Abstract
Background: In this study, we identify the nature of the immunological response of human peripheral blood mononuclear cells (PBMC) and lamina propria gastric lymphocytes (LPL) to two Helicobacter pylori antigens, the neutrophil activating protein (NapA) and alkyl hydroperoxide reductase (AphC). These antigens were identified and selected for study based on the observation that serological recognition of these proteins was associated with H pylori negative status in humans.
Aims: The aim was to study the serological, proliferative, and cytokine responses of PBMC and LPL, obtained from H pylori infected and uninfected individuals, to these antigens.
Methods: Patient serum, PBMC, and LPL were used to determine antibody isotype, and proliferative and cytokine responses to recombinant forms of NapA and AphC using western blotting and ELISA.
Results: Western blotting revealed antibody reactivity to recombinant NapA and AphC among the H pylori negative population studied. Both the proliferative and interferon γ responses of PBMC and LPL to NapA and AphC were significantly higher in H pylori negative compared with H pylori positive subjects. Analysis of the IgG subclass profiles to both antigens revealed a T helper 1 associated IgG3 antibody response in uninfected individuals. However, interleukin 10 production was greater in H pylori positive individuals in response to these antigens.
Conclusions: Taken together these data are consistent with an immune response to these antigens skewed towards a T helper 1 response in the uninfected cohort.
Keywords: Helicobacter pylori, lamina propria, lymphocytes, immune response
Helicobacter pylori specifically colonises human gastric epithelium, is a major cause of chronic gastritis, and is strongly associated with peptic ulcer disease and the development of gastric cancer.1–3 Colonisation of the gastric epithelium by the bacterium results in an inflammatory reaction consisting of elements of both the humoral and cellular immune response. However, the immune response mounted by the host is ineffective in eliminating H pylori from the stomach lumen.4 Eradication of the organism is believed to be a rare event once colonisation is established. In addition to strain dependent gene expression by H pylori, host factors are also thought to influence disease outcome. The vast majority of individuals colonised by H pylori elicit a measurable systemic antibody response that may reflect the specificity of those antibodies produced at the gastric mucosa.5 The Ig classes and subclasses of these circulating anti-H pylori antibodies are consistent with a prolonged chronic mucosal infection, with IgG and IgA predominating and IgM antibodies rarely observed.6–9 Despite the production of such antibodies, the infection persists and gastritis progresses chronically. However, following eradication of H pylori, specific antibody levels decline slowly10 but can be detected by immunoblot for at least two years post eradication.11 Reinfection is accompanied by a rapid rise in antibody titre.12 These observations support the view that anti-H pylori antibodies are not protective and only reflect the chronicity of infection. Of note, reports in the literature indicate that spontaneous eradication of H pylori can occur, particularly in the paediatric population8,13–19 Of the two documented ingestion studies20,21 one reported elimination of an acute infection whereas the other proceeded to develop chronic colonisation. Little attention has been paid however to the systemic and humoral immune responses of H pylori uninfected seropositive individuals to H pylori antigens.
In this paper, we demonstrate that H pylori negative individuals have detectable antibody responses to several H pylori antigens, including the neutrophil activating protein (NapA; HP0243, The Institute for Genomic Research annotation, www.tigr.org) and alkyl hydroperoxide reductase (AphC, HP1563). We present the proliferative and cytokine (interleukin 10 (IL-10), interferon γ (IFN-γ)) responses of human peripheral blood mononuclear cells (PBMC) and lamina propria lymphocytes (LPL) to NapA and AphC in H pylori positive and negative individuals. The different immune responses to these antigens by both cohorts may have implications for disease progression.
MATERIALS AND METHODS
Materials
All antibodies were obtained from Sigma Chemical Co. (Poole, Dorset, UK), Dako Ltd (High Wycombe, UK), or the Binding Site Ltd (Birmingham, UK). All other chemicals and solvents, except where indicated, were obtained from Sigma. Reagents for DNA manipulation were obtained from either Promega Corporation (Madison, Wisconsin, USA) or New England Biolabs (Beverly, Massachusetts, USA). Recombinant urease B subunit (UreB) was obtained from Austral Biologicals (California, USA).
Sera samples
Serum samples were obtained from individuals undergoing gastrointestinal endoscopy at St James’s Hospital, Dublin. Infection in these patients was determined and confirmed by histological examination of endoscopic biopsy specimens, CLO testing, and culture of the bacterium in vitro. The studies described herein were approved by the ethics committee of the Federated Dublin Voluntary Hospitals. Serum samples were also collected from the cohort of patients described below for PBMC and LPL and additional immunoblotting studies.
