Abstract
Background: Endogenous cyclooxygenase (COX) activity is required to maintain a relatively alkaline surface pH at the gastric luminal surface.
Aims: The purpose of this study was to determine which COX isoform, COX-1 or COX-2, is responsible for regulating the protective surface pH gradient and to test if COX inhibitors also had non-COX mediated effects in vivo.
Methods: Immunofluorescence and western blot analysis showed constitutive expression of both COX isoforms in the normal mouse stomach. We used in vivo confocal microscopy to measure pH near the mucosal surface of anaesthetised COX-1 (−/−), COX-2 (−/−), or wild-type mice of the same genetic background.
Results: When the gastric mucosal surface was exposed and superfused (0.2 ml/min) with a weakly buffered saline solution (pH 3) containing the pH indicator Cl-NERF, the pH directly at the gastric surface and thickness of the pH gradient were similar in wild-type and COX-2 (−/−) mice, but COX-1 (−/−) mice had a significantly thinner pH gradient. Addition of indomethacin had minimal effects on the residual surface pH gradient in COX-1 (−/−) mice, suggesting no role for COX-2 in surface pH regulation. Whole stomach perfusion studies demonstrated diminished net alkali secretion in COX-1 (−/−) mice, and application of SC-560 or rofecoxib to wild-type mice and mutant mice confirmed that only COX-1 inhibition reduced alkali secretion.
Conclusion: COX-1 is the dominant isoform regulating the normal thickness of the protective surface pH gradient in mouse stomach.
Keywords: gastric pH, confocal microscopy, cyclooxygenase isoform, immunofluorescence, mucosal, defence
Between meals, the stomach lumen becomes extremely acidic. One gastric defence mechanism against damaging back diffusing acid is an alkaline pH environment at the tissue surface that helps buffer the luminal acid.1–3 This protective pH gradient is the result of regulated bicarbonate secretion in combination with an unstirred layer that is enhanced at the interface between the aqueous luminal content and the non-aqueous mucus gel layer and/or tissue surface.4 We and others have observed that the pH gradient extends beyond the boundaries of the mucus gel layer, suggesting an extensive unstirred layer and avid secretion.3,4 Previously, we have shown that cyclooxygenase (COX) activity regulates the alkaline surface pH that is formed in response to fasted luminal pH in mice.5 COX is the rate limiting enzyme involved in the synthesis of prostaglandins from arachidonic acid. Prostaglandins are known to act as important second messengers in many regulatory mechanisms in the body and have been implicated as crucial to cytoprotection in the stomach.1,6 The goal of this study was to better understand the requirement for COX activity in regulating surface pH in the stomach.
The COX enzyme has two well defined isoforms: COX-1 and COX-2. These enzymes are 70% homologous in structure and are identical at the active site.7 The major structural difference between the two isoforms is that COX-2 has a side pocket in the hydrophobic channel that leads to the active site.7 Both isoforms have similar Km values for arachidonic acid8 and both, through a cyclooxygenase reaction followed by a hydroperoxidase reaction, convert arachidonic acid to prostaglandin H2 (PGH2). Depending on the complement of enzymes expressed in an individual cell type, PGH2 can then be converted to prostacyclin, thromboxane, or the closely related prostaglandins via various isomerases and reductases. Although COX-1 and COX-2 catalyse the same reaction, there are some differences found in the substrates used and the products made by both isoforms. It has been shown that COX-2 is able to use a wider variety of substrate structures compared with COX-1.9 Reddy and Hershman8 have also found evidence that arachidonic acid released under certain conditions is only converted to prostaglandin by COX-2 and not COX-1, even though COX-1 is present and functional (suggesting that the isoforms may utilise different pools of arachidonic acid). Studies have also shown that lypoxin A4 is synthesised from the acetylated COX-2 isoform only.10,11
With the emerging complexity of these isoforms, it has been difficult to clearly define the role of each isoform in gastric protection. It is generally believed that COX-1 is the constitutively expressed isoform found in most tissues, including the stomach, during normal physiological conditions and COX-2 is induced during pathological conditions such as stress or inflammation. However, some studies have shown that COX-1 can also be induced6,12 and that COX-2 is constitutively expressed and plays a homeostatic role in many tissues.6,12–18 When both COX isoforms are inhibited, gastric lesions form.19,20 However, when either COX-1 or COX-2 alone is inhibited (or genetically disrupted) no gastric damage is seen.19,20 This suggests that both isoforms play a role in gastric mucosal defence. One way that COX activity may defend the gastric mucosa is by regulating pH at the gastric surface. Previously, we have shown that net alkali secretion by the whole mouse stomach can be significantly reduced by treatment with a general COX inhibitor (indomethacin) or COX-1 inhibitor (SC-560). Inhibiting COX-2 (NS-398) had no effect on net alkali secretion. Although these studies suggest that COX-1 plays a key role in luminal pH regulation, rigorous testing to confirm the specificity of the drugs in vivo and the effect of eliminating COX-1 or COX-2 activity on pH gradients at the gastric surface have not been performed.
To better understand the role of COX activity in regulating gastric surface pH, we first determined expression of COX-1 and COX-2 in the mouse corpus. We then determined which COX isoform was responsible for pH control in the stomach, using wild-type, COX-1 knockout (−/−), and COX-2 knockout (−/−) mice in combination with either in vivo confocal microscopy to directly examine the surface pH or whole stomach perfusion to measure net gastric acid/alkali secretion. As our previous study5 has shown that inhibition of COX activity does not affect basal acid secretion in the pH 3 luminal condition (approximating the pH in fasted rodent stomach), we focused on the COX isoform specificity for regulation of alkali secretion.
