Abstract
Background: Many patients with familial adenomatous polyposis (FAP) die from desmoid tumours which can arise spontaneously but often appear to be surgically induced by prophylactic colectomy. FAP results from germline adenomatous polyposis coli (APC) gene mutations and desmoids arise following biallelic APC mutation, with one change usually occurring distal to the second β-catenin binding/degradation repeat of the gene (3′ to codon 1399). We have suggested that because families with germline mutations in this region already have the requisite change, they are more likely to develop desmoids. However, there are families with 5′ germline mutations where desmoids are common.
Patients and methods: We examined desmoid risk dependent on germline APC mutation, sex, history of abdominal surgery, and family history in FAP patients from the St Mark’s Hospital Polyposis Registry.
Results: Overall desmoid prevalence was 15%. Desmoids tended to cluster in susceptible individuals, irrespective of the germline APC mutation. Independent predictors of increased desmoid risk were: germline mutation distal to codon 1399; any family history of disease; and a strong family history of desmoids. A family history of multiple desmoids (>1) increased an individual’s own risk of multiplicity. Females had twice the odds of developing desmoids compared with males. There was no significant interaction between any of the three explanatory variables.
Conclusions: Our results indicate the influence of unknown genetic factors independent of APC in susceptibility to desmoid tumours in FAP. The data have implications in terms of clinical management of FAP patients and assessing the balance between chemoprevention and prophylactic colectomy.
Keywords: familial adenomatous polyposis, desmoid tumours, adenomatous polyposis coli gene, family history
Desmoid tumours are rare non-metastasising but aggressive fibromatoses which may occur in normal individuals (where a somatic β-catenin mutation usually seems to be the explanation1,2) or in association with the hereditary colorectal cancer syndrome familial adenomatous polyposis (FAP). FAP is an autosomal dominant condition caused by germline mutation of the adenomatous polyposis coli (APC) gene and characterised by the development of hundreds to thousands of adenomatous polyps in the colon and rectum. Progression to colorectal cancer is inevitable if the colon is not surgically removed. However, even after colectomy, FAP patients remain at increased risk of other tumours, including duodenal adenomas and carcinomas, and desmoid tumours. Desmoids have a prevalence of 10–20% in FAP, arising with a frequency approximately 850 times that of the general population.3
Desmoid tumours are a major cause of morbidity and mortality in FAP patients. In a series at St Mark’s Hospital, London, UK, looking at FAP patients who had undergone prophylactic colectomy and ileorectal anastomosis, desmoid tumours presented as the third equal most common cause of death after duodenal tumours and metastases from an unknown primary.4 Other investigators have reported deaths attributable to desmoids to be the second commonest cause of death in FAP patients after colorectal cancer.5,6 The majority of tumours arise in the abdominal wall or small bowel mesentery, with smaller numbers arising on the trunk or limbs and causing morbidity and mortality by pressure on surrounding structures. The number of tumours per individual has been reported to range from 1 to 10.7,8 Current management strategies include non-steroidal anti-inflammatory drugs, anti-oestrogens, cytotoxic chemotherapy, and surgery. Radiotherapy has been used in the past but is unpopular in intra-abdominal tumours because of small bowel side effects. Trials of new drugs such as the antifibrinolytic agent imantinib mesylate are also being undertaken.9 However, these therapies are of very limited efficacy; consequently, there is urgent need for new modalities of treatment and greater understanding of the disease.