Subjects used for PBMC/LPL studies
Sixty patients with dyspepsia (30 females, 30 males; age range 18–67 years (median 40)) were studied. All of these patients were attending for upper gastrointestinal endoscopy. All patients had antral biopsies performed to obtain gastric LPL. None of the patients had received non-steroidal anti-inflammatory drugs, bismuth compounds, or antibiotics in the preceding 12 months. Patients with evidence of malignant disease or immunosuppression were excluded. H pylori was identifiable in tissue sections by haematoxylin-eosin staining. Seropositivity for H pylori was determined by ELISA.
Absorption of sera
Sera (diluted 1/50 with phosphate buffered saline (PBS)) were absorbed with a pooled mixture of two clinical isolates of H pylori in addition to the reference strain NCTC 11638, Esherichia coli (K12), or Campylobacter jejuni (clinical isolate) by incubating a suspension of the bacteria (109 bacteria/ml; McFarland standards) in PBS (pH 7.5) with patient sera for two hours at room temperature with gentle mixing. The bacteria were removed from suspension by centrifugation (12 000 g, three minutes). Additionally, for some experiments (figs 2 ▶, 4 ▶), sera were adsorbed with a whole cell sonicate of H pylori (pooled strains N6 and NCTC 26695) or sonicates of C jejuni, Enterobacter aerogenes, Salmonella typhimurium, or Yersinia pseudotuberculosis. In this case, bacteria were harvested in PBS and subjected to sonication (3×30 second bursts, amplitude setting 10 μm on a MSE Soniprep 150). The sonicates were diluted to an OD of 0.8 (600 nm) and used for adsorption studies. Sera were diluted 1/50 in the sonicate and incubated overnight (4°C) with rotary mixing. Prior to use for immunoblotting, the various adsorbed sera were diluted with blocking buffer (PBS, 10% (w/v) non-fat milk powder and Tween-20 (0.01%, v/v)) to give a final 1/100 dilution of each serum sample.
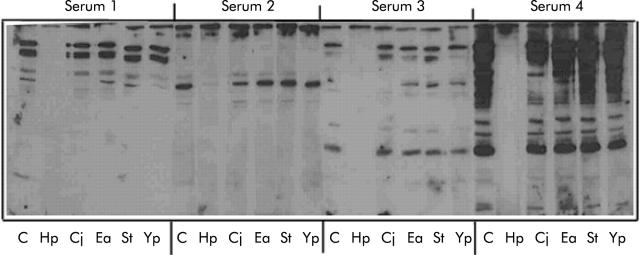
Figure 2.
Adsorption of sera from infected and uninfected seropositive subjects. Serum (40 μl) from either uninfected (sera 1, 2, 3) or infected (serum 4) individuals was either untreated (C) or adsorbed with sonicates of H pylori (Hp), C jejuni (Cj), E aerogenes (Ea), S typhimurium (St), or Y pseudotuberculosis (Yp) prior to probing blots of whole Helicobacter pylori (NCTC26695) with each serum sample. Primary IgG was detected with horseradish peroxidase conjugated rabbit antihuman IgG (1/3000) and developed by enhanced chemiluminescence.
Figure 4.

Immunoreactivity of neutrophil activating protein (NapA) and alkyl hydroperoxide reductase (AphC) with sera from uninfected seropositive subjects. Untreated sera (1/50) were used to probe blots of recombinant NapA (A, B) or AphC (E). Selected sera from these individuals were also adsorbed with the bacterial sonicates described in the legend to fig 2 ▶, prior to probing blots of NapA (C, three sera and D, three sera) or AphC (F, four sera). The blots were processed as described in fig 2 ▶.
Bacterial strains and growth conditions
The clinical isolates of H pylori used in this study were isolated from antral biopsies obtained from patients attending the Gastroenterology Clinic at St James’s Hospital, Dublin. H pylori was grown as described previously.22 A clinical isolate of C jejuni from a patient with C jejuni enteritis and a reference strain (HS:19) were grown for two days on campylobacter selective supplement (Oxoid, Basingstoke, UK) at 42°C. E coli K12 was purchased from Gibco (Grand Island, USA) and was grown under standard conditions on LB agar plates. E aerogenes (NCTC 9528), S typhimurium (ATCC 19585), and Y pseudotuberculosis (IP 2627) were grown on LB agar.
Western blotting and SDS-PAGE
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting were performed essentially as described previously.22,23 Immunoblots were processed and developed by enhanced chemiluminescence. For N terminal sequencing, proteins were electroblotted to ProBlott.
Purification of NapA and AphC
Both the NapA and AphC antigens were purified from whole H pylori (strain 11638; 25 mg) on the basis of molecular weight using preparative continuous elution SDS-PAGE on a Model 491 Prep-Cell (BioRad, Hemel Hempstead, UK), as recommended by the manufacturer.