METHODS
Animals
COX-2 knockout mice (B6;129S-Ptgs2tm1Jed) were purchased from Jackson Laboratories (Bar Harbor, Maine, USA) and bred as heterozygotes.21 COX-1 knockout mice (B6;129P-Ptgs1tm1) were purchased from Taconic (Germantown, New York, USA) and bred as female heterozygotes (+/−) mated with male homozygote knockout (−/−) mice.22 At 10 days old, animals were ear tagged and tail clipped for genotyping. DNA was isolated (DNeasy Tissue Kit; Qiagen, Valencia, California, USA) and genotype determined by polymerase chain reaction (PCR) amplification of the wild-type and/or mutated alleles. Genotyping of COX-1 knockout mice was performed exactly according to the protocol supplied by Taconic. For genotyping COX-2 knockout mice, we used primers (100P (5′ GCC ACC TCC GCT GCC ACC TCT GCG A 3′) and 200M (5′ CAT ACA TTC CCC ACG GTT TTG A 3′); Sigma, St Louis, Missouri, USA), suggested by SK Dey (Department of Molecular and Integrative Physiology, University of Kansas Medical Center), to amplify the wild-type COX-2 allele. To detect the mutated COX-2 allele, we used a 5′ primer (MDNEO-5, 5′ GAC AAT AGC AGG CAT GCT GG 3′; Sigma) that recognised the poly A tail of the genomically inserted neomycin resistance gene and a 3′ primer (MR547; Sigma) suggested by Jackson Laboratories that recognised a sequence within the COX-2 gene. The primer reaction mix was composed of 10× PCR buffer, 25 mM MgCl2, 2.5 mM dNTP, 5 U/μM Amplitaq Gold (Applied Biosystems, Foster City, California, USA), and either 10 μM 200 M + 10 μM 100P or 20 μM MR547 + 20 μM MDNEO-5. The COX-2 amplification was run for 37 cycles. The first cycle was run at 95°C for three minutes and then 35 cycles of: 94°C for 35 seconds, 62/52°C for wild-type/mutant, respectively, for 45 seconds, and then 72°C for 45 seconds. In the final cycle, 72°C extension was run for five minutes. The Animal Care and Use Committee of Indiana University approved all experimental procedures.
Immunostaining for COX-1 and COX-2 expression
Mice were anaesthetised with thiobutabarbitol sodium (Inactin; 100–150 mg/kg intraperitoneally). After laparatomy and catheterisation of the abdominal aorta, blood was washed out of the vasculature by perfusing phosphate buffered saline (PBS), pH 7.4, followed by 10 ml of 4% paraformaldehyde in PBS. After in situ fixation, the stomach was excised, opened along the lesser curvature, gastric contents were washed out, and small segments of the corpus region were dissected and kept in the same fixative for 2–4 hours. After washing in PBS, specimens were gradually infiltrated with increasing sucrose concentrations by immersion in PBS containing 5% sucrose for 15 minutes, 10%, 15%, and 20% sucrose for one hour each, and 30% sucrose overnight at 4°C. Tissues were finally embedded in Tissue-Tek OCT compound, frozen in isopentane precooled with liquid nitrogen, and stored at −70°C. Sections 8–10 μm thick were cut on a cryostat at −20°C, mounted on poly-lysine coated slides, and kept at −20°C until use.
Cryosections were rehydrated in PBS for 20 minutes at room temperature (RT). The residual aldehyde groups were quenched with 0.05 M NH4Cl in 0.01 M PBS (pH 7.4) for 20 minutes at RT. Non-specific antibody binding was blocked by incubation of the cryosections with 1% bovine serum albumin (BSA) or 10% normal sera of the host species for the secondary antibodies (as comparative blocking agents) in PBS for one hour at RT before addition of primary antibodies. Primary antibodies were diluted in PBS containing 0.1% BSA or 1% normal serum, respectively, as blocking buffer. Between incubations with antibodies, sections were washed thoroughly (5×15 min). Wash after quenching and final washing were with PBS.
Primary antisera were affinity purified antimouse polyclonals: goat anti-COX-1 (Santa Cruz Biotechnology, California, USA) and rabbit anti-COX 2 (Cayman Chemical, Ann Arbor, Michigan, USA). For double immunostaining, sections were incubated simultaneously or sequentially with both primary antisera at 2 μg/ml each (anti-cox-1 diluted 1:100 and anti-cox-2 diluted 1:200, respectively) overnight at 4°C. Immunoreactivity for COX-1 and COX-2 was detected with an Alexa 488 or 546 conjugated antigoat and antirabbit, respectively, secondary antibody (hosts: rabbit or donkey, goat or chicken, respectively; Molecular Probes, Eugene, Oregon, USA), 1:1000 diluted in PBS. Incubation with the secondary antibodies was performed for two hours at RT. Control tissues (data not shown) were prepared in an identical manner but omitting primary or secondary antibodies. Normal sera from the host species contributing the primary antibodies were used as non-immune controls. Additional controls were performed with primary antibodies preabsorbed 1:1 with a blocking peptide, either identical to the immunising peptide used to generate the antibody or the peptide used to generate the antibody for the other isoform. Cryosections were mounted in an aqueous anti-fade medium (ProLong; Molecular Probes). Sections were examined with a LSM 510 confocal microscope using excitation and emission wavelengths appropriate for Alexa 488 and 546. Final images were constructed with MetaMorph and Adobe Photoshop software. For each fluorescent probe, identical settings were used for image collection and all image processing, to assure valid comparisons between genotypes.
