) | 2.2 (0.7) |
| Serum albumin (mg/dl) | 4.1 (0.3) | 4.4 (0.3) | 2.8 (0.2) |
| INR | 0.99 (0.1) | 1.05 (0.05) | 1.5 (0.2) |
| Viral genotype (1/non-1) | 16/6 | 3/2 | 3/2 |
| Viral load (high/low) | 11/11 | 3/2 | 2/3 |
Values are mean (SEM) or number.
HCV, hepatitis C virus; MCH, mild chronic hepatitis; SCH, severe chronic hepatitis; CIR, cirrhosis; INR, International normalised ratio; low viral load, ⩽800 000 IU/ml; high viral load, >800 000 IU/ml.
Viral markers
HBsAg and antibodies to HIV were determined by commercially available enzyme immunoassay kits (Abbott Laboratories, North Chicago, Illinois, USA). Antibodies to HCV were detected using a commercially available second generation enzyme linked immunoassay kit (Ortho Diagnostic Systems, Raritan, New Jersey, USA). HCV RNA was detected by a nested polymerase chain reaction technique using primers from the highly conserved 5′ non-coding region of the HCV genome, as described elsewhere.24
Chemicals
LPS from Salmonella typhimurium and cytokines (tumour necrosis factor α (TNF-α) and interleukin (IL)-1β) were from Sigma Chemical Co. (St Louis, Missouri, USA) and Roche Diagnostics (Mannheim, Germany). Antibodies were obtained from Santa Cruz Laboratories (Santa Cruz, California, USA) and Cayman Chemical (Ann Arbor, Michigan, USA). DFU [(5, 5-dimethyl-3(3-fluorophenyl)-4-(4-methyl sulphonyl)phenyl-2(5H)-furanone)] is a highly selective COX-2 inhibitor25,26 from Merck (Rahway, New York, USA). Reagents for electrophoresis were obtained from Bio-Rad (Hercules, California, USA). Tissue culture dishes were from Falcon (Lincoln Park, New Jersey, USA). Tissue culture media were from BioWhittaker (Walkersville, Maryland, USA).
Liver tissue studies
Liver histology
Liver biopsy specimens were evaluated by a single pathologist, recording the conventional histological diagnoses, and in biopsy samples from patients with HCV+ chronic hepatitis, the stage of fibrosis was scored on a scale of 0–4 using the histological classification proposed by Scheuer.27 Accordingly, we classified chronic hepatitis as mild or severe, defining mild chronic hepatitis (MCH) when the stage score was ⩽2 (22 cases) and severe (SCH) when >2 (five cases). All cases of explanted HCV+ livers had histologically proven cirrhosis (five cases).
Protein extraction and western blot analysis
Liver biopsy samples from 32 HCV+ patients (22 MCH, five SCH, and five cirrhosis) and from 10 HCV− patients (six non-alcoholic steatosis and four normal livers) were homogenised in a medium containing: 10 mmol/l Tris-HCl, pH 7.5; 1 mmol/l MgCl2, 1 mmol/l EGTA, 10% glycerol, 0.5% 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulphonate, 1 mmol/l β-mercaptoethanol, and 0.1 mmol/l PMSF. Extracts were vortexed for 30 minutes at 4°C, and after centrifuging for 20 minutes at 13 000 g the supernatants were stored at −20°C.
For western blot analysis, total proteins were boiled in Laemmli sample buffer and equal amounts of protein (20–30 μg) were loaded onto a 10–12% sodium dodecyl sulphate polyacrylamide gel electrophoresis. Equal amounts of protein were size fractionated in 10–12% acrylamide gel, transferred to a polyvinylidene difluoride membrane, and after blocking with 5% non-fat dry milk, incubated with the corresponding antibodies anti-COX-2, anti-MMP-2, anti-MMP-9 (Cayman Chemical), and anti-actin (Sigma Chemical Co.) at 1:1000. Blots were revealed after incubation with the horseradish peroxidase conjugate and were normalised by measuring the amount of β-actin. Densitometric analysis of the bands was performed using a scanner, and expressed in arbitrary units.
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA of liver biopsy samples from all HCV+ and HCV− patients included in the study was extracted by the guanidinium isothiocyanate method using TRIzol reagent.28 RNA (1 μg) was reverse transcribed using oligo (dT)16 sequence as primer and 50 U of expand reverse transcriptase (Roche Diagnostics, Mannheim, Germany). PCR amplification of the reverse transcribed RNA was carried out with 1 unit of DNA polymerase in a 50 μl reaction mixture containing 200 μM of each dNTPs, 0.3 μM of specific primers, and 1× reaction buffer using a DNA Thermal Cycler. Cycling conditions were three minutes at 94°C, 20 seconds at 94°C, 20 seconds at 58°C, 30 seconds at 72°C, 35 cycles, and a final elongation for five minutes at 72°C. Amplified DNA fragments were analysed by electrophoresis in a 1.5% agarose gel. Quantification of the bands was performed by laser densitometry after normalisation with 18S ribosomal RNA.
The primers specific for COX-2 were based on published sequences.29 The primers specific for MMP-9 were 5′-CCA GCA TCT GTA TGG TCG TG-3′ (sense) and 5′-CAG AAG GAC CAG CAG TAG GG-3′ (antisense) and for 18S rRNA were 5′-GCA ATT ATT CCC CAT GAA CGA (sense) and 5′-CAA AGG GCA GGG ACT TAA TCA A-3′ (antisense). The specificity of the amplified bands was validated by their predicted size.
Determination of PGE2 and MMP-9 levels
Liver biopsy samples from all HCV+ and HCV− patients were homogenised in assay buffer: 0.1 mol/l phosphate buffer, pH 7.5, containing 0.9% (w/v) bovine serum albumin. After treating the homogenates with a 1:4 water:ethanol solution and 10 μl of glacial acetic acid, samples were purified through Amprep C18 minicolumn, eluted with ethyl acetate, and evaporated to dryness under nitrogen. Then, PGE2 levels were determined using a specific enzyme immunoassay system (Amersham Biosciences UK Ltd, Buckinghamshire, UK). For MMP-9 activity, another piece of the same liver biopsy samples used for PGE2 determination was homogenised in 50 mmol/l Tris-ClH, pH 7.4, with 1 mmol/l glycerol. After centrifugation for 10 minutes at 5000 g, the supernatant was used in an assay system with a specific chromogenic peptide substrate.
Immunohistochemical analysis
Liver biopsy samples from all HCV+ and HCV− patients were assessed using an indirect immunoperoxidase staining technique, as detailed elsewhere.30 Briefly, cryostat liver sections (5 μm thick) were incubated with a rabbit serum that recognises a peptide fragment corresponding to amino acids 584–598 from the C terminus region of human/murine COX-2 (Cayman Chemical) at a working dilution of 1:300. In parallel, each tissue section was also incubated with a rabbit antimouse antiserum (Dakopatts, Copenhagen, Denmark), at a working dilution of 1:300, and was used as a negative control.