Cloning of the H pylorinap A gene
Genomic DNA was extracted from H pylori (NCTC 11638) as described previously.24 Oligonucleotide primers specific for the 5′ and 3′ termini of the napA gene were generated. The forward primer (F) was designed to incorporate an Nde1 restriction endonuclease site while the reverse primer (R) incorporates a BamH1 restriction site. The primer sequences were: F, 5′-GAA GGA CTT CAT ATG AAG ACA TTT G -3′ and R, 5′-CGT GAA TGG ATC CTC ATG CTG ACT TCT-3′. The napA gene sequence was amplified in a “hotstart” polymerase chain reaction (PCR) using 50–100 ng of H pylori DNA. A “touchdown” PCR procedure25 was utilised. The reaction products were purified on a 4% low melting point agarose gel and recovered following β-agarase 1 digestion. Approximately 3 μg of purified DNA fragment corresponding to the napA gene was then digested with the restriction enzymes Nde1 and BamH1, each of which occurs only once on the amplified fragment.
Cloning of aphC
The following primers were used to amplify the entire sequence of aphC for cloning in an expression vector (pET16b: Novagen, Madison, USA). Forward primer: 5′ GAC TGA TAG CAT ATG TTA GTT ACA AAA CTT GC-3′; reverse primer: 5′-AGC TTA ATG GAT CCT TCT TAA AGA TAT TCT GCA ACG-3′. The forward primer was modified to include an Nde1 site and the reverse had a built in BamH1 site. The insert was amplified, digested with the appropriate enzymes, and ligated into the expression vector pET16b.
Expression and purification of the recombinant products
The expression vector used was pET16b (Novagen); 1.6 μg of the vector were digested using Nde1 and BamH1. Approximately 200 ng of pET16b were ligated to approximately 100 ng of the appropriate insert DNA with 3 units of T4 DNA ligase at 20°C for 16 hours. The products of this reaction were used to transform competent E coli XL1-blue cells. Plasmids with appropriate inserts were used to transform E coli expression hosts (BL21 DE3 and NOVAblue DE3). Overexpression was induced by the addition of IPTG (1 mM). The antigens were purified as recommended by the manufacturer on Ni-NTA agarose.
IgG ELISA
Polyclonal IgG ELISA
All steps were performed at room temperature. ELISA plates (Nunc Maxisorp, Roskilde, Denmark) were coated with recombinant NapA or AphC (1 μg/ml; 50 μl/well) in PBS (pH 7.4) for three hours. After washing with PBS and blocking with bovine serum albumin (3% w/v; 150 μl/well) in PBS for one hour the plates were washed with PBS and serum (50 μl; diluted 1/50 in PBS) was added to duplicate wells and incubated for one hour. Controls consisted of wells with PBS alone and H pylori sonicate (1 μg/ml) as negative and positive controls, respectively. Peroxidase conjugated antihuman IgG (1/5000) was added and incubated for one hour after which time the plates were washed with PBS and the colour reaction was initiated by addition of TMB (50 μl). After 10 minutes the reaction was terminated by addition of 1 M-H2SO4 (50 μl) and the colour intensity was measured at 450 nm.
IgG subclass ELISA
Detection of specific IgG subclasses was achieved by adding 50 μl of alkaline phosphatase conjugated anti-IgG subclasses (IgG 1–4) at a dilution of 1/5000 in PBS. The colour reaction was initiated by addition of 50 μl p-nitrophenyl-phosphate (1 mg/ml in 10% diethanolamine buffer, pH 9.8) and incubated in the dark for 10 minutes. The reaction was terminated by addition of 50 μl NaOH (3 M). The plates were read at 405 nm.
PBMC and LPL proliferation studies
Venesections were performed for isolation of PBMC which were subsequently separated from other blood products by Ficoll hypaque density gradient centrifugation as described previously.26 Viability of PBMC was consistently >95%. To assess antigen specific lymphocyte proliferation, 1×106/ml PBMC were cultured at 37°C in 5% CO2 in 96 well U bottom microplates in a total volume of 200 μl for three days either alone or in the presence of OKT3 (1:50 dilution), PHA (10 μg/ml), H pylori sonicate (3 μg/ml for PBMC and 300 μg/ml for LPL), NapA (1 μg/ml), AphC (1 μg/ml), recombinant urease B (rUreB) (1 μg/ml), or β-galactosidase (1 μg/ml), essentially as described previously.26 Gastric LPL were isolated and used in proliferation studies as previously described.26 The optimal stimulatory concentration for each recombinant antigen was predetermined for both recombinant NapA and AphC (range 0.05–3 μg/ml) using samples of PBMC and LPL obtained from H pylori infected (n = 4) and uninfected individuals (n = 4) and in both cases was found to be 1 μg/ml (data not shown).
Measurement of IFN-γ and IL-10 secretion by PBMC and LPL
PBMC (1×106/ml) and LPL (4×105/ml) were cultured either alone or in the presence of the antigens described above for three days at 37°C in 5% CO2. The culture supernatants were collected and stored at −80°C prior to quantifying the amounts of IFN-γ and IL-10 present using commercially available ELISA kits (Cambridge Bioscience, UK).
