Abstract
Background/Aims: The gut flora may play an important role in the pathogenesis of inflammatory bowel disease. An ileal reservoir or pouch can be created to replace the excised rectum after proctocolectomy. In patients with ulcerative colitis this is subject to inflammation and termed pouchitis. Using bacteria from patients the authors sought evidence for the presence rather than the identity of a pathogenic species in pouchitis, and for its absence in healthy pouches by the differential effect on lymphocyte proliferation.
Methods: An ex vivo cell culture assay was used in which peripheral blood mononuclear cells or lamina propria mononuclear cells were cultured with sterile sonicates of gut flora from patients with or without pouchitis in the presence of antigen presenting cells.
Results: Sonicated pouchitis flora produced a consistent and intense proliferation of the mononuclear cells but that produced by sonicates from healthy pouches was minimal (p = 0.012 or 0.018, peripheral blood or lamina propria mononuclear cells). Preparation of the sonicates with the antibiotic metronidazole abolished their stimulatory ability (p = 0.005, peripheral blood mononuclear cells). In separate assays neither direct addition of metronidazole nor of its hydroxy metabolite affected the mononuclear cells’ proliferation with alternative stimuli.
Conclusions: These results strongly support a bacterial aetiology for pouchitis.
Keywords: intestinal flora, lymphocytes, metronidazole, mucosal immunity, pouchitis
The construction of a neorectum from the distal ileum and its anastomosis to the anus provides restoration of intestinal continuity in patients who have undergone total proctocolectomy.1 This ileal neorectum, or pouch, is prone to inflammation which is known as pouchitis and which almost exclusively affects those with a diagnosis of ulcerative colitis (UC) rather than familial adenomatous polyposis.2
Evidence suggests a role for bacteria in the pathogenesis of inflammatory bowel disease (IBD).3–5 In animal models both B27 transgenic rats6 and knockout mice predisposed to colitis require the presence of bacteria to provoke that colitis.7 In human disease, improvement is seen in Crohn’s disease when the faecal stream is diverted away from the diseased area.8 In addition, there is benefit from manipulation of the flora by using antibiotics9 or probiotics in pouchitis10–12 and in animal models13 although in Crohn’s disease the proven efficacy of antibiotics is limited to the specific situations of avoiding postoperative recurrence14 and perianal disease.15 Despite the findings in experimental colitis, there is little evidence for antibiotic use in UC.16
Several candidate species have been investigated as potential aetiological agents. For example, increased numbers of E coli and bacteroides have been found adherent to the mucosa in IBD patients, although moderately raised levels of adherent bacteria in subjects with self-limiting inflammation weaken the case for causation.17 Increased concentrations of the same bacteria together with fusobacteria in the neoterminal ileum have been found to correlate with early postoperative relapse in Crohn’s disease.18 Fusobacterium varium has also been observed within the mucosa of UC patients where it seems to stimulate a species specific antibody. Interestingly, when cultured, the supernatants from the cultures of these human isolates produce ulcers in the colons of mice.19
Investigation of bacteria with distinct metabolic traits has revealed that whereas a majority of UC pouches harbour sulphate reducing species, these are absent from familial adenomatous polyposis pouches.20 However, the intuitive approach of identifying a specific pathogen by comparing the results of culture in IBD and controls is rendered impractical by the number of species present and the lack of universal survival outside the body.21
More indirect methods have been used in attempting to identify a pathogen. Serological studies of excreted and circulating antibacterial antibodies,22,23 and reactivity of bacterial components with commonly found disease related antibodies such as ANCA in UC24 or ASCA in Crohn’s disease have implicated B vulgatus (53% v 1% seroreactivity in controls) and E coli in active UC. These are found among many commensals also stimulating a serological response. The response to commensals suggests these findings may reflect secondary colonisation of lesions rather than the cause. The same interpretation can be made for the identification of bacterial components from lymphoid follicles or gut lesions.21,25 This argument weakens the otherwise strong case made for Mycobacterium paratuberculosis, a putative pathogen in Crohn’s disease, by the recent demonstration of its DNA in 92% of cases versus 26% of controls using the most sensitive methods.26 DNA fingerprinting using 16S rRNA technology holds more promise than culture27 but remains a costly method in terms of time and cannot identify all bacteria present.