Cell culture studies
Plasmid constructs
The expression vector pEF1α has been described previously as pcDEF.31 The expression vector pEF-core was obtained by subcloning into pEF1α a PCR fragment encoding full length core protein from HCV genotype 1b. The plasmid pcDNA3.1-NS5A was generated by subcloning into pcDNA3.1 (Invitrogen Corp., Carlsbad, California, USA) a PCR fragment encoding full length NS5A protein and was kindly provided by Dr Lai (University of Southern California, Los Angeles, California, USA). The construct pUHD10-3-core was generated by subcloning a DNA fragment coding for core protein into the blunt ended XbaI site of the tetracycline controlled expression plasmid pUHD10-3.32 The expression vector pUHD10-3-Flag-NS5A was obtained by subcloning a DNA fragment coding for Flag-NS5A protein into a blunt ended EcoRI site of pUHD10-3. The reporter plasmid harbouring the human COX-2 promoter (−1796 to +104) linked to the luciferase gene has been described previously.33
Stable transfectants
Polyclonal transfectants Chang Liver (CHL) cells core and CHL-NS5A were generated by stable transfection of CHL cells (CCL13; American Type Culture Collection, Manassas, Virginia, USA)34 with pEF-core and pcDNA3.1-NS5A expression vectors, respectively. Expression of core protein was confirmed by western blot assay (anticore MA1080 from Affinity BioReagents) and that of NS5A by RT-PCR. We also generated transfectants CHL-cat by stable transfection of CHL cells with a vector which drives expression of the bacterial chloramphenicol acetyltransferase (cat) gene, which were used as controls. Where indicated, cells were stimulated for 24−48 hours with 0.5 μg/ml of LPS, a cytokine mixture (CK) (0.5 μg/ml of LPS, 20 ng/ml of TNF-α, 20 ng/ml of IL-1β), or 10 nM of phorbol myristate acetate (PMA). In some experiments cells were treated with 50 μM DFU (Merck, Raway, New York, USA) in order to inhibit COX-2 activity.
Polyclonal transfectants AML12-core and AML12-NS5A, which express the viral proteins in a tetracycline (tet)-off inducible manner, were generated by stable transfection of the previously reported clone 3 of the murine hepatocyte cell line AML1235 with pUHD10-3-core and pUHD10-3-Flag-NS5A, respectively.
Transient transfection and luciferase assays
CHL cells were transfected with 0.1 μg of COX-2 promoter containing plasmid and increasing amounts (μg) of pEF-core or pcDNA3.1-NS5A employing the DOSPER liposomal reagent (Roche Diagnostics). A snail gene promoter was used as a control for specificity in all luciferase assays. Transfected cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) with 2% fetal calf serum (FCS) for 24 hours. Cells were lysed and cell extracts were assayed for luciferase activity using a Lumat LB9501 luminometer.
Protein extraction and Western blot analysis
The experimental procedure used was identical to that described for liver biopsy samples.
RT-PCR assay
AML12-core and AML12-NS5A cells were grown, in the presence or absence of tet, for 24 hours in 1% FCS. RNA isolation and cDNA synthesis were then performed as described for liver biopsy samples. PCR conditions for COX-2 were five minutes at 95°C, 30 seconds at 95°C, 30 seconds at 60°C, 20 seconds at 72°C, 31 cycles, and a final elongation for five minutes at 72°C. PCR conditions for β-actin were five minutes at 95°C, 30 seconds at 95°C, 45 seconds at 60°C, one minute at 72°C, 25 cycles, and a final elongation for five minutes at 72°C. Amplified DNA fragments were analysed by electrophoresis in a 1.5% agarose gel. The primers specific for murine COX-2 were 5′-TGA GTA CCG CAA ACG CTT CTC-3′ (sense) and 5′-TGG ACG AGG TTT TTC CAC CAG-3′ (antisense) and yielded a 151 bp PCR product. The primers for β-actin were 5′-ATC TGG CAC CAC ACC TTC TAC AAT GAG CTG CG-3′ (sense) and 5′-CGT CAT ACT CCT GCT TGC TGA TCC ACA TCT GC-3′ (antisense).
Determination of culture supernatant PGE2 levels
Experiments were carried out following the same protocol used for liver biopsy samples but without the purification step.
Statistical analysis
The significance of differences was determined using the Kruskal-Wallis ANOVA test for data from COX-2 and MMPs experiments of protein, mRNA, and enzyme activity. Statistical significance was established at a p value <0.05.
RESULTS
Increased intrahepatic COX-2 expression in HCV induced chronic liver disease
Western blot analysis from liver biopsy extracts of all HCV+ patients included in this study demonstrated that COX-2 expression levels were significantly higher in MCH (n = 22, mean 2.4-fold), SCH (n = 5, mean 3.3-fold), and in cirrhosis (CIR) (n = 5, mean 3.9-fold) than in HCV CT samples (n = 10) (fig 1A ▶, 1B ▶ top). When PGE2 levels were measured (fig 1B ▶ bottom), a statistically significant increase was found in liver samples from HCV+ SCH (n = 5, mean 1.9-fold) and CIR (n = 5, mean 3.3-fold) compared with CT samples (n = 10). Likewise, COX-2 mRNA levels were significantly higher in MCH (n = 22, mean 2.6-fold), SCH (n = 5, mean 3.5-fold), and CIR (n = 5, mean 5.3-fold) than in CT liver biopsy samples (n = 10) (fig 1C ▶). We also determined NOS-2 protein by western blot as a surrogate marker of inflammation (fig 1D ▶), showing that NOS-2 expression was significantly higher in MCH (n = 22, mean 1.6-fold), SCH (n = 5, mean 2-fold), and cirrhosis (n = 5, mean 3.2-fold) than in CT liver biopsy samples (n = 10).
Figure 1.