Statistical analysis
The significance of the difference between the results obtained with H pylori positive and H pylori negative individuals was evaluated using the Mann-Whitney U test/Wilcoxon and independent Student’s t test.
RESULTS
Immunoblotting of serum obtained from H pylori infected and uninfected subjects
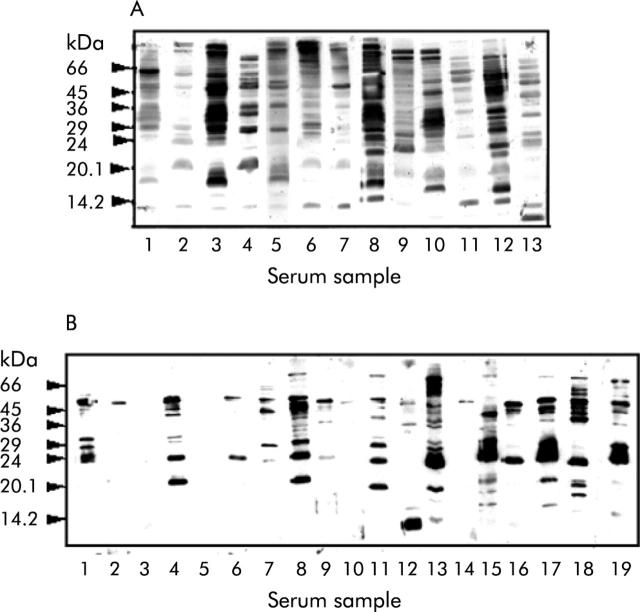
Cohorts of sera obtained from H pylori infected and uninfected individuals were screened for anti-H pylori IgG antibodies by western blotting. All of the H pylori infected individuals examined recognised a heterogeneous population of H pylori antigens (fig 1A ▶). Similarly, sera obtained from subjects known to be uninfected were found to be immunoreactive against H pylori antigens but to a lesser extent (fig 1B ▶ compared with fig 1A ▶). The anti-H pylori immunoreactivity of sera from both infected and uninfected cohorts was immunodepleted almost completely only by pre-adsorption of the sera with whole H pylori extracts (fig 2 ▶, lane Hp in all panels). Adsorption of the same sera with C jejuni resulted in some but considerably less immunodepletion compared with adsorption with H pylori, and very little was seen when the samples were adsorbed with E aerogenes, S typhimurium, or Y pseudotuberculosis (fig 2 ▶). Also, the ability of rabbit polyclonal anti-whole H pylori antiserum to cross react with C jejuni and E coli antigens was examined by western blotting (fig 3A ▶). Anti-H pylori antiserum recognised a reduced number of antigens on both E coli and C jejuni compared with H pylori itself. Specifically, the antiserum recognises proteins of molecular mass 72, 50, 40, 36, and 25 kDa on C jejuni and proteins of molecular mass 200, 116, 45, and 38 kDa on E coli (fig 3A ▶). Of these, only three proteins (70 and 25 kDa from C jejuni and 200 kDa from E coli) showed pronounced cross reactivity. Additional adsorption experiments demonstrated that E coli also failed to significantly deplete anti-H pylori seroreactivity (fig 3B ▶).
Figure 1.
Serum samples screened for the presence of anti-Helicobacter pylori IgG antibodies. Western blots of whole H pylori (50 μg/lane) probed with serum obtained from H pylori infected (A) and H pylori uninfected (B) cohorts are shown. All sera were diluted 1:100 in phosphate buffered saline containing fat free dried skimmed milk (5% w/v). Each track represents an individual strip of PVDF membrane which had been incubated with a different serum sample. Blots were developed by enhanced chemiluminescence.
Figure 3.