Our aim was to show the presence rather than the identity of a pathogen or pathogens in the gut flora of pouchitis sufferers by an ex vivo lymphocyte stimulation assay.
We tested the hypothesis that lamina propria mononuclear cells (LPMC) and peripheral blood mononuclear cells (PBMC) would proliferate more with a sonicate made from the bacterial flora present in pouchitis than they would with one made from the flora in healthy pouches. Secondly, we tested the hypothesis that the antibiotic metronidazole (MTZ) might prevent the growth of the stimulatory bacteria.
MATERIALS AND METHODS
Subjects
Patients aged 18–70 years who had undergone ileal pouch formation and ileostomy reversal for ulcerative colitis (UC) and under regular follow up at a single centre were recruited by letter followed by a telephone call. Pouchitis was defined as a total score of 7 or more on the pouch disease activity index (PDAI)28 in patients with clinical, macroscopic, and histological changes. Exclusion criteria were significant comorbidity leading to increased hazard with extra biopsies such as ischaemic heart disease, chronic airways disease, insulin dependent diabetes mellitus, and current anticoagulant use. The subjects gave written informed consent and the Harrow Research Ethics Committee approved the study. Twenty two patients were recruited. One patient failed to attend. Bacterial contamination of the sterile lymphocyte cultures occurred in three. Thus, data from 18 were analysed (10 male, 8 female). The median age range was 38.5 years (range 27–68 years). Median pouch age was 126 months (range 2–266 months) and median PDAI score was 1.75 (range 0–9). One patient had active pouchitis (PDAI score 9) with a history of recurrent episodes and ankylosing spondylitis. The yield of lymphocytes from biopsies was variable but the greater the yield the greater the number of experiments which could be performed. This means that the data for some experiments come from fewer than 18 subjects.
Bacterial sonicate preparation
Four biopsies were taken via a sterile rigid sigmoidoscope from the lower posterior portion of the pouch. Using sterile forceps each biopsy was immediately smeared onto an agar plate preheated to 37°C and kept in an insulated bag. For aerobes, CHB agar (Oxoid, Basingstoke, UK) was used. For anaerobes two plates of fastidious anaerobe agar (E&O Labs, Bonnybridge, UK) preincubated in the “anaerogen compact” system (Oxoid) were used and immediately replaced in the “anaerogen” system to optimise conditions for anaerobic growth. One of these was impregnated with MTZ at 20 μg/ml (E&O Labs), equating to reported plasma concentrations.29 The fourth biopsy was sent for histological analysis.
The plates were cultured for eight days at 37°C in a humidified incubator and in 5% CO2 for aerobes. Bacteria were harvested in a laminar flow cabinet and transported in HBSS (Gibco, Paisley, UK) on ice. In initial experiments cell yields limited the number of sonicates which could be tested. In view of the effect of metronidazole in pouchitis we anticipated a greater response from anaerobes and concentrated on these. So as not to miss an effect from aerobes, half of the yield from the aerobic plate was added to each of the anaerobic harvests. All colonies growing on any one plate were mixed on harvesting.
Sonication of harvested bacteria was performed for 30 minutes on ice using a W385 sonicator (Heat Systems-Ultrasonics Inc, NY, USA) at a 50% duty cycle on power 3 with 10 mM phenylmethylsulphonyl fluoride (Sigma-Aldrich, Gillingham, UK) as a protease inhibitor. Electron microscopy revealed >99.9% fragmentation of bacteria. After centrifugation at 6000 g for 15 minutes the supernatant was collected and the pellet discarded. Sterility was achieved by 2600 Gy of gamma irradiation. Protein content was assessed using the Bradford method (BioRad Labs Ltd, Hemel Hempstead, UK). Sonicates were aliquoted and stored at −80°C until used. Sonicates were designated as autologous or heterologous to indicate their origin as being from the patient whose mononuclear cells were under stimulation or from another patient; as P to designate that they derived from pouchitis and not non-pouchitis patients who were diagnosed as non; and as M to designate that they were derived from agar plates with metronidazole rather than without. Pure strains of Bacteroides vulgatus (11154), Bacteroides thetaiotaomicron (10582), Bifidobacterium bifidum (10472) (NTCC, PHLS, London, UK), and Escherichia coli (25922, ATCC, Manassas, VA, USA) were obtained and prepared similarly.