Sporadic desmoids are known to be associated with oestrogens while their role in the aetiology of FAP associated tumours is less clear. FAP associated desmoids have been linked to trauma, particularly abdominal surgery such as prophylactic colectomy,7,10,11 and also the position of the APC germline mutation. Caspari and colleagues12 found that desmoid disease in FAP was more common in patients with a germline APC mutation distal to codon 1444, an association supported by a number of other studies.13,14 Although only a limited number of desmoids have been examined, somatic APC mutations have been identified in these tumours.15–17 In all cases, one of the two APC mutations has been shown to lie near or beyond codon 1444. An association between the “two hits” at APC exists, as patients with 3′ germline mutations distal to codon 1444 tend to show allelic loss (loss of heterozygosity (LOH)) of the somatic allele in their desmoids whereas other patients’ tumours generally acquire a protein truncating mutation after codon 1444.17 Such a model would explain the more severe desmoid disease associated with germline mutations after codon 1444 on the basis that the “second hit” at APC is rate limiting for tumorigenesis. Initially, it was suggested that a germline mutation distal to codon 1444 led to a high prevalence of desmoid disease because any somatic mutation of the second allele of vulnerable cells led to desmoid formation.17 In patients with germline mutations before codon 1444, somatic mutations in desmoid cells had to occur after codon 1444, a relatively rare mutational event. Subsequently, this idea has been modified such that a combination of “first” and “second” hits is the important factor in providing an optimal level of β-catenin dysregulation for tumour growth. This requires retention of only two β-catenin binding repeats that lie immediately proximal to codon 1399. Thus patients with germline mutations distal to codon 1444 cannot simply acquire any type of “second hit”. Rather, LOH occurs spontaneously in vulnerable cells, at a relatively high frequency when compared with truncating APC mutations, and is only selected in these patients. The result is more severe desmoid disease.18,19
Despite this, and as is the case for gastrointestinal tumorigenesis, the position of the germline APC mutation does not explain all the phenotypic variation of desmoid disease in FAP. It remains to be seen whether there is interdependence or independence between a strong family history of desmoid disease and position of the germline mutation. To date, there is only one study that provides evidence of possible independence between the two risk factors.10
In this study, we have set out to refine associations between APC genotype and desmoid disease, and to find further evidence of genetic influences associated with desmoid development independent of the APC gene, similar to the modifier genes already proposed for the colorectum.20 We chose codon 1399 rather than codon 1444 as the cut off for assessing associations between germline APC mutations and desmoid disease for the following reasons: the number of β-catenin repeats remaining in the truncated APC protein has been shown to have functional importance in colorectal18,19 and upper gastrointestinal polyposis21 in FAP and codon 1399 lies immediately after the second 20 amino acid β-catenin binding repeat; and the original boundary at codon 1444 was chosen by Caspari and colleagues12 although there was no patient in their study with a germline mutation between codons 1380 and 1443.
On clinical grounds, desmoid disease is a severe, largely untreatable illness which is often the consequence of prophylactic colonic surgery to reduce cancer risk.7,10,11 Specific 3′ APC germline mutations associated with a high risk of desmoid disease are frequently associated with a lower density of colonic polyposis22–24 and hence a later and reduced cancer risk. In such cases, might the clinician elect to manage the colon by surveillance, resorting only to surgery when there is sufficient concern over dysplasia? And how should the clinician deal with cases where there is a strong family history of desmoid disease? In this study, we have set out to further define risk factors for desmoid disease in FAP so that further information can be gained when deciding how to manage at risk patients.
MATERIALS AND METHODS
Patients
All patients who attended St Mark’s Hospital for follow up of their FAP in whom the germline mutation was known were identified and family histories were obtained. In the vast majority of cases, genetic testing for the APC mutation was performed by the North West Thames Regional Genetics Service, and comprised evaluation of the entire gene. In those cases tested elsewhere it was confirmed by correspondence from the departments involved that analysis of the whole gene had been performed. Desmoid history within this cohort was then ascertained from Polyposis Registry records, with further data collected from patients’ notes, and x ray and histopathology reports. The following data were collected: site of germline mutation (before/after 1399); sex; history of previous abdominal surgery (any or none); whether tumours were single or multiple; number of at risk (that is, APC mutation positive) family members; and proportion of desmoid affected family members. Desmoid diagnosis was made according to a radiologist’s report of computed tomography scans (with or without magnetic resonance imaging scans) or as a surgical finding. A finding of putative desmoid precursor lesions at laparotomy or an equivocal radiological finding was not counted as a tumour. In order for desmoids to be considered multiple, there had to be clear radiological or surgical evidence of two or more separate tumours. Where this was equivocal, the patient was counted as having a single tumour.