Protein expression in fasted versus fed stomach
COX-1 and COX-2 expression were determined using western blot analysis. Whole stomachs were harvested from COX-1 (+/−), COX-1 (−/−), COX-2 (+/−), and COX-2 (−/−) mice that were fed or fasted overnight. Stomach tissue was placed in liquid nitrogen and ground into a powder. Protein was extracted in Halt protease inhibitor cocktail (Pierce, Rockford, Illinois, USA). Protein concentration was determined using a BCA Protein Assay Kit (Pierce). Protein (20 μg) was separated on a 7.5% acrylamide gel and then transferred to a PVDF membrane (Immobilon-P; Millipore Corporation, Billerica, Massachusetts, USA). Membranes were blocked in 3% milk buffer (150 mM NaCl + 10 mM Tris + 0.05% Tween-20) for two hours and then incubated with murine polyclonal antisera against COX-1 or COX-2 (1:1000 dilution; Cayman Chemical Co.) for two hours. Membranes were then washed in buffer and incubated in goat antirabbit secondary antibody conjugated to horseradish peroxidase (1:5000 dilution; Jackson Immunoresearch Laboratories, West Grove, Pennsylvania, USA) for one hour. An ECL Western Blotting Analysis System Kit (Amersham Biosciences, Little Chalfont, UK) was used to detect the secondary antibody. Images of the blots were developed using Digital Fuji Phosphoimager.
Confocal microscopy
Animal preparation
The surgical procedure has been described previously.5 Briefly, adult animals (3–4 months old) were fasted overnight with free access to water in raised bottom cages to prevent coprophagia. The stomachs of anaesthetised mice were exteriorised and everted to expose the gastric mucosa. Tracheotomy was performed to facilitate breathing. The mouse was placed supine on the stage of an inverted confocal microscope (Zeiss LSM 510) such that a portion of the exposed mucosa protruded into a perfusion chamber. The perfusion chamber and stage were warmed to keep the animal’s body temperature at 37°C. At the end of the experiment, animals were sacrificed by halothane overdose.
Cl-NERF pH imaging
The protocol for imaging mouse gastric surface pH has been described previously.5 Briefly, a pH sensitive extracellular dye, Cl-NERF (Molecular Probes), was placed in the solution (pH 3, 150 mM NaCl + 4 mM Homopipes + 10 μM Cl-NERF) flowing over the tissue at 0.2 ml/min (KD Scientific push/pull syringe pump). Tissue was visualised in the Zeiss LSM510 confocal microscope (Zeiss C-Apo 10× objective), while alternately exciting Cl-NERF at 514 nm and 458 nm to obtain a ratiometric image (>530 nm fluorescence) that was calibrated to extracellular pH values.
Measuring whole stomach acid/base secretion
The surgical procedure has been described previously.5 Briefly, using anaesthetised mice, a cannula was inserted down the oesophagus and into the stomach to flow fresh perfusate into the stomach, and gastric effluent was directed out through a pyloric cannula. A lightly buffered saline solution (pH 3, 150 mM NaCl + 4 mM Homopipes) was perfused through the stomach and a flow-thru electrode measured pH in the effluent flow line. The measured buffering capacity of the perfusate solution was used in combination with the measured change in pH (as perfusion rate was changed from 0.2 to 0.05 ml/min) to determine the net acid/alkali secretion of the whole stomach per minute. As described previously,5 transient changes in perfusion flow rate were used to amplify the contribution of gastric secretions and thereby increase the resolution of small pH changes. Body temperature was maintained at 37°C by placing a heating lamp over the animal and monitoring temperature with a thermister (YSI, Colorado Springs, Colorado, USA). Net acid/alkali secretion was measured under control conditions or one hour after a COX-1 inhibitor, SC-560 (10 mg/kg intraperitoneally) or a COX-2 inhibitor, rofecoxib (10 mg/kg intraperitoneally).
Chemicals
Drugs used were thiobutabarbitol (Inactin; RBI, Natick Massachusetts, USA), indomethacin (Sigma Chemical Co.), Homopipes (Research Organics Inc., Cleveland, Ohio, USA), Cl-NERF (Molecular Probes Inc.), SC-560 (Cayman Chemical Co.), rofecoxib (Merck, Rahway, New Jersey, USA), and 16,16-dimethyl prostaglandin E2 (ICN, Costa Mesa, California, USA). Indomethacin was dissolved in absolute ethanol and then diluted with saline to the desired concentration (final EtOH <0.1%). SC-560 and rofecoxib were suspended in saline with Tween 80 (7.5%) and were heated to dissolve. All other agents were in saline.
Statistics
Data are presented as mean (SEM). Paired two tailed Student’s t test was used for statistical comparisons between paired results where each animal was its own control before and after treatment. Unpaired two tailed t test was used for comparison of results between genotypes. A p value of <0.05 was considered significant.
