We have recently described1 mucosal ultrastructural impairments, such as height and thickness of microvilli, space between microvilli, and thickness of tight junctions, in non-coeliac type 1 diabetic patients after a preliminary report of an alteration in intestinal mucosal permeability (IP) evaluated by the lactulose/mannitol (LA/MA) test.2,3
Therefore, in the “aetiological” classification of autoimmunity based on initiating factors,4 the category of diet induced diseases could be expanded to include type 1 diabetes and, perhaps, other endocrine autoimmune diseases.
Thyroiditis is the most frequently associated autoimmune endocrine disease with type 1 diabetes. Moreover, type 1 diabetes and Hashimoto thyroiditis present similar pathogenetic mechanisms of cellular damage, a cell mediated autoimmunity induced by Th1 cytokines. However, mucosal intestinal morphology and function have not yet been studied in autoimmune thyroiditis patients. Hence we investigated intestinal mucosal ultrastructural morphology and IP in a group of patients with autoimmune thyroiditis. The study was approved by the local ethics committee.
Fourteen patients (12 females and 2 males; mean age 33.2 (SD 10.2) years) and 23 controls (12 females and 11 males; mean age 27.9 (SD 8.01) years) were enrolled into the study after giving written informed consent.
The diagnosis of autoimmune thyroiditis was based on the following criteria: plasma autoantibody TPO positive at high titre and a typical thyroiditis ultrasound pattern. All patients were in euthyroidism (normal FT3, FT4, and TSH plasma levels without hormonal therapy). Mean duration of known disease was 5.2 (2.5) years. All patients were negative for the presence of antigliadin antibodies IgA and IgG, antiendomysium antibodies IgA, as well as antihuman transglutaminase IgA following a gluten rich Mediterranean diet. Type 1 diabetes mellitus was excluded according to the 1997 American Diabetes Association criteria, and none of the participants had a family history of diabetes mellitus. Other intestinal and endocrine diseases were excluded through clinical and, when indicated, laboratory evaluation. Food or other allergies were excluded. None of the subjects reported gastrointestinal signs or symptoms, or was a habitual smoker, abuser of alcohol, or regularly took non-steroidal anti-inflammatory drugs.
Only four of 14 patients and nine of 23 age matched controls consented to undergo standard oesophagogastroduodenal endoscopy, and biopsy specimens were collected from the distal portion of the duodenum. Biopsies were then processed for standard histological examination by light microscopy (LM), after staining with haematoxylin and eosin, and for transmission electron microscopy (TEM) analysis.
The following enterocyte parameters were measured in a blinded fashion by two different pathologists: height of microvilli, thickness of microvilli, space between two adjacent microvilli in the same cell, and total thickness of the tight junction. The value expressed for each of the above parameters represents the mean of eight such measurements. TEM analysis was performed with a ZEISS EM 109 Transmission Electron Microscope.
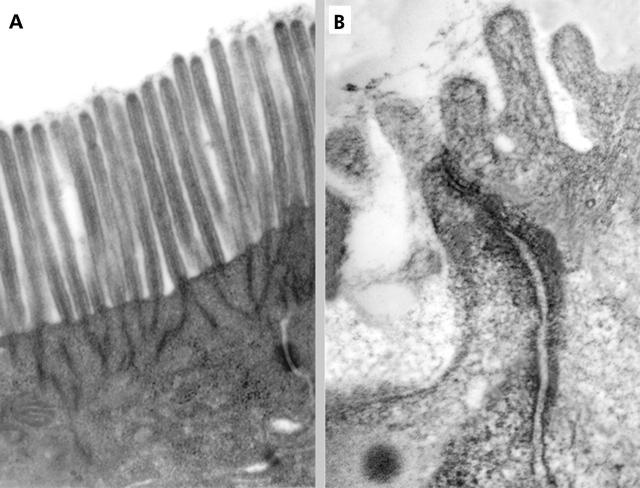
At LM, all four intestinal biopsies from thyroiditis patients showed a normal histological pattern. In particular, intraepithelial lymphocytes were within the normal range (Marsh grade = 0: <30 lymphocytes per 100 epithelial cells). TEM showed some alterations, particularly evident on the apical side of enterocytes where rarefaction and partial disappearance of microvilli were observed (fig 1 ▶).