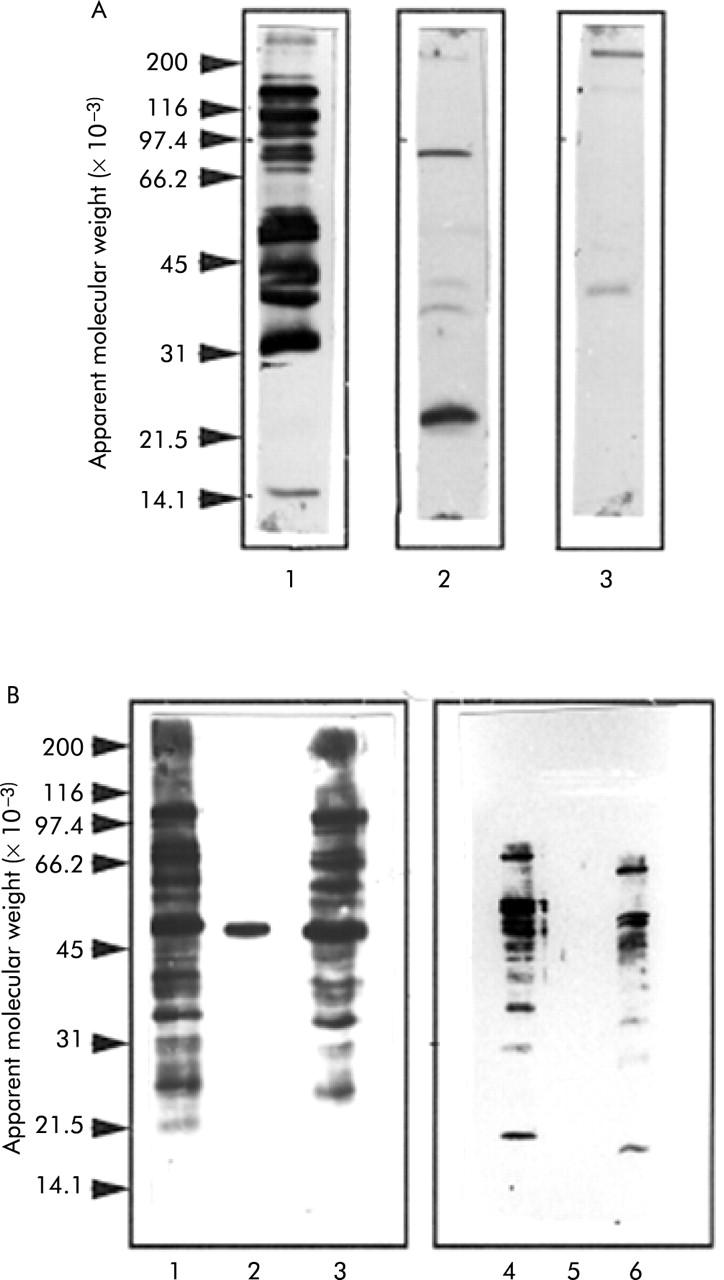
C jejuni and E coli antigens recognised by anti-Helicobacter pylori antiserum and elimination of cross reactivity by adsorption with H pylori. (A) Western blot of H pylori (lane 1), C jejuni (lane 2), and E coli (lane 3) probed with rabbit anti-H pylori antiserum. A sonicate of each bacterium (50 μg) was subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis and immunoblotting, as described in the methods section. (B) Effect of adsorbing serum obtained from a H pylori infected subject (lanes 1–3) and a subject negative for H pylori (lanes 4–6) with E coli (lanes 1 and 4), H pylori (lanes 2 and 5), or C jejuni (lanes 3 and 6).
Identification of two antigens recognised by serum from H pylori negative subjects
Preparative continuous elution SDS-PAGE was used to fractionate whole H pylori on the basis of molecular size. Two immunoreactive antigens were identified by probing immunoblots of protein fractions with serum from H pylori uninfected individuals. N terminal amino acid sequencing of two of the seroreactive antigens revealed one to be NapA and the other, AphC. Recombinant forms of both antigens were subsequently generated and used for further studies. Almost all sera from uninfected subjects had IgG that reacted with both recombinant NapA (fig 4A ▶, B) and AphC (fig 4E ▶). Furthermore, this immunoreactivity was completely depleted only when the sera were pre-adsorbed with H pylori. Some immunoreactivity against NapA (fig 4C ▶, D) and AphC (fig 4F ▶) appears to be adsorbed partially, but incompletely, by other bacteria.
IgG subclass responses to Nap A and AphC
Both total IgG and IgG subclass responses to H pylori recombinant NapA and AphC were analysed in sera from H pylori infected and uninfected individuals by ELISA. IgG immunoglobulins to NapA and AphC were present in the serum of both H pylori positive and H pylori negative subjects (fig 5A ▶, B). Interestingly, H pylori negative individuals had a significantly greater total IgG response to both NapA (p<0.01) and AphC (p<0.01) compared with the infected cohort. Analysis of the subclass specificity of the IgG response to NapA demonstrated that uninfected subjects had a significantly higher IgG2 (p<0.05) and IgG3 response (p<0.01) whereas the infected cohort had a higher IgG4 response (p<0.01) (fig 5A ▶). A similar pattern was seen in the subclass responses to AphC (fig 5B ▶). There were no significant differences in the IgG1 responses to these antigens in either study group.
Figure 5.