RESULTS
Immunostaining for COX-1 and COX-2 expression
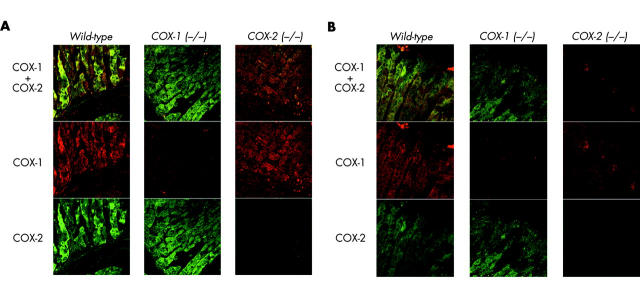
To understand how COX activity regulates gastric surface pH, we first determined if both COX isoforms were present in tissue mediating active acid and alkali secretion. Immunofluorescence analysis of the wild-type mouse corpus showed that both COX-1 and COX-2 were expressed in epithelial cells at the base of gastric glands (fig 1A ▶) and near the mucosal surface (fig 1B ▶). The upper portion of the glands and some neck and surface cells showed weaker and more sporadic immunostaining for both COX isoforms, with COX-1 expression mainly in the stroma (fibroblast-like cells, together with macrophages and polymorphonuclear cells, by size and shape). Dual immunostaining for COX-1 and COX-2 showed that both isoforms were frequently expressed in the same cells. Results using COX-1 (−/−) and COX-2 (−/−) mice showed that the anti-COX antisera were specific, and that there was no strong induction of the remaining COX isoform in COX knockout animals.
Figure 1.
Cyclooxygenase (COX)-1 and COX-2 immunofluorescence. Sections of fixed mouse corpus were immunolabelled with goat antimouse COX-1 and rabbit antimouse COX-2 polyclonal antisera. Immunofluorescence for COX-1 (red, Alexa 546) and COX-2 (green, Alexa 488) was evaluated in wild-type, COX-1 (−/−), and COX-2 (−/−) mice. Confocal microscopy imaged immunofluorescence at the base of gastric glands (A) and at the gastric surface (B).
COX-1 and COX-2 expression in the fasted versus fed condition
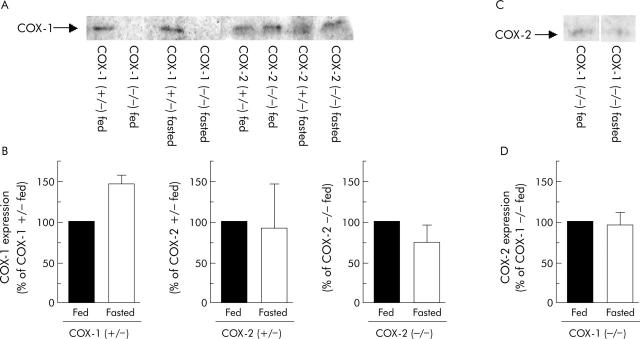
Because mice were fasted before each experiment to clear the stomach, we needed to determine if COX expression levels were different in the fasted versus the fed stomach. Western blot analysis showed that COX-1 was expressed in COX-1 (+/−), COX-2 (+/−), and COX-2 (−/−) mice with no significant differences in any genotype between the fasted and fed condition. Representative results are shown in fig 2A ▶ and the results compiled in fig 2B ▶. Due to cross reactivity of the anti-COX-2 antibody for the COX-1 isoform in western analyses (data not shown), the only reliable measure of COX-2 expression was in COX-1 (−/−) mice. In these mice, results showed that COX-2 was expressed and protein levels did not change with fasting (fig 2C and 2D ▶ ▶, respectively).
Figure 2.
Cyclooxygenase (COX)-1 and COX-2 expression in fasted versus fed mouse stomach. Western blot analysis was used to determine COX-1 expression (A, B) in the fasted and fed stomach of wild-type, COX-1 (+/−), COX-1 (−/−), COX-2 (+/−), and COX-2 (−/−) mice. COX-2 expression (C, D) was examined in the fasted and fed stomach of COX-1 (−/−) mice. Kodak Digital Science image analysis was used to quantify COX expression (expressed as per cent of COX isoform expression in fed condition—B, D). Values are mean (SEM), n = 3 for each genotype.
Surface pH response in knockout mice
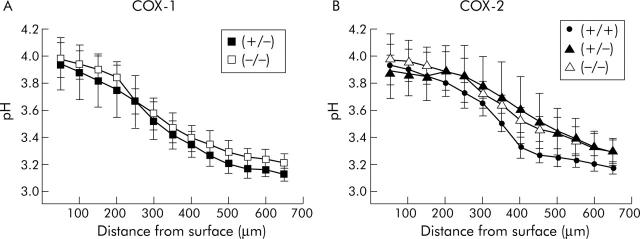
We first determined whether each mutant mouse genotype had equivalent capacity for surface pH regulation in response to exogenous prostaglandins. The gastric mucosa was superfused with a pH 3 solution containing Cl-NERF, a dye previously shown to diffuse rapidly into the juxtamucosal space,4 and allowing measurement of pH directly at the gastric surface. When alkali secretion was stimulated downstream of COX activity with exogenous dimethyl-prostaglandin E2 (dm-PGE2 0.1 mg/kg intraperitoneally), all genotypes showed a similar alkaline pH gradient at the gastric surface (fig 3 ▶). Each genotype had a similar pH directly at the tissue surface (pHs 3.94 (0.20), 3.97 (0.13), 3.93 (0.08), 3.89 (0.20), and 3.98 (0.19) for COX-1 (+/−), COX-1 (−/−), wild-type, COX-2 (+/−), and COX-2 (−/−) respectively) and a similar thickness of the pH gradient (d0.5), defined as the distance from the tissue surface at which pH is halfway between the pH directly at the tissue surface and pH 3.0 (d0.5 280 (51), 367 (53), 367 (17), 475 (48), 440 (56) μm for COX-1 (+/−), COX-1 (−/−), wild-type, COX-2 (+/−), and COX-2 (−/−), respectively). Results indicate that alkali secretory mechanisms downstream of COX are functionally equivalent in all genotypes.
Figure 3.