Figure 1.
(A) Transmission electron microscopy (TEM) of a normal duodenal mucosa in a sample from a healthy control subject. Both microvilli and the visible tight junction (TJ) are normal with respect to thickness and height. Original magnification: 24 000×. (B) TEM micrograph showing features of adjacent enterocytes in a mucosal sample from a thyroiditis affected patient. TJ complexes are characterised by dilated intercellular spaces; some microvilli are also visible, clearly shorter and thicker than normal. Original magnification: 60 000×.
Among the four parameters investigated, the space between two adjacent microvilli and the thickness of microvilli were significantly different in patients compared with controls (0.045 (0.019) v 0.024 (0.006) μm (p = 0.012); 0.132 (0.012) v 0.098 (0.036) μm (p = 0.032), respectively; t test for independent samples). Moreover, mean height of the microvilli and mean thickness of the tight junctions were different in the two groups, although these differences were not statistically significant (thyroiditis patients v controls 1.18 (0.16) v 1.33 (0.23) μm (p = 0.224); 0.031 (0.005) v 0.023 (0.010) μm (p = 0.114), respectively). Therefore, these findings suggest some alterations in the morphology of the enterocytes of patients with Hashimoto’s thyroiditis that make them much more similar to type 1 diabetic subjects1 than to controls.
To investigate if these morphological findings were related to functional mucosal alterations, similarly to type 1 diabetes, IP was evaluated by the LA/MA test in all subjects. After an oral load, the percentage of sugar probe recovery from urine was measured. The ratio %LA/%MA is commonly referred to as the IP index. Detection and measurement of the two sugar probes in urine was performed by high performance anion exchange chromatography coupled with pulsed amperometric detection, as described previously.5 LA/MA values were significantly different in thyroiditis patients compared with controls (0.024 (0.018) v 0.014 (0.06) (p = 0.022); t test for independent samples). Increased LA/MA values are consistent with impaired IP in subjects with chronic autoimmune thyroiditis compared with controls. These functional data further support the ultrastructural observations.
These original findings are similar to impairments previously observed in non-coeliac type 1 diabetes. This novel observation suggests that endocrine autoimmune diseases with similar autoimmune mechanisms of cellular damage show impaired intestinal morphology and function. Thus a pathogenetic role for mucosal intestinal damage at the onset of such diseases can be proposed. Further investigations are necessary to confirm this hypothesis.
References
- 1.Secondulfo M , Iafusco D, Carratù R, et al. Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type I diabetic patients. Dig Liver Dis 2004;36, 1:35–45. [DOI] [PubMed] [Google Scholar]
- 2.Carratù R , Secondulfo M, deMagistris L, et al. Altered intestinal permeability to mannitol in diabetes mellitus type I. J Pediatr Gastroenterol Nutr 1999;28:264–9. [DOI] [PubMed] [Google Scholar]
- 3.Secondulfo M , deMagistris L, Sapone A, et al. Diabetes mellitus type 1: a new enteropathy? J Gastroenterol Hepatol 2002;17 (suppl) :A68. [Google Scholar]
- 4.Eisenbarth G , Bellgrau D. Autoimmunity. Sci Med 1994;1:38–47. [Google Scholar]
- 5.Generoso M , De Rosa M, De Rosa R, et al. Cellobiose-mannitol and lactulose-mannitol tests determined by high-performance anion exchange chromatography with pulsed amperometric detection are similarly reliable in the in vivo investigation of intestinal permeability. J Chromatogr B 2003;783:349–57. [DOI] [PubMed] [Google Scholar]