Cell preparation and harvest
After at least two weeks patients returned for further biopsies and venesection. LPMC: biopsies were collected into HBSS-CMF (Gibco) and immediately treated with DTT and EDTA followed by a 14 hour collagenase digestion with DNAase I (Boehringer Ingelheim, Bracknell, UK) in AIM V lymphocyte medium (Gibco). Medium and cells were collected through a cell strainer (Falcon, via Marathon LS, London, UK) and washed before density gradient centrifugation over Ficoll-paque plus (Amersham Pharmacia Biotech, St Alban’s, UK). PBMC: Ficoll density gradient centrifugation was performed.
Cells were >95% viable by trypan blue exclusion. FACS analysis revealed that the CD45+ PBMC were 81% CD3+ of which 67% were CD4+ and 14% CD8+. CD45+ LPMC were 89% CD3+ (49% CD4+; 40% CD8+). To avoid degradation of cell surface ligands further purification was not performed. Antigen presenting cells were PBMC that had been irradiated with 8.6 Gy of gamma irradiation.
Cell culture assays
Each assay was performed in triplicate in AIM V medium with added Plasmocin antibiotic (Invivogen, Cayla, Toulouse, France) at 1 in 10 000 with 5×104 LPMC or PBMC and 5×104 antigen presenting cells per well in 96 well U-bottomed plates (Falcon). The antibiotic was in routine use in the cell culture laboratory, and should not have influenced results as it acts on synthetic pathways of non-eukaryotes. Where cell yields allowed, all six sonicates were tested: AutM, Aut, HetPM, HetP, HetNonM, and HetNon all at 50 μg/ml. As a positive control 0.2 μg of phytohaemagglutinin (PHA) was added, and albumin was added in lieu of sonicate as an unstimulated control.
In parallel experiments MTZ and its hydroxy metabolite (kindly donated by Aventis Pharma, Vitry-sur-Seine, France) were added to PHA and B vulgatus cultures at 100 μM and 50 μM, respectively, which equate to reported plasma concentrations. These were to examine the direct effect of MTZ on cell culture, which is debated. They also serve as controls to eliminate possible carry over of MTZ with the bacteria from the culture plates to the cell cultures as the explanation of any effect seen in the sonicate stimulation assays. Precautions were observed to avoid accidental direct contact with PHA. Sonicates were plated on agar to test for sterility. Wells which generated colony forming units overtly or when plated were discarded. Incubation in a humidified incubator at 37°C and in 5% CO2 lasted 96 hours with 3H added at 1 μCi/well for a further 16 hours. 3H incorporation was assessed. Institutional safety guidelines for the handling and disposal of radioactive reagents were adhered to.
Proliferation was recorded as the stimulation index (SI) which is the mean scintillation of the triplicate wells divided by the mean of the three unstimulated wells. An SI >3 was taken to represent increased proliferation beyond experimental error.
The Wilcoxon signed rank test was used for comparison of treatment conditions. Significance for a two tailed test was chosen as p<0.05.
RESULTS
Comparison of proliferation with bacterial sonicates of different origins
In general, mononuclear cells proliferated little when they were stimulated with sonicates derived from either autologous or heterologous pouch bacteria if that pouch was healthy, nor was there a significant difference between the level of proliferation they each induced (p = 0.247, n = 12 PBMC; p = 0.678, n = 12 LPMC) (fig 1A ▶).
Figure 1.