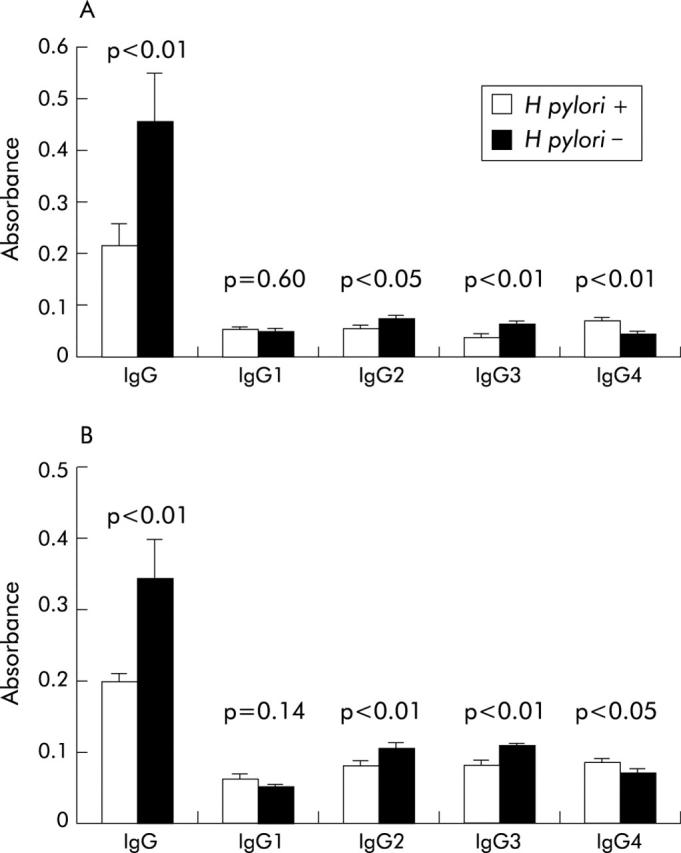
Total IgG and IgG subclass responses to neutrophil activating protein (NapA) and alkyl hydroperoxide reductase (AphC). Total IgG response and the various IgG subclass responses to NapA (A) and AphC (B) in serum obtained from Helicobacter pylori positive and H pylori negative individuals are shown. IgG levels were determined by ELISA, as described in the methods section and the absorbance axes represent readings obtained at 450 nm for total IgG and 405 nm for the IgG subclasses.
Proliferative responses of PBMC and LPL to NapA and AphC
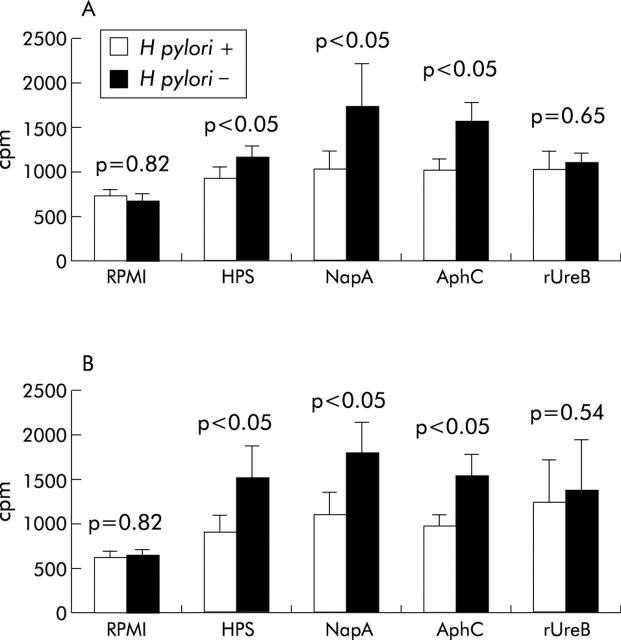
The proliferative responses of PBMC to NapA, AphC, and H pylori sonicate (HPS) were significantly higher (p<0.05 in all three cases) in H pylori negative compared with H pylori positive subjects (fig 6A ▶). In contrast, the proliferative responses to rUreB were not significantly different between H pylori positive (n = 10) and H pylori negative (n = 10) patients. Similarly, the proliferative responses of LPL to NapA, AphC, and HPS were significantly higher (p<0.05 in all three cases) in H pylori negative compared with H pylori positive patients (fig 6B ▶) whereas there were no significant differences between the two cohorts in the proliferative responses observed to rUreB. No significant differences were found between the two groups after stimulation with PHA, OKT3, or β-galactosidase (table 1 ▶). β-Galactosidase was included as a control histidine tagged fusion protein.
Figure 6.
Proliferative responses of peripheral blood mononuclear cells (PBMC) and lamina propria lymphocytes (LPL) to neutrophil activating protein (NapA) and alkyl hydroperoxide reductase (AphC). The proliferative responses of PBMC (A) and LPL (B) to Helicobacter pylori sonicate (HPS) (3 μg/ml for PBMC and 300 μg/ml for LPL), NapA (1 μg/ml), AphC (1 μg/ml), and recombinant urease B subunit (rUreB) (1 μg/ml) are shown for both H pylori positive H pylori uninfected subjects. Results are expressed as [3H]-thymidine incorporation (cpm) into PBMC (2×106/ml) and LPL (4×105/ml) cultured for three days in the presence of the indicated antigens. All samples were measured in triplicate.