Response to prostaglandin E2 (PGE2) in all cyclooxygenase (COX) mouse genotypes. Results were compiled from confocal measurements of extracellular pH (Cl-NERF ratio images) during continuous superfusion (0.2 ml/min) of the gastric mucosa, as described in the methods. pH was measured at various distances tangential to the mucosal surface 10–15 minutes after administration of exogenous dimethyl-PGE2 in mice derived from COX-1 (A) and COX-2 (B) knockout colonies (+, wild-type; −, mutated allele). Values are mean (SEM), n = 3–6 per genotype.
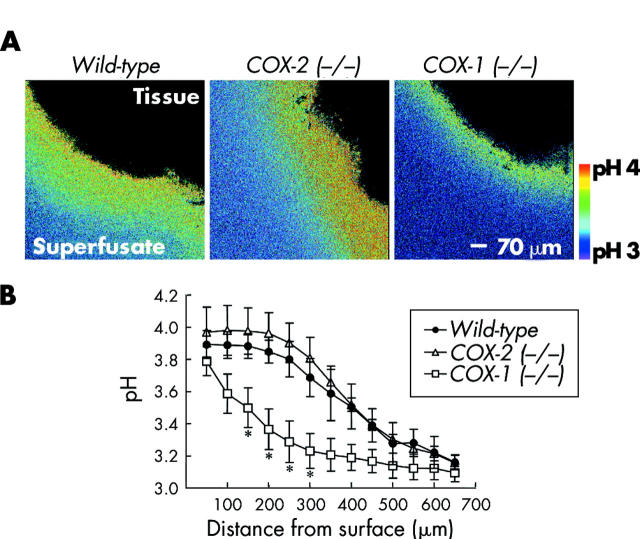
To determine which COX isoform regulates basal alkali secretion in response to low luminal pH, we measured unstimulated baseline surface pH in wild-type, COX-1 (−/−), and COX-2 (−/−) mice. Figure 4A ▶ shows qualitative ratio images of the relatively alkaline pH at the gastric surface in these mice (region imaged within tissue shown in black with extracellular Cl-NERF containing superfusate in colour). Quantitative analysis of the pH gradients at the gastric surface (fig 4B ▶) confirmed that the pH gradient of COX-2 (−/−) mice (pHs 3.97 (0.16), d0.5 380 (25) μm, n = 5) was not significantly different from wild-type mice (pHs 3.89 (0.08), d0.5 383 (44) μm, n = 3). COX-1 (−/−) mice showed a surface pH (pHs 3.79 (0.09), n = 6) not significantly different from wild-type mice. However, the thickness of the pH gradient in COX-1 (−/−) mice (d0.5 183±31 μm) was significantly reduced compared with wild-type, indicating weaker alkali secretion.
Figure 4.
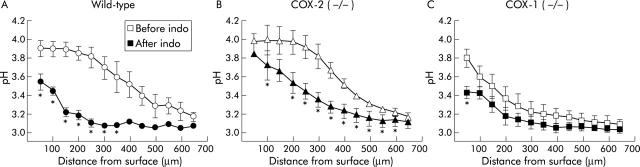
Surface pH gradients in unstimulated wild-type, cyclooxygenase (COX)-1 (−/−), and COX-2 (−/−) mice. Confocal imaging of surface pH during superfusion of unstimulated tissue at pH 3, as described in the methods. (A) Representative ratio images of Cl-NERF in wild-type, COX-2 (−/−), and COX-1 (−/−) mice are shown. (B) As in fig 3 ▶, pH was measured at various distances tangential to the mucosal surface in each genotype. Values are mean (SEM), n = 3–6 per genotype. *p<0.05 versus wild-type pH value at the same distance from the surface.
Results indicated that some level of surface pH control was retained in all genotypes in the basal state. To test if this was due to residual COX activity, indomethacin (5 mg/kg intraperitoneally) was added to block all COX enzyme activity and imaged the surface pH gradient response 60 minutes later. Figure 5A ▶ shows the baseline pH gradient of wild-type mice (pHs 3.89 (0.08), d0.5 383 (44) μm) and confirms that indomethacin significantly reduced the pH directly at the gastric surface (pHs 3.54 (0.08)) as well as the thickness of the pH gradient (d0.5 166 (33) μm) compared with baseline. When indomethacin was added to COX-2 (−/−) mice, the pH gradient was also disrupted, albeit less prominently (fig 5B ▶). The pH directly at the gastric surface (pHs 3.84 (0.20)) was not significantly reduced compared with baseline (pHs 3.97 (0.16)) but the thickness of the pH gradient was significantly reduced (d0.5 310 (56) μm) compared with baseline (d0.5 380 (25) μm). Results indicate that in COX-2 (−/−) mice, the remaining COX-1 is significantly contributing to maintenance of the pH gradient. In contrast, when indomethacin was added to COX-1 (−/−) mice (fig 5C ▶), the pH gradient did not significantly change (except for the data point closest to the gastric surface).
Figure 5.
Effect of indomethacin in wild-type, cyclooxygenase (COX)-1 (−/−), and COX-2 (−/−) mice. Results were compiled from confocal images of extracellular pH at the gastric surface in response to luminal pH 3 superfusion before and after treatment with indomethacin (indo 5 mg/kg intraperitoneally). pH gradients were measured in wild-type mice (A), COX-2 (−/−) mice (B), and COX-1 (−/−) mice (C). Values are mean (SEM), n = 3–6 per genotype. *p<0.05 versus pH value at the same distance from the surface in the absence of inhibitor.