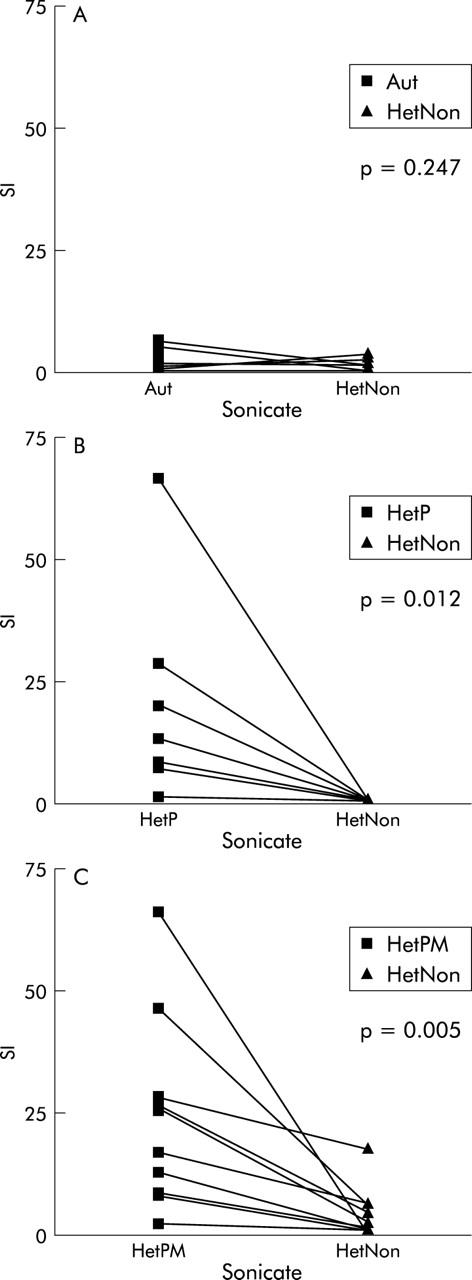
Comparison of proliferation of PBMC with (A) sonicate of autologous (Aut) or heterologous healthy pouch (HetNon) flora; (B) sonicate of heterologous pouchitis flora (HetP) or heterologous healthy pouch flora (HetNon); (C) sonicate of pouchitis flora grown on agar with metronidazole (HetPM) or without (HetP).
However, in the majority of cases strong proliferation was seen with sonicate derived from pouchitis flora whereas weak proliferation close to baseline was observed with non-pouchitis sonicate. This was in eight patients whose mononuclear cells were tested under both conditions in parallel (p = 0.012; PBMC) (fig 1B ▶).
The proliferation was less marked when LPMC were stimulated but was still significantly greater than that provoked by the non-pouchitis sonicate (p = 0.018, n = 7).
Metronidazole affects proliferation when present at the bacterial culture stage but not when added to the mononuclear cell culture with the sonicate
When pouchitis bacteria were cultured simultaneously in the presence or absence of MTZ the sonicate derived from the MTZ impregnated plate lost the capacity to induce proliferation almost entirely (p = 0.005, n = 10; PBMC) (fig 1C ▶). The proliferation was less for LPMC with no significant difference in that provoked between the two types of sonicate (p = 0.314, n = 10).
Proliferation with non-pouchitis sonicate was close to baseline levels regardless of the presence or absence of MTZ in the original agar.
To examine whether MTZ has a profound effect on lymphocyte proliferation, as has been claimed, in a smaller number of parallel experiments MTZ or its hydroxy metabolite were added to PBMC and LPMC cultures together with medium, B vulgatus sonicate or PHA.
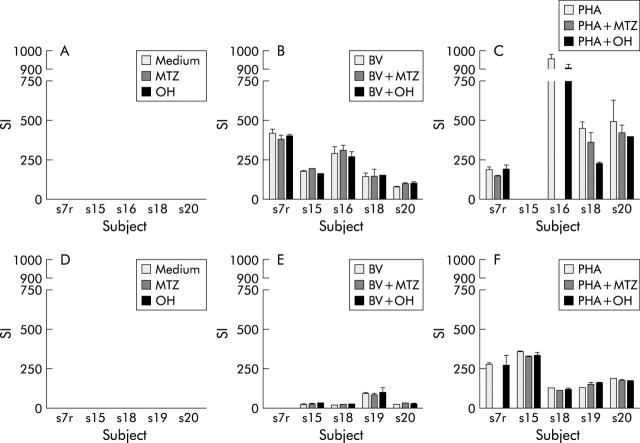
For each of the subjects, lymphocyte proliferation with progressively stronger stimuli (medium, then B vulgatus sonicate, then PHA) increased as expected. However, for a given subject under any one of those conditions the addition of MTZ or its hydroxy metabolite made little difference to proliferation. No consistent effect was seen in all subjects under any one condition. This was true for PBMC (fig 2A ▶–C) and LPMC (fig 2D ▶–F).