Table 1.
Proliferative responses of PBMC and LPL to PHA, OKT3, and β-galactosidase: [3H]-thymidine incorporation (cpm)
| PBMC | LPL | |||||
| HP+ve | HP−ve | p Value | HP+ve | HP−ve | p Value | |
| PHA | 20574 (3974) | 25645 (4735) | 0.14 | 8378 (2086) | 7360 (1744) | 0.34 |
| OKT3 | 10112 (2318) | 12556 (2166) | 0.31 | 5475 (666) | 4745 (1043) | 0.56 |
| β-gal | 651 (110) | 670 (124) | 0.39 | 601 (165) | 605 (173) | 0.96 |
HP, Helicobacter pylori; PBMC, peripheral blood mononuclear cells; LPL, lamina propria lymphocytes; β-gal, β-galactosidase.
Results are expressed as [3H]-thymidine incorporation (cpm) into PBMC and LPL cultured for three days. LPL (4×105/ml) were cultured with autologous irradiated (2500 rads) PBMC (2×106/ml) in the presence of IL-2 (2 IU/ml). All samples were measured in triplicate and are shown as mean (SEM) (n = 30). PHA was used at a concentration of 10 μg/ml and 5 μg/ml for PBMC and LPL, respectively. OKT3 was used at a dilution of 1:50 and β-galactosidase was 1 μg/ml.
Induction of IFN-γ and IL-10 production by H pylori HPS, NapA, AphC, and rUreB in PBMC and LPL
Human PBMC and LPL were incubated with NapA, AphC, rUreB, and HPS to determine the effect of these antigens on cytokine production. IFN-γ production by PBMC from H pylori negative patients stimulated with either NapA (p<0.05), AphC (p<0.01), or HPS (p<0.05) was significantly higher compared with H pylori positive patients (fig 7A ▶). In contrast, PBMC from H pylori positive subjects produced significantly more IL-10 when activated with either NapA (p<0.05), AphC (p<0.05), or HPS (p<0.05) compared with H pylori negative patients (fig 8A ▶).
Figure 7.
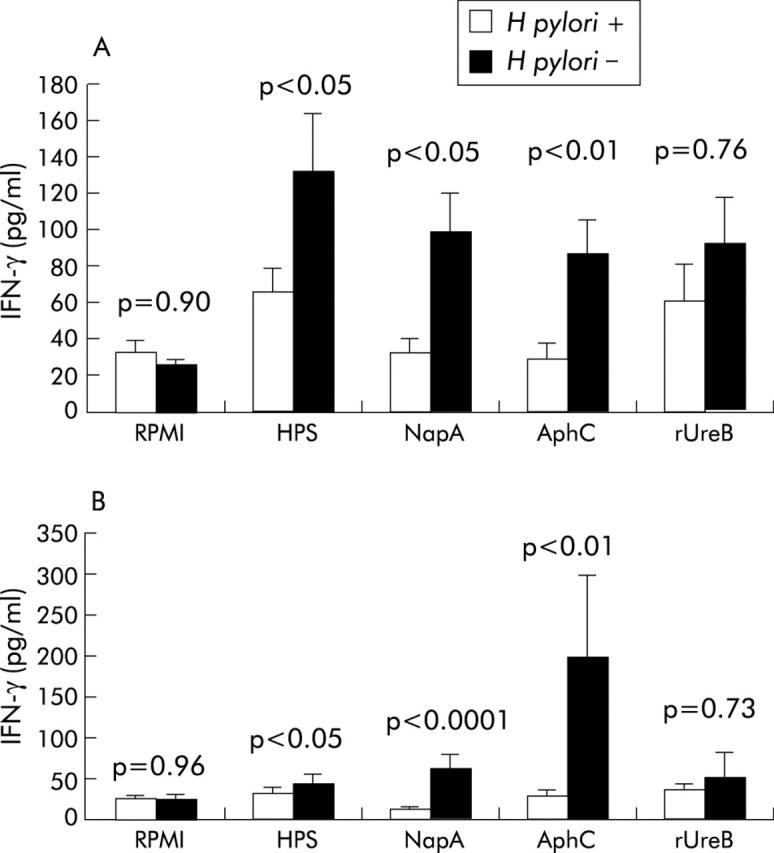
Interferon γ (IFN-γ) production by peripheral blood mononuclear cells (PBMC) and lamina propria lymphocytes (LPL) in response to neutrophil activating protein (NapA) and alkyl hydroperoxide reductase (AphC). IFN-γ production by PBMC (A) and LPL (B) obtained from Helicobacter pylori positive or H pylori negative individuals in response to the indicated antigens. All antigens were used at 1 μg/ml. Supernatants were collected from cultured PBMC and LPL after 72 hours and stored at −80°C. IFN-γ was measured in the supernatant by ELISA. All samples were measured in duplicate. Results are expressed as mean (SEM). rUreB, recombinant urease B subunit; HPS, H pylori sonicate.