Statistical analysis
Logistic regression was used to examine the effect of mutation location, sex, history of previous abdominal surgery, and family history on occurrence of desmoid tumours. Family history was defined according to the proportion of other APC mutation positive family members affected by desmoid disease. This was classified as no others affected, 1–49% of other members affected, or 50% or more affected. All family members, rather than just the index case, were included in the analysis. This was done both to improve the power of the study and also because the index case is in itself arbitrary. It was recognised that results from the same family members are likely to be more similar than results from different families. However, the advance in statistical methods means that there are methods that will cope with lack of independence between observations. We used a population averaged method for the analysis, by including robust standard errors with the logistic regression procedure. This method works by inflating the standard errors if the results from family members are more similar than results from different families and is designed to counter the potential bias encountered.
RESULTS
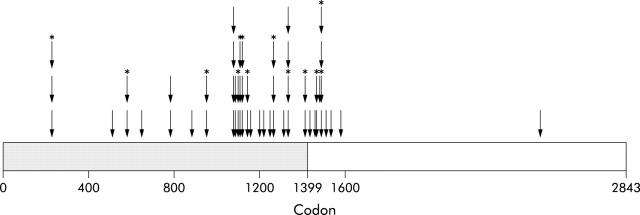
The APC germline mutation was known in 379 patients from 143 families. Of these, 94% (357/379) had a 5′ mutation (before codon 1399) and 6% (22/379) had a 3′ mutation (after codon 1399). The overall prevalence of desmoid disease was 15% (57/379), with affected individuals identified in 38 families (27%). The prevalence of disease in the 5′ (<1399) and 3′ groups was 12% (43/357) and 64% (14/22), respectively. The site of the germline APC mutation for each of these affected individuals is shown in fig 1 ▶. The influence of four variables (mutation location, sex, history of previous abdominal surgery, and family history) on desmoid occurrence was tested after exclusion of individuals who were the only FAP positive members of their family. In those individuals with no at risk relatives it was not possible to determine a family history. Therefore, it would be incorrect to include these individuals in the no family history of desmoids group. They could have been included in the univariate analyses of other factors but would then have to have been omitted from the multivariate analyses that included family history. For consistency, this small number of subjects was therefore completely omitted from all analyses. When such individuals were excluded, a total of 50 patients were identified from a total of 320 FAP positive individuals from 84 families, a prevalence of 16%.
Figure 1.
Position of germline adenomatous polyposis coli gene mutations in individuals with desmoids. Arrows indicate position of germline mutation in individuals affected by desmoid tumours. Arrows below *denote affected members of the same family. Gene subdivided into region before and after codon 1399 by shading.
In a univariate analysis, germline APC mutation after codon 1399, female sex, and family history were found to increase the risk of desmoid disease (table 1 ▶). Subjects with a germline mutation after codon 1399 were found to have a sevenfold increase in the odds of presenting with desmoid tumours (odds ratio (OR) 7.19 (95% confidence interval (CI) 1.27–40.67); p = 0.03). Females had twice the odds of developing desmoids compared with males (OR 2.15 (95% CI 1.02–4.65); p = 0.04). Abdominal surgery was not a predictor (p = 0.35) probably because almost all patients had undergone prophylactic colectomy. For analyses involving family history, three patient groups were created as described in the methods section. A family history of desmoids where 1–49% of family members were affected increased the risk threefold (OR 3.25 (95% CI 0.76–13.87); p = 0.001). Furthermore, a stronger family history of desmoids (⩾50% affected members) increased the odds over sevenfold (OR 7.66 (95% CI 2.53–23.25); p = 0.006).
Table 1.