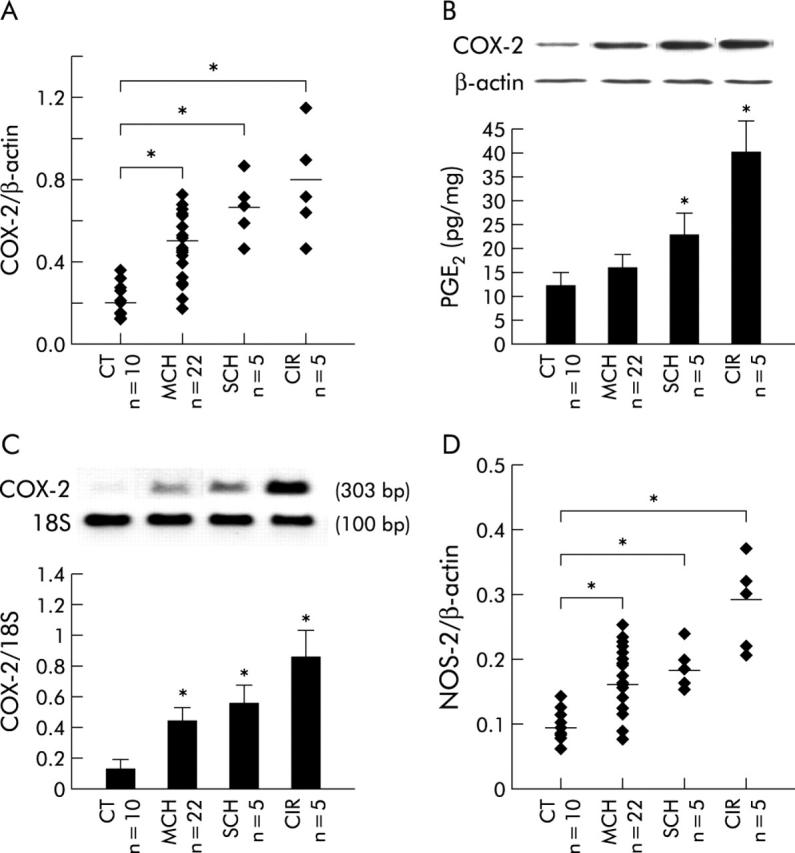
Cyclooxygenase 2 (COX-2) was overexpressed in hepatitis C virus (HCV) induced chronic liver disease. Total extracts of liver biopsy samples from patients with HCV related mild chronic hepatitis (MCH), severe chronic hepatitis (SCH), and cirrhosis (CIR) as well as control liver samples (CT) were analysed by western blot to determine COX-2 protein levels (72 kDa). (A) Points represent the COX-2 value of each liver biopsy sample analysed. COX-2 expression levels were significantly higher in MCH (n = 22, mean 2.4-fold), SCH (n = 5, mean 3.3-fold), and CIR (n = 5, mean 3.9-fold) than in CT liver samples (n = 10). (B) Top: representative western blot of COX-2 is shown. Blots were normalised by measuring the amount of β-actin. Bottom: prostaglandin E2 (PGE2) levels were determined in the same liver samples by enzyme immunoassay. Bars are mean (SEM) of all liver biopsy samples analysed in each histological group. A statistically significant increase in PGE2 levels was found in liver samples from SCH (n = 5, mean 1.9-fold) and CIR (n = 5, mean 3.3-fold) compared with CT samples (n = 10). (C) Top: representative reverse transcription-polymerase chain reaction (RT-PCR) of COX-2 is shown. Bottom: bars are mean (SEM) of all liver biopsy samples analysed in each histological group. RT-PCR analysis showed that COX-2 mRNA levels were significantly higher in MCH (n = 22, mean 2.6-fold), SCH (n = 5, mean 3.5-fold), and CIR (n = 5, mean 5.3-fold) than in CT liver biopsy samples (n = 10), in agreement with the protein analysis. (D) The same samples as in (A) were used to determine NOS-2 protein levels. Points represent the NOS-2 value of each liver biopsy sample analysed. NOS-2 expression was significantly higher in MCH (n = 22, mean 1.6-fold), SCH (n = 5, mean 2-fold), and cirrhosis (n = 5, mean 3.2-fold) than in CT liver biopsy samples (n = 10). *p<0.05 versus the CT group.
Immunohistochemical experiments performed on liver biopsy sections from patients with HCV+ end stage cirrhosis detected COX-2 expression in a number of cell types, including hepatocytes, which showed a predominant cytoplasmic staining, and although some COX-2-positive hepatocytes were observed at the edge of hepatic lobules, the majority were mainly restricted to the regenerative nodules (fig 2A, 2B ▶ ▶). In contrast, in patients with SCH, hepatocellular COX-2 immunoreactivity was observed in some hepatocytes located in periportal areas (fig 2C ▶). Notably, hepatocytes from patients with MCH were negative for COX-2, with the positive cells restricted to sinusoidal lining cells (SLC) and scattered mononuclear cells (MNC) (fig 2D ▶). In all liver sections from patients with non-alcoholic steatosis (fig 2E ▶) and with a normal liver (fig 2F ▶), no hepatocellular COX-2 staining was observed, being only detected in scattered SLC.
Figure 2.
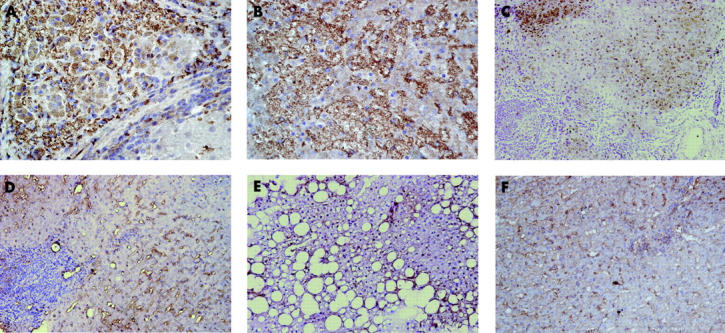
Immunohistochemical detection of cyclooxygenase 2 (COX-2) in liver sections. An intense hepatocellular COX-2 expression is shown within a regenerative nodule from different patients with hepatitis C virus (HCV)+ end stage cirrhosis (A, B, original magnification ×450). In contrast, hepatocellular COX-2 immunoreactivity was only observed in some hepatocytes located in periportal areas of severe chronic hepatitis (SCH) (C, original magnification ×250). Noticeable, hepatocytes from patients with mild chronic hepatitis (MCH) were negative for COX-2, with the positive cells being restricted to sinusoidal lining cells and scattered mononuclear cells (D, original magnification ×250). Interestingly, in the liver sections from patients with non-alcoholic steatosis (E, original magnification ×250) and a normal liver (F, original magnification ×250), no hepatocellular COX-2 staining was observed, being detected only in scattered sinusoidal lining cells.
Enhanced MMP-2 and MMP-9 expression and activity in HCV related chronic liver disease
A statistically significant increase in MMP-9 and MMP-2 proteins was observed in MCH (n = 22, mean 2- and 3.2-fold, respectively), SCH (n = 5, mean 2.6- and 4.7-fold), and CIR (n = 5, mean 6.6- and 6.9-fold) compared with CT samples (n = 10) (fig 3A, 3B ▶ ▶). Moreover, a significant increase in MMP-9 activity was found in SCH (n = 5, mean 2-fold) and CIR (n = 5, mean 2.8-fold) compared with CT samples (n = 10) (fig 3C ▶). RT-PCR analysis demonstrated that intrahepatic MMP-9 mRNA levels were significantly higher in MCH (n = 22, mean 2.6-fold), SCH (n = 5, mean 3.6-fold), and CIR (n = 5, mean 4.5-fold) than in CT samples (n = 10) (fig 3D ▶). A comparative analysis of COX-2, MMP-2, and MMP-9 expression levels in liver biopsies from all HCV patients was carried out. Interestingly, the intrahepatic content of these proteins increased in parallel with the fibrotic stage of HCV induced liver disease, being maximal in cirrhosis (fig 3E ▶). However, no relationship between intrahepatic expression levels of COX-2, MMP-2, and MMP-9 and viral load of HCV patients studied was found (see table 2 ▶).