Whole stomach secretion response in COX knockout mice
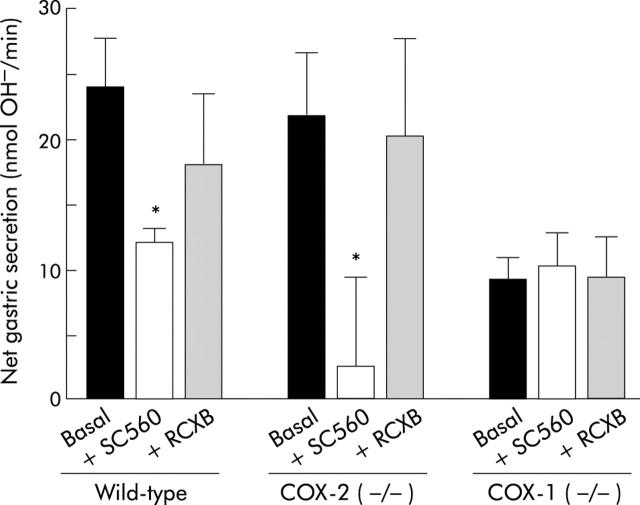
To confirm the roles of COX-1 and COX-2 in the alkali secretion that affects bulk luminal contents, net acid/alkali secretion by the whole stomach was measured in wild-type, COX-2 (−/−), and COX-1 (−/−) mice treated with inhibitors SC-560 (COX-1 inhibitor) and rofecoxib (COX-2 inhibitor). Stomachs were perfused with a pH 3 solution and net nmol OH− equivalents secreted by the stomach per minute were determined. Figure 6 ▶ shows the response of the whole stomach of three different genotypes during baseline conditions, one hour after COX-1 inhibition with SC-560 (10 mg/kg intraperitoneally), and one hour after COX-2 inhibition with rofecoxib (10 mg/kg intraperitoneally). Wild-type mice showed net alkali secretion (24.0 (3.8) nmol OH−/min, n = 7) in the baseline response to pH 3 perfusion. Net alkali secretion was significantly reduced after treatment with SC-560 but not with rofecoxib. Presuming isoform specificity of the drugs, these results suggest that the COX-1 isoform is required for maintaining basal alkali secretion. Baseline net alkali secretion in COX-2 (−/−) mice (21.7 (5.1) nmol OH−/min, n = 7) was similar in magnitude to that in wild-type mice, and administration of SC-560, but not rofecoxib, significantly reduced net alkali secretion. In contrast, baseline net alkali secretion in COX-1 (−/−) mice (9.1 (1.9) nmol OH−/min, n = 8) was significantly reduced compared with baseline secretion of wild-type mice, and neither SC-560 nor rofecoxib had any effect. This is an important control documenting the action of SC-560 as COX-1 specific in vivo.
Figure 6.
Effect of cyclooxygenase (COX)-1 and COX-2 inhibitors in wild-type, COX-2 (−/−), and COX-1 (−/−) mice. Intact stomachs of anaesthetised mice of the indicated genotype were perfused with a pH 3 solution. Net alkali secreted into the gastric effluent was calculated as described in the methods.5 For each genotype, results are shown before (Basal) or 60 minutes after addition of either a presumptive COX-1 inhibitor (SC-560 10 mg/kg) or a COX-2 inhibitor (rofecoxib 10 mg/kg (RCXB)). Values are mean (SEM), n = 3–8 animals per group. *p<0.05 versus basal.
DISCUSSION
Our results confirm previous work suggesting that COX-1 is the more critical COX isoform required for regulation of whole stomach alkali secretion in healthy tissue.5 Here we extended our results to examine COX isoform specificity for pH regulation at the gastric surface. Mice that did not express COX-1 showed a reduced basal alkali pH gradient at the gastric surface as well as decreased basal net alkali secretion by the whole stomach compared with mice with normal COX-1 and COX-2 expression. Conversely, disruption of the COX-2 gene or inhibition of the COX-2 enzyme (rofecoxib) did not affect the surface pH gradient or net alkali secretion compared with wild-type. COX-1 knockout mice were used to rigorously test the specificity of SC-560 for the COX-1 isoform in vivo. Inhibition of alkali secretion with SC-560 in mice with functional COX-1 and lack of effect in COX-1 (−/−) mice indicates that the effects of SC-560 are fully specific for the COX-1 isoform in vivo and do not effect net alkali secretion in a COX independent manner. Results agree with prior observations showing that stomach content pH was significantly more acidic in COX-1 (−/−) compared with wild-type mice.12 Langenbach and colleagues22 have also shown that gastric PGE2 levels in COX-1 (−/−) mice were not significantly different from indomethacin treated wild-type mice (both conditions were <1% of normal wild-type gastric PGE2 levels), fuelling speculation that COX-2 was either less important or perhaps not even present in normal gastric tissues.
When using constitutive knockout models, it is important to acknowledge that results may be due, wholly or in part, to potential secondary effects of inactivating the specific genes. For instance, COX-2 (−/−) mice have renal dysplasia,21 which may alter systemic acid/base homeostasis and thereby affect basal gastric secretions. However, such concerns are tempered by the observation that topical addition of indomethacin and/or PGE2 suggests that the gastric machinery remains intact in all genotypes for mediating alkali secretion and regulating alkali secretion by COX/PGs.