Figure 2.
Effect of addition of metronidazole or its hydroxy metabolite direct to cultures Of mononuclear cells with no stimulus or bacterial sonicate or PHA (A–C, PBMC; D–F, LPMC). (Insufficient initial cell yields meant subject 15’s PBMC not tested with PHA stimuli; similarly subject 16’s PBMC with PHA + MTZ. Subject 7r’s LPMC not tested with BV stimuli nor with PHA + MTZ).
Mononuclear cell proliferation with other stimuli
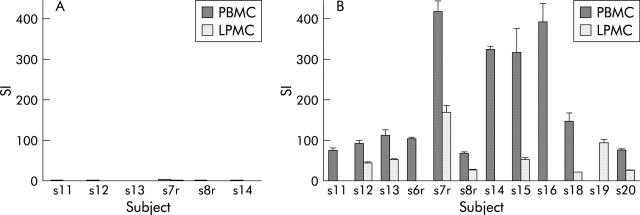
PHA was used as a positive control to confirm viability of the mononuclear cells. The median SI was 560 for PBMC and 177 for LPMC. Sonicate prepared from E coli reduced proliferation below SI of 1 in nearly all cases—that is, below the spontaneous level of proliferation of the mononuclear cells (fig 3A ▶). The same pattern was seen with sonicate from Bifidobacterium bifidum and from Bacteroides thetaiotaomicron in three patients in whom they were tested. In contrast, the sonicate from B vulgatus proved an exceptionally strong stimulus to mononuclear cell proliferation. (fig 3B ▶)
Figure 3.
Proliferation by cell type with E coli sonicate (A) and B vulgatus sonicate (B) (where tested). No LPMC were available for subjects 11, 6r, 14, or 16; no PBMC for 19.
DISCUSSION
Interaction of the gastrointestinal flora and the gut wall is essential for health. The observation of a specific gut bacterial profile which seems to persist,30,31 like a part of one’s phenotype, supports the theory that flora probably interact with the host genotype.32,33 Certain bacteria may interact with certain genotypes to produce disease states: germ free HLA B27 transgenic rats developed severe colitis only when they were exposed to Bacteroides vulgatus.6 Therefore, it seems reasonable to propose that some bacterial species or their products interact with hosts with a susceptible genotype to produce mucosal disease.
Given the technological constraints on identifying the different species present in the gut flora and on comparing the total flora of any case and control, we sought to establish the presence of pathogenic species rather than their identity.
Using an ex vivo lymphocyte stimulation assay we examined the flora in pouchitis. We found that bacterial sonicate from a heterologous but healthy pouch, HetNon, did not stimulate lymphocytes (PBMC or LPMC) from patients with healthy pouches. Similarly, there was a seemingly anergic response to sonicate of their own bacteria, Aut (fig 1A ▶). The latter result is consistent with the theory that individuals are tolerant of their own gastrointestinal flora. However, our finding of tolerance to the flora of strangers differs from the findings of Duchmann,34 who found a proliferative response to foreign flora. Our use of feeder cells may reflect the mucosal environment more faithfully, as feeder cells (rather than T cells) have been shown to be the main source of the anti-inflammatory cytokine IL-10 when rodent cells are stimulated with commensal bacterial products.35 In addition, it is not clear whether their sonicates were derived from inflamed areas. More simply, the explanation may be a dosage effect as their sonicate was 10 times more concentrated than ours.