Figure 3.
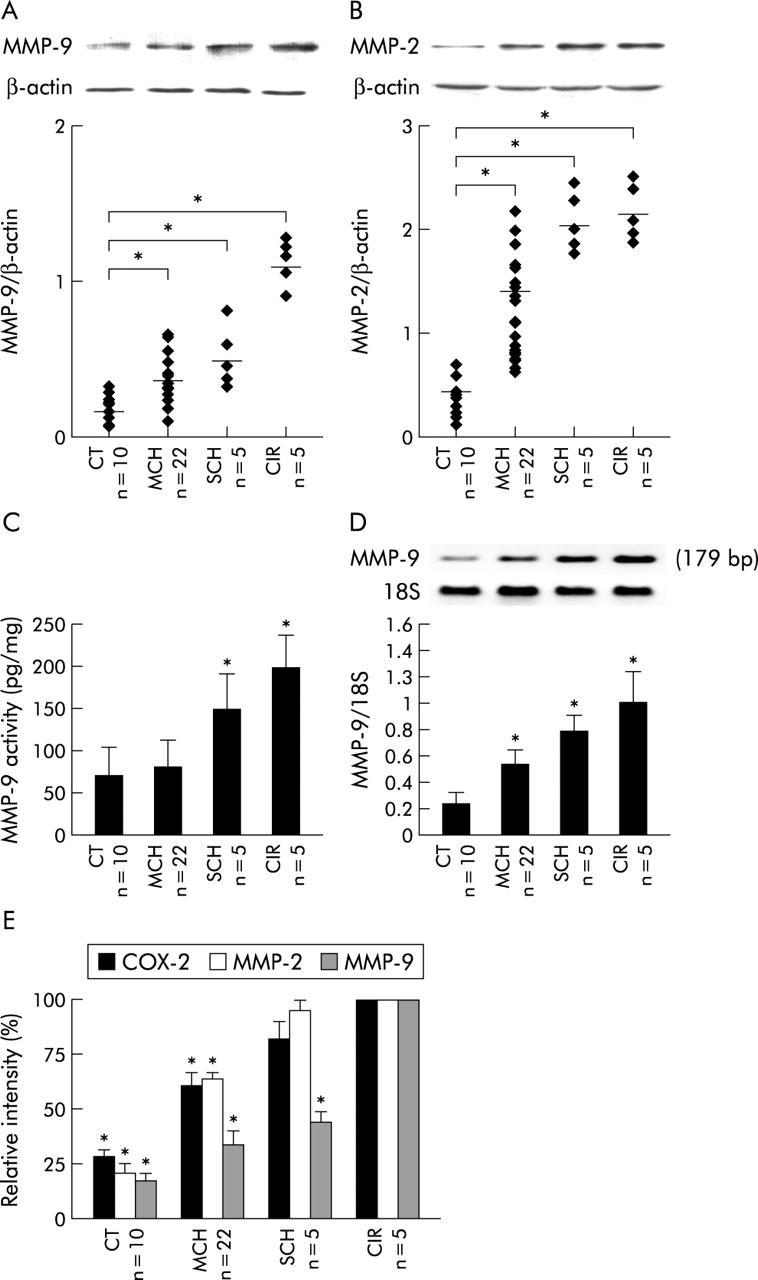
Matrix metalloproteinase (MMP)-2 and MMP-9 expression in hepatitis C virus (HCV) related chronic liver disease. Biopsy samples from all HCV+ and HCV− control (CT) patients were analysed by western blot to measure MMP-9 and MMP-2 proteins (82 and 66 kDa, respectively). (A) Top: representative western blot of MMP-9 is shown. Bottom: points represent the MMP-9 value of each liver biopsy sample analysed. There was a statistically significant increase in MMP-9 protein in mild chronic hepatitis (MCH) (n = 22, mean 2-fold), severe chronic hepatitis (SCH) (n = 5, mean 2.6-fold), and cirrhosis (CIR) (n = 5, mean 6.6-fold) compared with CT samples (n = 10). (B) Top: representative western blot of MMP-2 is shown. Bottom: points represent the MMP-2 value of each liver biopsy sample analysed. There was a statistically significant increase in MMP-2 protein in MCH (n = 22, mean 3.2-fold), SCH (n = 5, mean 4.7-fold), and CIR (n = 5, mean 6.9-fold) compared with CT samples (n = 10). (C) MMP-9 activity was determined using a specific assay system. Bars are mean (SEM) of all liver biopsy samples analysed in each histological group. A significant increase in MMP-9 activity was found in SCH (n = 5, mean 2-fold) and CIR (n = 5, mean 2.8-fold) compared with CT samples (n = 10). (D) Top: representative reverse transcription-polymerase chain reaction (RT-PCR) of MMP-9 is shown. Bottom: bars are mean (SEM) of all liver biopsy samples analysed in each histological group. RT-PCR analysis demonstrated that intrahepatic MMP-9 mRNA levels were significantly higher in MCH (n = 22, mean 2.6-fold), SCH (n = 5, mean 3.6-fold), and CIR (n = 5, mean 4.5-fold) than in CT samples (n = 10). (E) Comparative analysis of intrahepatic COX-2, MMP-2, and MMP-9 expression levels in all HCV patients studied, showing that the intrahepatic content of these proteins increased in parallel with the fibrotic stage of HCV induced liver disease, being maximal in cirrhosis. Results are expressed as percentage, being 100 in the CIR group. *p<0.05 versus the CIR group.
Table 2.
Comparative analysis of intrahepatic expression levels of COX-2, MMP-2, and MMP-9 between high and low viral load patients
| High viral load | Low viral load | p Value | |
| COX-2 | 0.48 (0.16) | 0.58 (0.16) | 0.13 |
| MMP-2 | 1.63 (0.8) | 1.56 (0.76) | 0.83 |
| MMP-9 | 0.43 (0.3) | 0.26 (0.2) | 0.15 |
COX-2, cyclooxygenase 2; MMP, matrix metalloproteinase.
High viral load, >800 000 IU/ml; Low viral load, ⩽800 000 IU/ml.