Studies have found both COX-1 and COX-2 mRNA expression in the normal rat13,14,23–25 and in human17,26 and mouse15 stomach. COX-1 protein has been found in the normal stomach and has been immunohistochemically localised to the neck13,23,25 and base24 of the gastric gland in the fundus region of the rat stomach. Electron microscopy has further defined COX-1 localisation to the apical cytoplasm and the endoplasmic reticulum membrane in the apical region of these cells.13 COX-2 expression has been more controversial. Looking at mRNA expression and immunohistochemistry, Tatsuguchi and colleagues26 were unable to detect COX-2 expression in the normal human stomach but did see expression in mesenchymal cells of the lamina propria or macrophages and fibroblast cells that were located near necrotic tissue in ulcerated gastric tissue. Several other investigators have also been unable to detect COX-2 expression in the normal stomach.23,27–29 Conversely, some studies have shown COX-2 expression in the normal stomach via northern blot analysis and reverse transcription-PCR techniques18 as well as immunohistochemical staining.13–16 Strongest COX-2 expression was found in the apical cytoplasm of the antral surface of mucous cells of rat13 and the base of gastric glands in the corpus of both rats and humans.14,16,17 The only previous study that examined COX isoform expression in the mouse stomach showed that COX-1 and COX-2 were constitutively expressed in gastric muscle.15 However, expression of the COX isoforms in the gastric glands of the corpus (site of acid and alkali secretion) was not examined. Our results for the first time used dual label immunofluorescence and COX knockout mice to test and compare the presence of both COX-1 and COX-2 in the gastric corpus of mice. Although present and frequently located in the same cells as COX-1, COX-2 does not appear to be playing a significant role in compensating for the decrease in alkali secretion when COX-1 is inactive.
Our results revealed that a COX independent mechanism is also important in supporting surface pH and net alkali secretion. Previously, we have shown that inhibiting both COX isoforms with indomethacin significantly reduces, but does not eliminate, the surface pH gradient and net alkali secretion in the stomach.5 It was unclear if the residual alkali secretion was due to incomplete inhibition of COX-1 enzyme or COX independent mechanisms. COX-1 (−/−) mice in either the presence or absence of COX-2 inhibition showed a similar residual alkali secretion and surface pH gradient as wild-type mice after addition of indomethacin. These data indicate that the stomach has residual alkali secretion in the complete absence of COX-1 activity that defends surface pH. In COX-2 (−/−) mice, indomethacin had a significant effect in diminishing the surface pH gradient (d0.5) and individual pH values at fixed distances from the surface in COX-2 (−/−) mice. However, the effect of indomethacin appears visually less marked in COX-2 (−/−) mice compared with wild-type mice (fig 5B ▶v 5A). This appearance is difficult to confirm or deny. Many of the individual values for COX-2 (−/−) mice plus indomethacin were significantly higher than those found in wild-type mice plus indomethacin but only one data point was different versus COX-1 (−/−) plus indomethacin. Thus we cannot reject with certainty the hypothesis that mice activate COX independent (indomethacin insensitive) mechanisms to support surface pH in the absence of COX-2. If the hypothesis is true, then it becomes difficult to reconcile with complementary evidence that there is no significant change in total gastric COX-1 protein levels or basal rates of alkali secretion, and that SC-560 is able to potently inhibit net alkali secretion in COX-2 (−/−) mice. However, it remains possible that the absence of COX-2 might upregulate compensatory mechanisms within the animal that could specifically alter the metabolism of indomethacin, or that COX-1 protein specifically in the gastric surface cells is either reduced or inactivated as COX independent mechanisms activate. We speculate that COX independent net alkali secretion may be due to nitric oxide (NO) production. NO performs many of the same functions in mucosal defence as prostaglandins. NO can stimulate gastric bicarbonate secretion and has been shown to prevent ulcers induced by suppression of prostaglandin synthesis.1
In summary, our data suggest that although constitutive COX-1 and COX-2 expression occur in the corpus of healthy mice, COX-2 does not play a major role in regulating corpus alkali secretion in the fasted state. Our results also suggest that any additional “stress” of being in the fasted state (at least overnight) is not sufficient to induce COX-2 expression. COX-2 is unequivocally important in mucosal defence because the absence of both COX isoforms is required in order to create gastric damage, and upregulated COX-2 expression is important in gastric ulcer healing. The precise role that constitutive COX-2 expression plays in normal gastric function remains largely unknown.
Acknowledgments
We are grateful to SK Dey (Department of Molecular and Integrative Physiology, University of Kansas Medical Center) for providing his protocol for genotyping COX-2 knockout mice. We also acknowledge the National Institutes of Health (RO1DK 54940) for financial support. HK Baumgartner is also supported by a Predoctoral Fellowship from the American Heart Association.