Our findings with sonicate from inflamed tissue agree with theirs, and this stimulatory effect would be consistent with a more aggressive species being present in a greater predominance in inflamed tissue. The degree of inflammation in the bacterial donor bowel has been found to influence profoundly the stimulatory effect of the sonicate derived from it (Sartor RB, personal communication, 2002). This provides evidence for bacterial pathogenesis of the initial inflammation in that local change to a more pathogenic microflora may initiate activation of the local mononuclear cells. This theory is supported by a recent study showing that lymphocytes respond to the presence of pathogens before active inflammation appears. T cells isolated from mesenteric lymph nodes of E faecalis mono-associated IL-10 knockout mice produced more interferon gamma when pulsed with E faecalis than with B vulgatus. T cells from wild type mice without this exposure did not show the increase.36
Our results using other specific sonicates are consistent with other published data regarding intense stimulation with B vulgatus and inhibition of, or low level proliferation of, LPMC and PBMC with E coli.37,38
We have shown that pouchitis derived sonicate (HetP) induces more proliferation in another individual’s lymphocytes (PBMC or LPMC) than non-pouchitis derived sonicate (HetNon) (fig 1B ▶). Extraneous factors such as the “cytokine soup”, and other host factors in which the bacteria making up the sonicate may have existed before collection, were carefully excluded as colonies were grown from smears of biopsies and were later harvested from the agar. The constituent species of the sonicates was not something we set out to determine. Feasibly, a pathogenic species is present in all pouches but simply in greater number in inflamed ones. However, our examination of the role of the flora in pouches shows that key differences exist and that elements present in pouchitis flora may specifically activate inflammation.
Metronidazole is effective treatment for pouchitis.9 Because it has been suggested (although not all would agree39,40) that the action of MTZ might be as an immune modulator,41–43 we attempted to separate its effect on lymphocyte stimulation from its effect on the bacteria involved in the stimulation. Thus, the MTZ was introduced in the agar rather than into the cell culture. The concentration was similar to that in serum following oral dosing. A fall to baseline of the lymphocyte proliferation when pouchitis flora were grown on MTZ impregnated medium instead of conventional medium was seen (fig 1C ▶).
A smaller number of further assays was conducted to examine the effect of direct addition of MTZ or its main metabolite to cell cultures at a concentration found to exert an inhibitory effect in previous studies. No such reduction in proliferation was seen across assays using, variously, no stimulus, mitogen (PHA), or B vulgatus sonicate (fig 2 ▶ A–F). This makes it unlikely that our results reflect carry over of MTZ into cell cultures via incorporation into growing bacteria where it then directly inhibited lymphocyte proliferation. Rather, the organisms responsible for the proliferation appear to be inhibited by the MTZ. This suggests that the reason for the in vivo efficacy of MTZ is its antibacterial action.
The pathogenic species whose presence we propose is shown in our experiment and which are important in the aetiology of pouchitis are, therefore, metronidazole sensitive. MTZ has no action against aerobes or facultative anaerobes. It is active against anaerobes especially B fragilis and other bacteroides and is also bactericidal for fusobacteria, eubacteria, clostridia, and anaerobic cocci. Although it has an antiprotozoal action it is ineffective against mycobacteria.
CONCLUSION
We have shown that pouchitis derived bacterial sonicate stimulates healthy pouch patients’ mononuclear cells significantly more than non-pouchitis derived sonicate. Taking mononuclear cell proliferation as a surrogate marker for inflammation, this makes the eventual demonstration of Koch’s third postulate (which stipulates that disease be induced in a suitable host by inoculation with the isolated putative agent) more likely in pouchitis. Moreover, we have shown that the stimulatory species is or are MTZ sensitive.
We believe that this provides strong evidence for a bacterial aetiology in pouchitis. We feel this justifies investing in total 16S rRNA analysis of pouchitis, IBD, and control flora to determine the underlying differences in the microflora composition between healthy and inflamed tissue and, particularly, in the metronidazole sensitive population.
Abbreviations
IBD, inflammatory bowel disease
LPMC, lamina propria mononuclear cells
MTZ, metronidazole
PBMC, peripheral blood mononuclear cells
PDAI, pouch disease activity index
PHA, phytohaemagglutinin
SI, stimulation index
UC, ulcerative colitis
Aut, a sonicate derived from pouch flora of same patient whose LPMC/PBMC are under stimulation
Het, a sonicate derived from pouch flora of a different patient from the one whose LPMC/PBMC are under stimulation
P, a sonicate derived from pouch flora of a patient with pouchitis
Non, a sonicate derived from pouch flora of a patient without pouchitis
M, a sonicate derived from pouch flora grown in the presence of MTZ
These data were published in abstract form (Gastroenterology 2002;122:A261–2) and were presented orally at the Tripartite Colorectal Conference, Melbourne, 2002.