HCV induced COX-2 upregulation in hepatocyte derived cultured cells
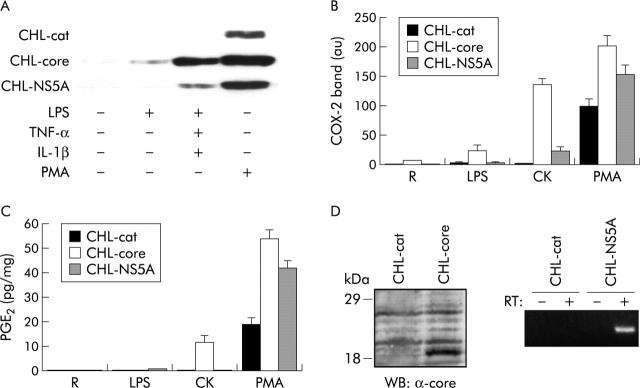
Experiments performed in control CHL-cat cells showed that COX-2 expression was only detected after 48 hours of treatment with PMA. In contrast, weak COX-2 expression in untreated CHL-core polyclonal transfectans was already observed, its expression being markedly enhanced after treatment with LPS, CK, and PMA (fig 4A, 4B ▶ ▶). Similar results, although to a lesser extent, were obtained in CHL-NS5A polyclonal cells. COX-2 protein was functionally active, as demonstrated by the increase in PGE2 concentrations in culture supernatants from control CHL-cat cells stimulated with PMA. Noteworthy, there was a further increase in PGE2 levels in culture supernatants from CHL-core and CHL-NS5A cells treated under the same conditions. However, PGE2 concentrations were lower than expected for COX-2 expression levels observed in core and NS5A transfected CHL cells, particularly in those treated with LPS and CK compared with PMA treated cells (fig 4C ▶).
Figure 4.
Increased cyclooxygenase 2 (COX-2) expression and activity in human hepatic cell lines in relation to the effect of hepatitis C virus (HCV) proteins. Resting (R) Chang Liver (CHL)-cat, CHL-core, and CHL-NS5A polyclonal transfectans were cultured in Dulbecco’s modified Eagle’s medium supplemented with 2% fetal calf serum and stimulated for 48 hours with 0.5 μg/ml lipopolysaccharide (LPS), a cytokine mixture (CK) (0.5 μg/ml LPS, 20 ng/ml tumour necrosis factor α (TNF-α) and 20 ng/ml interleukin 1β (IL-1β)), or 10 nM phorbol myristate acetate (PMA). (A) Representative western blot of COX-2 in total cellular extracts is shown. (B) Bars are mean (SEM) of band intensities obtained in three different experiments. COX-2 expression was markedly enhanced in CHL-core cells and, although to a lesser extent, in CHL-NS5A cells compared with control CHL-cat cells. (C) Prostaglandin E2 (PGE2) levels in cell culture supernatants were measured by enzyme immunoassay. Bars are mean (SEM) of PGE2 concentrations obtained in three different experiments. There was a further increase in PGE2 levels in the cell culture supernatants of CHL-core and CHL-NS5A cells, stimulated with CK and PMA, compared with control CHL-cat cells treated under the same conditions. (D) Expression of core protein in CHL-core cells was confirmed by western blot (WB) assay and the positive expression of NS5A in CHL-NS5A was tested by RT (reverse transcription)-polymerase chain reaction.
As shown in fig 5A ▶, both viral proteins were able to activate the COX-2 gene promoter in a dose dependent manner. The tet-off mediated expression of core and NS5A resulted in upregulation of COX-2 mRNA (fig 5B ▶).
Figure 5.
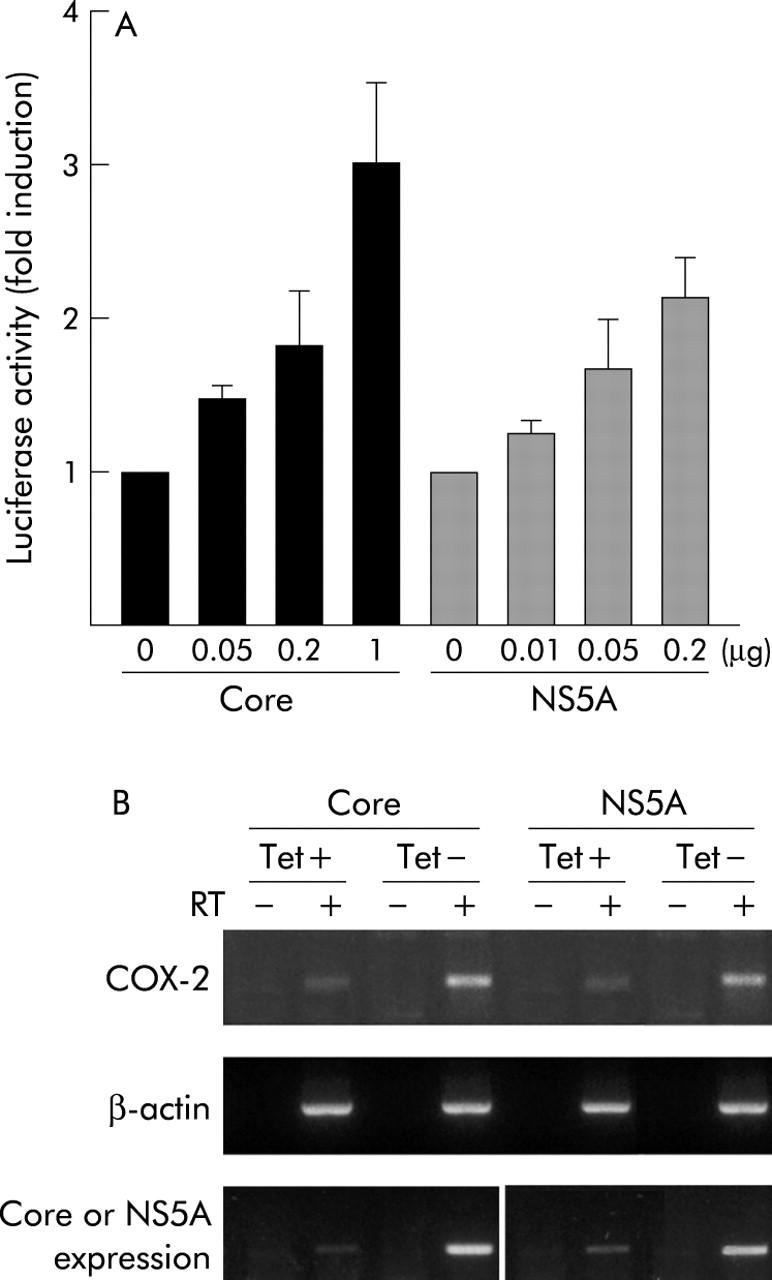
Hepatitis C virus (HCV) proteins transactivated cyclooxygenase 2 (COX-2) gene expression in hepatocyte derived cell lines. (A) Core and NS5A induced COX-2 promoter activity. Chang Liver (CHL) cells were transfected with 0.1 μg of COX-2 promoter plasmid along with increasing amounts (μg) of pEF-core or pcDNA3.1-Flag-NS5A. Luciferase activity was analysed 24 hours after transfection. Results are represented as fold induction over values obtained with the control plasmids, and bars are mean (SEM) of three independent experiments. (B) RT (reverse transcription)-polymerase chain reaction assay (RT+) showing COX-2 mRNA expression in murine stable transfectants AML12-core and AML12-NS5A grown for 24 hours in the presence (+) or absence (−) of tetracycline (Tet). The Tet-off mediated expression of core and NS5A resulted in upregulation of COX-2 mRNA. Control PCR reactions were with RNAs that had not been reverse transcribed (RT−), in the presence of the respective primers. Results are representative of three independent experiments.