Influence of family history, mutation location, sex, and history of abdominal surgery on desmoid occurrence
| Variable | No with desmoids/total No (%) | Univariate analysis | Multivariate analysis | ||
| Odds ratio (95% CI) | p Value | Odds ratio (95% CI) | p Value | ||
| Proportion of family members with desmoids | |||||
| None | 18/218 (8) | 1 | 0.001 | 1 | 0.006 |
| 1–49% | 12/53 (23) | 3.25 (0.76, 13.87) | 3.69 (0.86, 15.84) | ||
| 50%+ | 20/49 (41) | 7.66 (2.53, 23.25) | 6.29 (1.84, 21.53) | ||
| Mutation location | |||||
| Before 1399 | 41/303 (14) | 1 | 0.03 | 1 | 0.007 |
| After 1399 | 9/17 (53) | 7.19 (1.27, 40.67) | 6.06 (1.64, 22.48) | ||
| Sex | |||||
| Male | 17/159 (11) | 1 | 0.04 | 1 | 0.01 |
| Female | 33/161 (20) | 2.15 (1.02, 4.65) | 2.68 (1.22, 5.89) | ||
| History of abdominal surgery | |||||
| No | 2/23 (9) | 1 | 0.35 | – | – |
| Yes | 48/297 (16) | 2.02 (0.46, 8.90) | |||
Multivariate analysis showed female sex, family history (especially strong family history), and germline mutation after codon 1399 to be independent factors in desmoid disease occurrence (table 1 ▶). All three factors therefore have a significant influence on desmoid occurrence, and family history is a major factor even when the germline mutation is 5′ (<codon 1399).
We then determined whether, in those patients with mutations before codon 1399, there was evidence to support the possibility that desmoids cluster in susceptible individuals. Within this mutation group 12% of patients might be expected to show multiple tumours. A one sample test of proportions demonstrated that the actual occurrence of multiple desmoids (44%) differed significantly from this predicted chance frequency (p<0.001).
Next, we tested predictors (mutation location, sex, abdominal surgery, and family history) of multiple (>1) desmoids. Owing to the lower frequency with which multiple desmoids were found, two groups were created for the family history analysis (no other family members affected by multiple desmoids and any other family member so affected). Subjects in families where other members were affected by multiple lesions had a notable increase in the odds of multiple tumours compared with individuals with no other affected family member (OR 13.12 (95% CI 3.54–48.50); p<0.001; univariate analysis, table 2 ▶). A mutation location >codon 1399 increased the odds of multiple tumours 10-fold compared with patients with <codon 1399 mutations (OR 10.46 (95% CI 2.02–54.11); p = 0.005) (table 2 ▶). Multivariate analysis confirmed these as strong independent predictors (table 2 ▶). There was no evidence of an interaction between family history and mutation location (p = 0.64), suggesting the relationship between family history and occurrence of multiple desmoids did not differ for the two mutation location groups.
Table 2.
Influence of family history, mutation location, sex, and history of abdominal surgery on multiple desmoid occurrence
| Variable | No with desmoids/total No (%) | Univariate analysis | Multivariate analysis | ||
| Odds ratio (95% CI) | p Value | Odds ratio (95% CI) | p Value | ||
| Proportion of family members with multiple desmoids | |||||
| None | 9/266 (3) | 1 | <0.001 | 1 | <0.001 |
| Any | 17/54 (31) | 13.12 (3.54, 48.50) | 11.08 (3.02, 40.59) | ||
| Mutation location | |||||
| Before 1399 | 19/303 (6) | 1 | 0.005 | 1 | <0.001 |
| After 1399 | 7/17 (41) | 10.46 (2.02, 54.11) | 6.62 (2.34, 18.78) | ||
| Sex | |||||
| Male | 9/159 (6) | 1 | 0.24 | – | – |
| Female | 17/161 (11) | 1.96 (0.63, 6.10) | |||
| History of abdominal surgery | |||||
| No | 1/23 (4) | 1 | 0.49 | – | – |
| Yes | 8/297 (8) | 2.02 (0.26, 15.24) | |||
DISCUSSION
As more is understood about duodenal disease and its management, desmoid disease has become the greatest remaining challenge in the management of FAP. A frequently severe and largely untreatable illness, desmoids are often induced by the very abdominal surgery intended to prolong life and improve its quality. For this reason we performed a detailed analysis of risk factors for desmoid formation to further understanding and clinical management of the disease. Previous abdominal surgery is given as a risk factor and included in this study to ensure interdependence from other variables. However, as the majority of patients have had surgery, and the time between surgery and the study was not always known, quantifying the desmoid risk associated with abdominal surgery remains problematic.
We examined the desmoid history of all FAP patients in whom the germline mutation was known and who have attended the St Mark’s Registry for follow up. Patients were subdivided into those with an APC germline mutation 5′ of codon 1399 and those with a germline mutation 3′ of codon 1399. As mentioned previously, most previous studies have made this point of division at codon 1444. We chose codon 1399 for the reasons discussed in the introduction. In fact, only two patients had mutations between these two points, and altering the division to codon 1444 made no difference to the statistical analyses results (data not shown).