REFERENCES
- 1.Parks AG, Nicholls RJ. Proctocolectomy without ileostomy for ulcerative colitis. BMJ 1978;2:85–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madden MV, Farthing MJ, Nicholls RJ. Inflammation in ileal reservoirs: ‘pouchitis’. Gut 1990;31:247–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campieri M , Gionchetti P. Bacteria as the cause of ulcerative colitis. Gut 2001;48:132–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings JH, Macfarlane GT, Macfarlane S. Intestinal bacteria and ulcerative colitis. Curr Issues Intest Microbiol 2003;4:9–20. [PubMed] [Google Scholar]
- 5.Sartor RB. Targeting enteric bacteria in treatment of inflammatory bowel diseases: Why, how, and when. Curr Opin Gastroenterol 2003;19:358–65. [DOI] [PubMed] [Google Scholar]
- 6.Rath HC, Herfarth HH, Ikeda JS, et al. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest 1996;98:945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun 1998;66:5224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harper PH, Lee EC, Kettlewell MG, et al. Role of the faecal stream in the maintenance of Crohn’s colitis. Gut 1985;26:279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madden MV, McIntyre AS, Nicholls RJ. Double-blind crossover trial of metronidazole versus placebo in chronic unremitting pouchitis. Dig Dis Sci 1994;39:1193–6. [DOI] [PubMed] [Google Scholar]
- 10.Gionchetti P , Rizzello F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology 2000;119:305–9. [DOI] [PubMed] [Google Scholar]
- 11.Gionchetti P , Rizzello F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology 2003;124:1202–9. [DOI] [PubMed] [Google Scholar]
- 12.Mimura T , Rizzello F, Helwig U, et al. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut 2004;53:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrell RJ, LaMont JT. Microbial factors in inflammatory bowel disease. Gastroenterol Clin N America 2002;31:41–62. [DOI] [PubMed] [Google Scholar]
- 14.Rutgeerts P , Hiele M, Geboes K, et al. Controlled trial of metronidazole treatment for prevention of Crohn’s recurrence after ileal resection. Gastroenterology 1995;108:1617–21. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein LH, Frank MS, Brandt LJ, et al. Healing of perineal Crohn’s disease with metronidazole. Gastroenterology 1980;79:357–65. [PubMed] [Google Scholar]
- 16.Casellas F , Borruel N, Papo M, et al. Antiinflammatory effects of enterically coated amoxicillin-clavulanic acid in active ulcerative colitis. Inflamm Bowel Dis 1998;4:1–5. [DOI] [PubMed] [Google Scholar]
- 17.Swidsinski A , Ladhoff A, Pernthaler A, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology 2002;122:44–54. [DOI] [PubMed] [Google Scholar]
- 18.Neut C , Bulois P, Desreumaux P, et al. Changes in the bacterial flora of the neoterminal ileum after ileocolonic resection for Crohn’s disease. Am J Gastroenterol 2002;97:939–46. [DOI] [PubMed] [Google Scholar]
- 19.Ohkusa T , Okayasu I, Ogihara T, et al. Induction of experimental ulcerative colitis by Fusobacterium varium isolated from colonic mucosa of patients with ulcerative colitis. Gut 2003;52:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duffy MP, O’Mahony LP, Coffey JCA, et al. Sulfate-reducing bacteria colonize pouches formed for ulcerative colitis but not for familial adenomatous polyposis. Dis Colon Rectum 2002;45:384–8. [DOI] [PubMed] [Google Scholar]
- 21.Chiba M , Kono M, Hoshina S, et al. Presence of bacterial 16S ribosomal RNA gene segments in human intestinal lymph follicles. Scand J Gastroenterol 2000;35:824–31. [DOI] [PubMed] [Google Scholar]
- 22.Macpherson A , Khoo UY, Forgacs I, et al. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut 1996;38:365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda H , Fujiyama Y, Andoh A, et al. Characterization of antibody responses against rectal mucosa-associated bacterial flora in patients with ulcerative colitis. J Gastroenterol Hepatol 2000;15:61–8. [DOI] [PubMed] [Google Scholar]
- 24.Cohavy O , Bruckner D, Gordon LK, et al. Colonic bacteria express an ulcerative colitis pANCA-related protein epitope. Infect Immun 2000;68:1542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y , van Kruiningen HJ, West AB, et al. Immunocytochemical evidence of Listeria, Escherichia coli, and Streptococcus antigens in Crohn’s disease. Gastroenterology 1995;108:1396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bull TJ, McMinn EJ, Sidi-Boumedine K, et al. Detection and verification of Mycobacterium avium subsp. paratuberculosis in fresh ileocolonic mucosal biopsy specimens from individuals with and without Crohn’s disease. J Clin Microbiol 2003;41:2915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Relman DA. The search for unrecognized pathogens. Science 1999;284:1308–10. [DOI] [PubMed] [Google Scholar]
- 28.Sandborn WJ, Tremaine WJ, Batts KP, et al. Pouchitis after ileal pouch-anal anastomosis: a Pouchitis Disease Activity Index. Mayo Clin Proc 1994;69:409–15. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds JEF.ed. Martindale-The Extra Pharmacopoeia. 31st edn. London: Pharmaceutical Press, 1996.