Resting control CHL-cat cells expressed basal levels of MMP-9 protein but its expression was enhanced by the effect of LPS, CK, and PMA. Interestingly, a maximal increase in MMP-9 expression was observed in CHL-core cells, independent of the stimuli used (fig 6A, 6B ▶ ▶) whereas NS5A did not exert any effect on MMP-9 expression (data not shown), suggesting different roles for both viral proteins on MMP-9 expression. Noteworthy, DFU apart from inhibiting PGE2 levels (not shown) decreased MMP-9 expression induced by LPS, CK, and PMA in control CHL-cat cells, demonstrating a clear relationship between COX-2 expression and MMP-9 production. In contrast, inhibition of MMP-9 protein levels detected after DFU addition to CHL-core cells was markedly lower than that observed in control CHL-cat cells (fig 6A, 6B ▶ ▶), suggesting a direct effect of core protein on regulation of MMP-9 protein synthesis.
Figure 6.
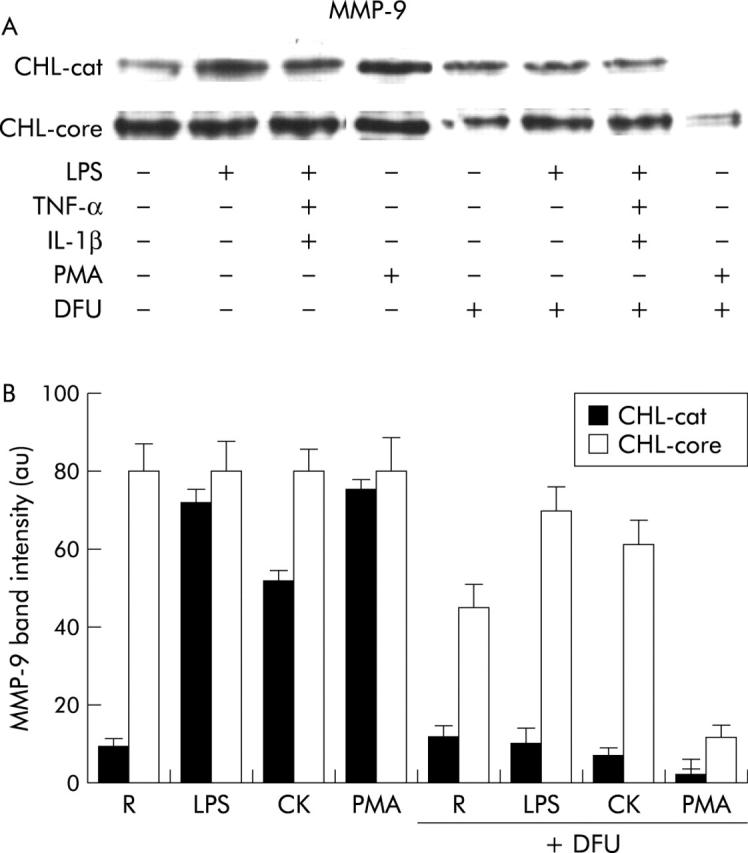
Hepatitis C virus (HCV) core protein induced matrix metalloproteinase (MMP)-9 synthesis in human hepatic cell lines. Resting (R) Chang Liver (CHL)-cat and CHL-core cells were preincubated or not for 30 minutes with 50 μM 5,5-dimethyl-3(3-fluorophenyl)-4-(4-methylsulphonyl)phenyl-2(5H)-furanone (DFU) followed by activation with lipopolysaccharide (LPS), cytokine mixture (CK), or phorbol myristate acetate (PMA). MMP-9 protein was analysed by western blot in total cellular extracts. (A) Representative experiment is shown. (B) Bars are mean (SEM) of band intensities obtained in three different experiments. A marked induction of MMP-9 expression was observed in CHL-core cells, which was higher than in control CHL-cat cells and independent of the stimuli used. DFU preincubation decreased markedly MMP-9 expression induced by LPS, CK, and PMA in control CHL-cat cells. In contrast, inhibition of MMP-9 protein levels in CHL-core cells was lower that that observed in control CHL-cat cells.
DISCUSSION
The results of the present study provide evidence that COX-2 is overexpressed in the liver tissue of patients with chronic HCV infection, showing a positive correlation with the fibrotic stage of HCV induced liver disease. Interestingly, intrahepatic COX-2 expressed in HCV infected patients was functional, as demonstrated by the parallel increase in PGE2 levels detected in liver extracts from the same patients.
The molecular basis for hepatocellular COX-2 expression in patients with chronic HCV infection remains to be defined but there is increasing experimental evidence that certain HCV proteins, such as core and NS5A, can function as transcriptional transactivators for a wide number of cellular genes.36,37 To assess the role of HCV proteins on COX-2 expression during chronic HCV infection, we performed in vitro experiments using cultured hepatocyte derived cells stably and transiently transfected with core or NS5A proteins. We found that maximal hepatocellular COX-2 expression occurred by the synergistic inducing effect exerted by HCV proteins plus several inflammatory mediators, including TNF-α and IL-1β,? which are well known factors involved in the fibrotic process,38 supporting our in vivo findings that, independent of viral load, intrahepatic COX-2 expression increased in parallel with the stage of HCV induced chronic liver disease.
It has been reported that core and NS5A proteins are able to activate different transcription factors, such as nuclear factor κB, AP-1, SRE, and STAT-3, suggesting their potential role in upregulation of COX-2 gene expression.39–41 Moreover, both viral proteins alter intracellular calcium levels and it is well known that the NF-AT family of transcription factors, which are calcium regulated, is essential for activation of COX-2 gene expression.33 Alternative candidates transactivated by HCV proteins are PPAR-α ligands, which are able to induce COX-2 expression in hepatocytes.42 Transcriptional regulation by PPARs is achieved through PPAR-retinoid X receptor heterodimers and interaction of core protein with retinoid X receptor α modulates transcriptional activity.43 The role of C/EBP-α transcription factor in the regulation of COX-2 expression in hepatocytes has also been described,44 providing another potential target to interact with HCV proteins.