Germline mutation in the 3′ region was much less common than in the 5′ region (94% v 6%), a finding supporting that of Bertario et al who found a 93% versus 7% prevalence when making the division at codon 1444.10 The presence of further “mutational hotspots” for desmoid formation also seems unlikely as there were many other families with the same germline mutations and no desmoids.
Independent of germline mutation, a strong family history of desmoids is a significant predictor of desmoid risk. Thus as patients with a strong family history had varied mutations along the APC gene, germline mutation alone cannot be used to predict desmoid risk. Other workers have reported a family history in FAP associated desmoids. Gurbuz et al, for example, noted an increased risk of desmoids in first degree relatives of affected patients compared with third degree relatives and conjectured that additional genetic loci or shared environmental factors might be responsible.3 In this study, a system of statistical analysis has been used which incorporates a robust standard error and this corrects for the assumption that members of the same family have other similarities (such as shared environment). Our results were statistically significant and we believe, therefore, that environmental factors are unlikely to be the explanation. By considering mutation clustering and identifying families with susceptibility to multiple tumours (>1), our study points to independent genetic factors. The frequency of multiple tumours in the 5′ mutation group was far higher than that which would be expected by chance alone. Furthermore, a family history of multiple desmoids increases the risk of developing desmoid disease and is a significant risk, independent of APC germline mutation, of an individual and their relatives developing multiple rather than single tumours. We propose that specific modifier genes exist at which variation increases the risk of desmoid tumours in FAP.
Given our findings, it is worthwhile considering the role of the somatic APC mutation in the initiation of desmoid disease and the type of cell in which it arises. As mentioned in the introduction, an association has been found between the site of germline and somatic mutations in FAP associated desmoids, akin to an association found in colonic polyps.15–17 In patients with a 5′ germline mutation it may be that a 3′ somatic mutation in cells of desmoid origin confers a significant proliferation advantage over cells with 5′ somatic mutations. Whether other genetic factors have a role in the selection of 3′ somatic APC mutations with subsequent escape from cellular control mechanisms remains to be seen. Modifier genes may act by regulating cell proliferation. This proposal is supported by work with mice where genetic deletion of a gene important in wound healing, Rhamm, significantly reduces the number and size of desmoids arising as a result of an APC truncating mutation at codon 1638.25 Cell culture studies have shown that Rhamm regulates proliferation of cells with sparse cell to cell contact, as observed in desmoids.25 Clearly, further studies on the genetic basis of desmoid formation are necessary and such analysis should contrast mutational events in desmoid precursor lesions and desmoid tumours.
Our findings also indicate an increased desmoid risk in female patients. Female sex has previously been proposed to be a risk factor, with reports of an increased incidence in women taking oestrogens26,27 and laboratory evidence of therapeutic effects of anti-oestrogens on desmoid cells.28 However, earlier studies have differed in their findings, with many reporting increased risk in female FAP patients10,29,30 while other workers found no significant sex difference.3,8
In terms of patient management, what are the implications of our current study in balancing the risks of desmoid disease? Given the characteristic low colonic polyp phenotype associated with germline mutations after codon 1399,22–24 the clinician might elect to manage the colon by close surveillance and chemoprophylaxis (for example, using celecoxib), resorting to surgery only when there is sufficient concern about colonic dysplasia, and particularly so in women or when there is also a family history of desmoid disease. While this may be regarded as unacceptable in terms of large bowel cancer development, this policy has been suggested by a previous study.31 As an independent risk factor, patients with a 5′ mutation and a strong family history of desmoids should also be considered to be at very high risk. Management of this cohort of patients is more problematic because many will have a dense colonic phenotype (particularly those with a mutation close to codon 1300), and hence an enhanced cancer risk. Surveillance and chemoprophylaxis are unlikely to prevent the early onset of invasive colorectal cancer, and early prophylactic colectomy will probably still need to be performed (except in cases where polyp density is low and follow up is likely to be good). It may be worth considering studies in these cases of the effect of non-steroidal anti-inflammatory agents (for example, sulindac) and anti-oestrogens at the time of surgery as attempted prophylaxis against the development of desmoid disease.