- 30.Bouhnik Y , Pochart P, Marteau P, et al. Fecal recovery in humans of viable Bifidobacterium sp ingested in fermented milk. Gastroenterology 1992;102:875–8. [DOI] [PubMed] [Google Scholar]
- 31.Stebbings S , Munro K, Simon MA, et al. Comparison of the faecal microflora of patients with ankylosing spondylitis and controls using molecular methods of analysis. Rheumatology 2002;41:1395–401. [DOI] [PubMed] [Google Scholar]
- 32.Van De Merwe JP, Stegeman JH, Hazenberg MP. The resident faecal flora is determined by genetic characteristic of the host. Implications for Crohn’s disease? Antonie van Leeuwenhoek 1983;49:119–24. [DOI] [PubMed] [Google Scholar]
- 33.Toivanen P , Vaahtovuo J, Eerola E. Influence of major histocompatibility complex on bacterial composition of fecal flora. Infect Immun 2001;69:2372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duchmann R , Kaiser I, Hermann E, et al. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol 1995;102:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoentjen F , Tonkonogy SL, Sprengers D, et al. Different cytokine profiles in mesenteric lymph node cells from HLA B27 transgenic versus wild-type rats stimulated with cecal bacterial antigen. Gastroenterology 2001;120:A516. [Google Scholar]
- 36.Kim SC, Tonkonogy SL, Balish E, et al. Bacterial antigen specific T-cell activation precedes intestinal inflammation in Enterococcus faecalis monoassociated IL-10 deficient mice. Gastroenterology 2002;122 (Suppl 1) :A85–A86. [Google Scholar]
- 37.Khoo UY, Proctor IE, Macpherson AJ. CD4+ T cell down-regulation in human intestinal mucosa: evidence for intestinal tolerance to luminal bacterial antigens. J Immunol 1997;158:3626–34. [PubMed] [Google Scholar]
- 38.Klapproth JM, Scaletsky IC, McNamara BP, et al. A large toxin from pathogenic Escherichia coli strains that inhibits lymphocyte activation. Infect Immun 2000;68:2148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganrot-Norlin K , Stalhandske T, Karlstrom R. Lack of antiinflammatory activity of metronidazole. Acta Pharmacol Toxicol (Copenh) 1981;49:130–3. [DOI] [PubMed] [Google Scholar]
- 40.Anderson R , Oosthuizen R, Maritz C, et al. Effects of metronidazole on certain functions of human blood neutrophils and lymphocytes. S Afr Med J 1979;55:593–6. [PubMed] [Google Scholar]
- 41.Arndt H , Palitzsch K-D, Grisham MB, et al. Metronidazole inhibits leukocyte-endothelial cell adhesion in rat mesenteric venules. Gastroenterology 1994;106:1271–6. [DOI] [PubMed] [Google Scholar]
- 42.Elizondo G , Montero R, Herrera JE, et al. Lymphocyte proliferation kinetics and sister-chromatid exchanges in individuals treated with metronidazole. Mutat Res 1994;305:133–7. [DOI] [PubMed] [Google Scholar]
- 43.Grove DI, Mahmound AA, Warren KS. Suppression of cell-mediated immunity by metronidazole. Int Arch Allergy Appl Immunol 1977;54:422–7. [DOI] [PubMed] [Google Scholar]