Proteolytic enzymes, known as matrix metalloproteinases (MMPs), play a central role in cell migration by degrading cellular and extracellular components during cancer invasion, morphogenesis, organ development, and tissue damage.45,46 The relevance of MMPs for liver extracellular matrix remodelling can be deduced from the observation that pro-MMP-2 and pro-MMP-9 are activated during rat liver regeneration after partial hepatectomy, and both contribute to priming hepatocyte proliferation.47 In the present study, we showed that the intrahepatic content of MMP-2 and MMP-9 is increased in HCV infected patients, showing a close relationship with fibrotic stage of chronic liver disease, in a similar manner to that observed for COX-2 protein. In addition, an inducing effect on MMP-9 synthesis in human liver cells is exerted by core protein, but not by NS5A, this induction being partially abrogated by a specific COX-2 inhibitor. Our results from HCV infected patients as well as from HCV transfected human hepatic cells are in accordance with our previous work demonstrating that COX-2 expression promotes the release of active MMP-2 and MMP-9 in fetal rat hepatocytes,22 favouring the notion that COX-2 may also be important in secretion of active MMPs by human liver cells.
We showed here that progressive hepatic fibrosis but not viral load is associated with increased intrahepatic COX-2 and MMPs expression during chronic HCV infection and that in vitro pharmacological inhibition of COX-2 partially abrogated HCV mediated hepatocellular MMP-9 synthesis. On the basis of these data, it is tempting to hypothesise that selective COX-2 inhibitors, in association with antiviral therapy, might be useful as antifibrotic drugs in chronic HCV infection by decreasing hepatocellular MMPs synthesis and secretion as well as by modulating hepatic stellate cell activation.14
Overexpression of COX-2 has been demonstrated in HCC and in human hepatoma cell lines,12–15 indicating its potential role in hepatocarcinogenesis. As shown in this study, increased intrahepatic expression of functional COX-2 in HCV+ end stage cirrhosis, a clearly established premalignant lesion,18 suggests that COX-2 may be a key factor in the development of HCC in HCV infected patients. It is conceivable, therefore, that large amounts of PGs abnormally produced in hepatocytes by the synergistic effect of HCV proteins plus inflammatory mediators might act on neighbouring liver cells, through autocrine and/or paracrine pathways, thus contributing to hepatocarcinogenesis.
In conclusion, we have shown that intrahepatic COX-2, MMP-2, and MMP-9 overexpression is associated with progressive liver disease in chronic HCV infection, suggesting their pathogenic role in the fibrotic process. We also demonstrated that HCV core and NS5A proteins, alone and with the synergistic effect of endotoxin and proinflammatory cytokines, were able to upregulate COX-2 and MMP-9 gene expression in cultured human hepatic cells, thus providing a potential mechanism by which chronic HCV infection could lead to liver fibrosis.
Acknowledgments
This work was supported by grants to CG-M and to GC from Instituto de Salud Carlos III (RTIC: G03/015; FIS: 01/0152; FIS 03/0417), to ML-C from Ministerio de Ciencia y Tecnología (SAF 2001-0305) and from Instituto de Salud Carlos III (RNIHG: C03/02), and to PM-S from Ministerio de Ciencia y Tecnología (SAF 2003-00342). An unrestricted educational grant to CG-M from Schering-Plough Biotech (Madrid, Spain) is also acknowledged. AFM is supported by a CNIC-BANCAJA fellowship. The authors thank Rocha Barroso for assistance in preparing this manuscript.
Abbreviations
COX, cyclooxygenase
CT, controls
DFU, 5,5-dimethyl-3(3-fluorophenyl)-4-(4-methylsulphonyl)phenyl-2(5H)-furanone
HCC, hepatocellular carcinoma
HCV, hepatitis C virus
LPS, lipopolysaccharide
MCH, mild chronic hepatitis
MMPs, matrix metalloproteinases
NOS, nitric oxide synthase
PG, prostaglandin
PMA, phorbol myristate acetate
RT-PCR, reverse transcription-polymerase chain reaction
SCH, severe chronic hepatitis
SLC, sinusoidal lining cells
MNC, mononuclear cells
HBsAg, hepatitis B surface antigen
HIV, human immunodeficiency virus
TNF-α, tumour necrosis factor α
IL, interleukin
CK, cytokine mixture
DMEM, Dulbecco’s modified Eagle’s medium
FCS, fetal calf serum
CIR, cirrhosis
REFERENCES
- 1.Xie WL, Chipman JG, Robertson DL, et al. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci U S A 1991;88:2692–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeWitt DL. Prostaglandin endoperoxide synthase: regulation of enzyme expression. Biochim Biophys Acta 1991;1083:121–34. [DOI] [PubMed] [Google Scholar]
- 3.Pilbeam CC, Kawaguchi H, Hakeda Y, et al. Differential regulation of inducible and constitutive prostaglandin endoperoxide synthase in osteoblastic MC3T3-E1 cells. J Biol Chem 1993;268:25643–9. [PubMed] [Google Scholar]
- 4.Kujubu DA, Fletcher BS, Varnum BC, et al. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem 1991;266:12866–72. [PubMed] [Google Scholar]
- 5.Feng L , Xia Y, García GE, et al. Involvement of reactive oxygen intermediates in cyclooxygenase-2 expression induced by interleukin-1, tumor necrosis factor-α, and lipopolysaccharide. J Clin Invest 1995;95:1669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer and development. Oncogene 1996;18:7908–16. [DOI] [PubMed] [Google Scholar]
- 7.Hinz B , Brune K. Cyclooxygenase-2—10 years later. J Pharmacol Exp Ther 2002;300:367–75. [DOI] [PubMed] [Google Scholar]
- 8.DuBois RN, Abramson SB, Crofford L, et al. Cyclooxygenase in biology and disease. FASEB J 1998;12:1063–73. [PubMed] [Google Scholar]
- 9.Nanji AA, Miao L, Thomas P, et al. Enhanced cyclooxygenase-2 gene expression in alcoholic liver disease in the rat. Gastroenterology 1997;112:943–51. [DOI] [PubMed] [Google Scholar]
- 10.Nanji A , Zakim D, Rahemtulla A, et al. Dietary saturated fatty acids down-regulate cyclooxygenase-2 and tumor necrosis factor alfa and reverse fibrosis in alcohol-induced liver disease in the rat. Hepatology 1997;26:1538–45. [DOI] [PubMed] [Google Scholar]
- 11.Liu CH, Chang SH, Narko K, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem 2001;276:18563–9. [DOI] [PubMed] [Google Scholar]
- 12.Koga H , Sakisaka S, Ohishi M, et al. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology 1999;29:688–96. [DOI] [PubMed] [Google Scholar]
- 13.Cheng J , Imanishi H, Iijima H, et al. Expression of cyclooxygenase-2 and cytosolic phospholipase A2 in the liver tissue of patients with chronic hepatitis and liver cirrhosis. Hepatology Res 2002;23:185–95. [DOI] [PubMed] [Google Scholar]