In summary, we have shown that the existence of genes independent of APC influencing desmoid formation in FAP is likely, and our data provide impetus for commencing a systematic search for such genes. Identification of controlling genetic factors is a prerequisite for individual risk assessment and it remains to be seen whether these factors are embodied in a few major genes or numerous low penetrance genes. Once all relevant genes and their contribution to desmoid formation have been specified, screening of FAP patients for such inherited genes would aid clinicians advising their patients about the risks and benefits of prophylactic surgery.
Acknowledgments
The authors gratefully acknowledge Cancer Research UK and the St Mark’s Hospital Foundation for the funding of this study.
Abbreviations
FAP, familial adenomatous polyposis
APC, adenomatous polyposis coli gene
LOH, loss of heterozygosity
REFERENCES
- 1.Miyoshi Y , Iwao K, Nawa G, et al. Frequent mutations in the beta-catenin gene in desmoid tumours from patients without familial adenomatous polyposis. Oncol Res 1998;10:591–4. [PubMed] [Google Scholar]
- 2.Tejpar S , Nollet F, Li C, et al. Predominance of beta-catenin mutations and beta-catenin dysregulation in sporadic aggressive fibromatosis. Oncogene 1999;18:6615–20. [DOI] [PubMed] [Google Scholar]
- 3.Gurbuz AK, Giardello FM, Petersen GM, et al. Desmoid tumours in familial adenomatous polyposis. Gut 1994;35:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nugent KP, Spigelman AD, Phillips RKS. Life expectancy after colectomy and ileorectal anastomosis for familial adenomatous polyposis. Dis Colon Rectum 1993;36:1059–62. [DOI] [PubMed] [Google Scholar]
- 5.Arvanitis ML, Jagelman DG, Fazio VW, et al. Mortality in patients with familial adenomatous polyposis. Dis Colon Rectum 1990;33:639–42. [DOI] [PubMed] [Google Scholar]
- 6.Bertario L , Presciuttini S, Sala P, et al. Causes of death and postsurgical survival in familial adenomatous polyposis: results from the Italian registry of familial polyposis writing committee. Semin Surg Oncol 1994;10:225–34. [DOI] [PubMed] [Google Scholar]
- 7.Clark SK, Neale KF, Landgrebe JC, et al. Desmoid tumours complicating familial adenomatous polyposis. Br J Surg 1999;86:1185–9. [DOI] [PubMed] [Google Scholar]
- 8.McAdam WA, Goligher JC. The occurrence of desmoids in patients with familial polyposis coli. Br J Surg 1970;57:618–31. [DOI] [PubMed] [Google Scholar]
- 9.Mace J , Sybil BJ, Sondak V, et al. Response of extraabdominal desmoid tumors to therapy with imatinib mesylate. Cancer 2002;95:2373–9. [DOI] [PubMed] [Google Scholar]
- 10.Bertario L , Russo A, Sala P, et al. Genotype and phenotype factors as determinants of desmoid tumours in patients with familial adenomatous polyposis. Int J Cancer 2001;95:102–7. [DOI] [PubMed] [Google Scholar]
- 11.Soravia C , Berk T, McLeod RS, et al. Desmoid disease in patients with familial adenomatous polyposis. Dis Colon Rectum 2000;43:363–9. [DOI] [PubMed] [Google Scholar]
- 12.Caspari R , Olschwang S, Friedl W, et al. Familial adenomatous polyposis: desmoid tumours and lack of opthalmic lesions (CHRPE) associated with APC mutations beyond codon 1444. Human Mol Genet 1995;4:337–340. [DOI] [PubMed] [Google Scholar]
- 13.Davies DR, Armstrong JG, Thakker N, et al. Severe Gardner syndrome in families with mutations restricted to a specific region of the APC gene. Am J Hum Genet 1995;57:1151–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Scott RJ, Froggatt NJ, Trembath RC, et al. Familial infiltrative fibromatosis (desmoid tumours) (MIM135290) caused by a recurrent 3′ APC gene mutation. Hum Mol Genet 1996;5:1921–4. [DOI] [PubMed] [Google Scholar]