Abbreviations
COX, cyclooxygenase
PGH2, prostaglandin H2
RT, room temperature
PBS, phosphate buffered saline
BSA, bovine serum albumin
dm-PGE2, dimethyl-prostaglandin E2
d0.5, half thickness
pHs, pH at tissue surface
NO, nitric oxide
PCR, polymerase chain reaction
REFERENCES
- 1.Wallace JL, Granger DN. The cellular and molecular basis of gastric mucosal defense. FASEB J 1996;10:731–40. [DOI] [PubMed] [Google Scholar]
- 2.Engel E , Guth PH, Nishizaki Y, et al. Barrier function of the gastric mucus gel. Am J Physiol 1995;269:G994–9. [DOI] [PubMed] [Google Scholar]
- 3.Allen A , Flemstrom G, Garner A, et al. Gastroduodenal mucosal protection. Physiol Rev 1993;73:823–57. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner HK, Montrose MH. Regulated alkali secretion acts in tandem with unstirred layers to regulate mouse gastric surface pH. Gastroenterology 2004;126:774–83. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner HK, Kirbiyik U, Coskun T, et al. Endogenous cyclo-oxygenase activity regulates mouse gastric surface pH. J Physiol (Lond) 2002;5443:871–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atay S , Tarnawski AS, Dubois A. Eicosanoids and the stomach. Prostaglandins Other Lipid Mediat 2000;61:105–24. [DOI] [PubMed] [Google Scholar]
- 7.Bjorkman DJ. Does Helicobacter pylori infection increase the risk for NSAID-associated ulcers? Rev Gastroenterol Dis 2001;1:121–7. [PubMed] [Google Scholar]
- 8.Reddy ST, Herschman HR. Ligand-induced prostaglandin synthesis requires expression of the TIS10/PGS-2 prostaglandin synthase gene in murine fibroblasts and macrophages. J Biol Chem 1994;269:15473–80. [PubMed] [Google Scholar]
- 9.Vane JR, Botting RM. New insights into the mode of action of anti-inflammatory drugs. Inflamm Res 1995;44:1–10. [DOI] [PubMed] [Google Scholar]
- 10.Fiorucci S , de Lima OM, Mencarelli A, et al. Cyclooxygenase-2-derived lipoxin A4 increases gastric resistance to aspirin-induced damage. Gastroenterology 2002;123:1598–606. [DOI] [PubMed] [Google Scholar]
- 11.Goh J , Godson C, Brady HR, et al. Lipoxins: pro-resolution lipid mediators in intestinal inflammation. Gastroenterology 2003;124:1043–54. [DOI] [PubMed] [Google Scholar]
- 12.Langenbach R , Loftin C, Lee C, et al. Cyclooxygenase knockout mice: models for elucidating isoform-specific functions. Biochem Pharmacol 1999;58:1237–46. [DOI] [PubMed] [Google Scholar]
- 13.Iseki S . Immunocytochemical localization of cyclooxygenase-1 and cyclooxygenase-2 in the rat stomach. Histochem J 1995;27:323–8. [DOI] [PubMed] [Google Scholar]
- 14.Loogna P , Franzen L, Sipponen P, et al. Cyclooxygenase-2 and Bcl-2 expression in the stomach mucosa of Wistar rats exposed to Helicobacter pylori, N’-methyl- N’-nitro- N-nitrosoguanidine and bile. Virchows Arch 2002;441:77–84. [DOI] [PubMed] [Google Scholar]
- 15.Porcher C , Horowitz B, Bayguinov O, et al. Constitutive expression and function of cyclooxygenase-2 in murine gastric muscles. Gastroenterology 2002;122:1442–54. [DOI] [PubMed] [Google Scholar]
- 16.Vogiagis D , Glare EM, Misajon A, et al. Cyclooxygenase-1 and an alternatively spliced mRNA in the rat stomach: effects of aging and ulcers. Am J Physiol Gastrointest Liver Physiol 2000;278:G820–7. [DOI] [PubMed] [Google Scholar]
- 17.Jackson LM, Wu KC, Mahida YR, et al. Cyclooxygenase (COX) 1 and 2 in normal, inflamed, and ulcerated human gastric mucosa. Gut 2000;47:762–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Neill GP, Ford-Hutchinson AW. Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissues. FEBS Lett 1993;330:156–60. [DOI] [PubMed] [Google Scholar]
- 19.Wallace JL, McKnight W, Reuter BK, et al. NSAID-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology 2000;119:706–14. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka A , Araki H, Hase S, et al. Up-regulation of COX-2 by inhibition of COX-1 in the rat: a key to NSAID-induced gastric injury. Aliment Pharmacol Ther 2002;16:90–101. [DOI] [PubMed] [Google Scholar]
- 21.Dinchuk JE, Car BD, Focht RJ, et al. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature 1995;378:406–9. [DOI] [PubMed] [Google Scholar]
- 22.Langenbach R , Morham SG, Tiano HF, et al. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell 1995;83:483–92. [DOI] [PubMed] [Google Scholar]
- 23.Kato S , Ogawa Y, Okayama M, et al. Ulcerogenic influence of selective cyclooxygenase-2 inhibitors in the rat stomach with adjuvant-induced arthritis. J Pharm Exp Ther 2002;303:503–9. [DOI] [PubMed] [Google Scholar]
- 24.Berenguer B , de la Lastra CA, Moreno FJ, et al. Chronic gastric ulcer healing in rats subjected to selective and non-selective cyclooxygenase-2 inhibitors. Eur J Pharmacol 2002;442:125–35. [DOI] [PubMed] [Google Scholar]
- 25.Sun W , Tsuji S, Tsujii M, et al. Induction of cyclooxygenase-2 in rat gastric mucosa by rebamipide, a mucoprotective agent. J Pharm Exp Ther 2000;295:447–52. [PubMed] [Google Scholar]
- 26.Tatsuguchi A , Sakamoto C, Wada K, et al. Localisation of cyclooxygenase 1 and cyclooxygenase 2 in Helicobacter pylori related gastritis and gastric ulcer tissues in humans. Gut 2000;46:782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmassmann A , Peskar BM, Stettler C, et al. Effects of inhibition of prostaglandin endoperoxide synthase-2 in chronic gastro-intestinal ulcer models in rats. Br J Pharmacol 1998;123:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komori M . Gastrin enhances gastric mucosal integrity through cyclooxygenase-2 upregulation in rats. Am J Physiol Gastrointest Liver Physiol 2002;283:G1368–78. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi S , Shigeta J, Inoue H, et al. Localization of cyclooxygenase-2 and regulation of its mRNA expression in gastric ulcers in rats. Am J Physiol Gastrointest Liver Physiol 1998;275:G1137–45. [DOI] [PubMed] [Google Scholar]