- 14.Cheng J , Imanishi H, Liu W, et al. Inhibition of the expression of α-smooth muscle actin in human hepatic stellate cell line, LI90, by a selective cyclooxygenase 2 inhibitor, NS-398. Biochem Biophys Res Commun 2003;297:1128–34. [DOI] [PubMed] [Google Scholar]
- 15.Hu KQ, Yu CH, Mineyama Y, et al. Inhibited proliferation of cyclooxygenase-2 expressing human hepatoma cells by NS-398, a selective COX-2 inhibitor. Int J Oncol 2003;22:757–63. [PubMed] [Google Scholar]
- 16.Alter MJ. Epidemiology of hepatitis C. Hepatology 1997;26:S62–5. [Google Scholar]
- 17.Poynard T , Bedossa P, Opolon P. For the OBSVIRC, METAVIR, CLINIVIR and DOSVIRC groups. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet 1997;349:825–32. [DOI] [PubMed] [Google Scholar]
- 18.Llovet JM, Beaugrand M. Hepatocellular carcinoma: present status and future prospects. J Hepatol 2003;38:S136–49. [DOI] [PubMed] [Google Scholar]
- 19.Friedman SL. Liver fibrosis—from bench to bedside. J Hepatol 2003;38:S38–53. [DOI] [PubMed] [Google Scholar]
- 20.Lichtinghagen R , Michels D, Haberkorn CI, et al. Matrix metalloproteinase (MMP)-2, MMP-7, and tissue inhibitor of metalloproteinase-1 are closely related to the fibroproliferative process in the liver during chronic hepatitis C. J Hepatol 2001;34:239–47. [DOI] [PubMed] [Google Scholar]
- 21.Theret N , Musso O, Turlin B, et al. Increased extracellular matrix remodeling is associated with tumor progression in human hepatocellular carcinomas. Hepatology 2001;34:82–8. [DOI] [PubMed] [Google Scholar]
- 22.Callejas NA, Casado M, Díaz-Guerra MJM, et al. Expression of cyclooxygenase-2 promotes the release of matrix metalloproteinase-2 and -9 in fetal rat hepatocytes. Hepatology 2001;33:860–7. [DOI] [PubMed] [Google Scholar]
- 23.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001;345:41–52. [DOI] [PubMed] [Google Scholar]
- 24.Young KK, Resnick R, Myers TH. Detection of hepatitis C virus RNA by a combined reverse-transcription-polymerase chain reaction assay. J Clin Microbiol 1993;31:882–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riendeau D , Percival MD, Boyce C, et al. Biochemical and pharmacological profile of a tetrasubstituted furanone as a highly selective COX-2 inhibitor. Br J Pharmacol 1997;121:105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yergey JA, Trimble LA, Silva J, et al. In vitro metabolism of the COX-2 inhibitor DFU, including a novel glutathione adduct rearomatization. Drug Metab Dispos 2001;29:638–44. [PubMed] [Google Scholar]
- 27.Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol 1991;13:372–4. [DOI] [PubMed] [Google Scholar]
- 28.Chomczynski P , Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987;162:156–9. [DOI] [PubMed] [Google Scholar]
- 29.Jones DA, Carlton DP, Mcyntire TM, et al. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J Biol Chem 1993;268:9049–54. [PubMed] [Google Scholar]
- 30.García-Monzón C , Majano PL, Zubia I, et al. Intrahepatic accumulation of nitrotyrosine in chronic viral hepatitis is associated with histological severity of liver disease. J Hepatol 2000;32:331–8. [DOI] [PubMed] [Google Scholar]
- 31.Zhu N , Khoshnan A, Schenider R, et al. Hepatitis C virus core protein binds to the cytoplasmatic domain of tumor necrosis factor (TNF) receptor 1 and enhances TNF-induced apoptosis. J Virol 1998;72:3691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gossen M , Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A 1992;89:5547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lara-Pezzi E , Gomez-Gaviro MV, Galvez BG, et al. The hepatitis B virus X protein promotes tumor cell invasion by inducing membrane-type matrix metalloproteinase-1 and cyclooxygenase-2 expression. J Clin Invest 2002;110:1821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JS, Thorgeirsson SS. Functional and genomic implications of global gene expression profiles in cell lines from human hepatocellular cancer. Hepatology 2002;35:1134–43. [DOI] [PubMed] [Google Scholar]
- 35.Tarn C , Bilodeau ML, Hullinger RL, et al. Differential immediate early gene expression in conditional hepatitis B virus pX-transforming versus nontransforming hepatocyte cell lines. J Biol Chem 1999;274:2327–36. [DOI] [PubMed] [Google Scholar]
- 36.Kato N , Yoshida H, Kioko Ono-Nita S, et al. Activation of intracellular signaling by hepatitis B and C viruses: C-viral core is the most potent signal inducer. Hepatology 2000;32:405–12. [DOI] [PubMed] [Google Scholar]
- 37.Reyes GR. The nonstructural NS5A protein of hepatitis C virus: an expanding, multifunctional role in enhancing hepatitis C virus pathogenesis. J Biomed Sci 2002;9:187–97. [DOI] [PubMed] [Google Scholar]
- 38.Olaso E , Friedman SL. Molecular mechanisms of hepatic fibrogenesis. J Hepatol 1998;29:836–47. [DOI] [PubMed] [Google Scholar]
- 39.Lerat H , Honda M, Beard MR, et al. Steatosis and liver cancer in transgenic mice expressing the structural and nonstructural proteins of hepatitis C virus. Gastroenterology 2002;122:352–65. [DOI] [PubMed] [Google Scholar]
- 40.Okuda M , Li K, Beard MR, et al. Mitochondrial injury oxidative stress and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology 2002;122:366–75. [DOI] [PubMed] [Google Scholar]
- 41.Gong G , Waris G, Tanveer R, et al. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci U S A 2001;98:9599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Callejas NA, Castrillo A, Bosca L, et al. Inhibition of prostaglandin synthesis up-regulates cyclooxygenase-2 induced by lipopolysaccharide and peroxisomal proliferators. J Pharmacol Exp Ther 1999;288:1235–40. [PubMed] [Google Scholar]
- 43.Tsutsumi T , Suzuki T, Shimoike T, et al. Interaction of hepatitis C virus core protein with retinoid X receptor modulates its transcriptional activity. Hepatology 2002;35:937–46. [DOI] [PubMed] [Google Scholar]
- 44.Callejas NA, Boscá L, Williams CS, et al. Regulation of cyclooxygenase 2 expression in hepatocytes by CCAAT/enhancer-binding proteins. Gastroenterology 2000;119:493–501. [DOI] [PubMed] [Google Scholar]
- 45.Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res 1991;51:5054s–9s. [PubMed] [Google Scholar]
- 46.Vaday GG, Lider O. Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation. J Leukoc Biol 2000;67:149–59. [DOI] [PubMed] [Google Scholar]
- 47.Kim TH, Mars WM, Stolz DB, et al. Expression and activation of pro-MMP-2 and pro-MMP-9 during rat liver regeneration. Hepatology 2000;31:75–82. [DOI] [PubMed] [Google Scholar]