- 15.Miyaki M , Konishi M, Kikuchi-Yanoshita R, et al. Coexistence of somatic and germ-line mutations of APC gene in desmoid tumours from patients with familial adenomatous polyposis. Cancer Res 1993;53:5079–82. [PubMed] [Google Scholar]
- 16.Palmirotta R , Curia MC, Esposito DL, et al. Novel mutations and inactivation of both alleles of the APC gene in desmoid tumours. Hum Mol Genet 1995;4:1979–81. [DOI] [PubMed] [Google Scholar]
- 17.Lamlum H , Ilyas M, Rowan A, et al. The type of somatic mutation at APC in familial adenomatous polyposis is determined by the site of germline mutation: a new facet to Knudson’s ‘two hit’ hypothesis. Nat Med 1999;5:1071–5. [DOI] [PubMed] [Google Scholar]
- 18.Albuquerque C , Breukel C, van der Luijt R, et al. The ‘just-right’ signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade. Hum Mol Genet 2002;11:1549–60. [DOI] [PubMed] [Google Scholar]
- 19.Crabtree M , Sieber OM, Lipton L, et al. Refining the relation between ‘first hits’ and ‘second hits’ at the APC locus: the ‘loose fit’ model and evidence for differences in somatic mutation spectra among patients. Oncogene 2003;22:4257–65. [DOI] [PubMed] [Google Scholar]
- 20.Houlston R , Crabtree M, Phillips R, et al. Explaining differences in the severity of familial adenomatous polyposis and the search for modifier genes. Gut 2000;48:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groves C , Lamlum H, Crabtree M, et al. Mutation cluster region, association between germline and somatic mutations and genotype-phenotype correlation in upper gastrointestinal familial adenomatous polyposis. Am J Pathol 2002;160:2055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagase H , Miyoshi Y, Horri A, et al. Correlation between the location of germline mutations in the APC gene and the number of colorectal polyps in familial adenomatous polyposis patients. Cancer Res 1992;52:4055–7. [PubMed] [Google Scholar]
- 23.van der Luijt RB, Meera Khan P, Vasen HFA, et al. Germline mutations in the 3′ part of APC exon 15 do not result in truncated proteins and are associated with attenuated adenomatous polyposis coli. Hum Genet 1996;98:727–34. [DOI] [PubMed] [Google Scholar]
- 24.Soravia C , Berk T, Madlensky L, et al. Genotype-phenotype correlations in attenuated adenomatous polyposis coli. Am J Hum Genet 1998;62:1290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolg C , Poon R, Fodde R, et al. Genetic deletion of receptor for hyaluronan-mediated motility (Rhamm) attenuates the formation of aggressive fibromatosis (desmoid tumour). Oncogene 2003;22:6873–82. [DOI] [PubMed] [Google Scholar]
- 26.Caldwell EH. Desmoid tumor: musculoaponeurotic fibrosis of the abdominal wall. Surgery 1976;79:104–6. [PubMed] [Google Scholar]
- 27.Eagel BA, Zentler-Munro P, Smith IE. Mesenteric desmoid tumours in Gardner’s syndrome—review of medical treatments. Postgrad Med J 1989;65:497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tonelli F , Valanzano R, Brandi ML. Pharmacologic treatment of desmoid tumors in familial adenomatous polyposis: results of an in vitro study. Surgery 1994;115:473–9. [PubMed] [Google Scholar]
- 29.Klemmer S , Pascoe L, Decosse J. Occurrence of desmoids in patients with familial adenomatous polyposis of the colon. Am J Med Genet 1987;28:385–92. [DOI] [PubMed] [Google Scholar]
- 30.Lofti AM, Dozois RR, Gordon H, et al. Mesenteric fibromatosis complicating familial adenomatous polyposis: predisposing factors and results of treatment. Int J Colorectal Dis 1989;4:30–6. [DOI] [PubMed] [Google Scholar]
- 31.Friedl W , Caspari R, Sengteller M, et al. Can APC mutation analysis contribute to therapeutic decisions in familial adenomatous polyposis? Experience from 680 FAP families. Gut 2001;48:515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]