Figure 8.
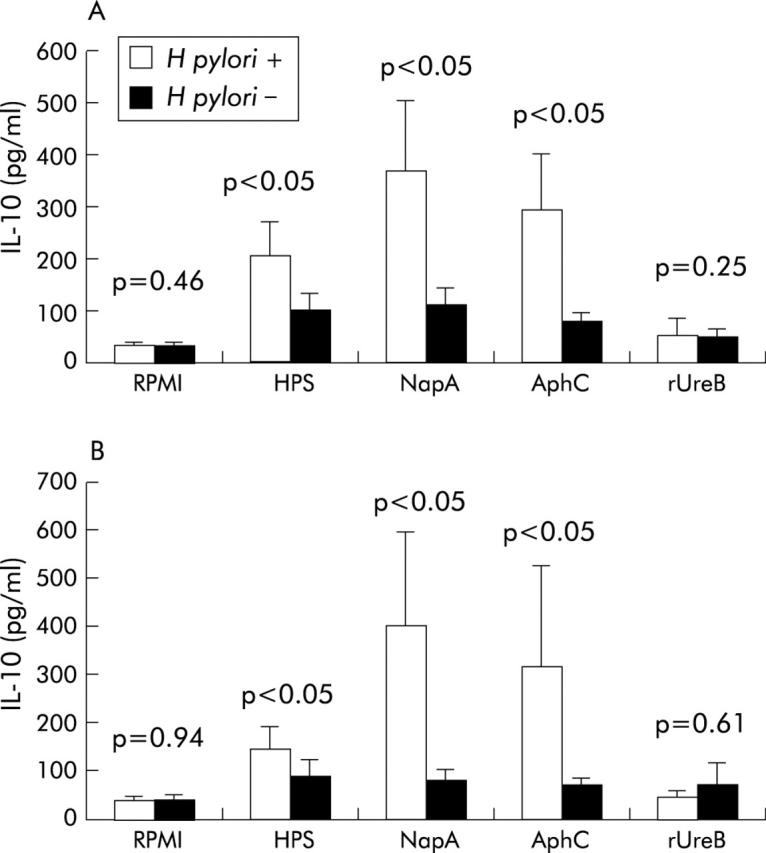
Interleukin 10 (IL-10) production by peripheral blood mononuclear cells (PBMC) and lamina propria lymphocytes (LPL) in response to neutrophil activating protein (NapA) and alkyl hydroperoxide reductase (AphC). Levels of IL-10 produced by PBMC (A) and LPL (B) from H pylori positive and H pylori negative subjects in response to the antigens indicated are shown. All antigens were used at 1 μg/ml. Supernatants were collected from cultured PBMC and LPL after 72 hours and stored at −80°C. IL-10 was measured in the supernatant by ELISA. All samples were determined in duplicate. Results were expressed as mean (SEM). rUreB, recombinant urease B subunit; HPS, H pylori sonicate.
IFN-γ production by LPL was significantly higher in the H pylori negative cohort after stimulation with NapA (p<0.0001), AphC (p<0.01), or HPS (p<0.05) compared with H pylori positive individuals (fig 7B ▶). IL-10 production by LPL was significantly higher in the H pylori positive group after stimulation with NapA (p<0.05), AphC (p<0.05), or HPS (p<0.05) compared with the H pylori negative group (fig 8B ▶).
Of note, NapA and AphC induced significantly higher IFN-γ production by both PBMC and LPL from H pylori negative subjects when compared with rUreB.
DISCUSSION
Eradication of H pylori is thought to be a rare event once colonisation is established, yet there are indications in the literature that this does occur.13–19 Given the high incidence of H pylori infection in the broad population it is likely that some individuals eliminate the infection without intervention. This is a contentious issue however but one that may have implications relating to the actual incidence of exposure as many such individuals would remain seropositive but undetected. Moreover, in many instances, H pylori negative individuals are classified as such based solely on serological EIA, a technique prone to error.27,28 In addition, cross reactivity with other bacterial species decreases specificity (for example, see Feldman and colleagues29). More sensitive detection techniques such as immunoblotting combined with enhanced chemiluminescence30 facilitate detection of low levels of specific antibodies not detected by ELISA.31–33 Additionally, immunoblotting has enabled investigators to differentiate between age related changes in antigen recognition.34–36
An antibody response to bacterial antigens is one indicator of prior exposure to an organism. Infection with H pylori at a subclinical level and consequent elimination of the infection has been proposed to account for serological recognition of H pylori antigens in some uninfected subjects.37 In this study, we showed that H pylori uninfected subjects had circulating antibodies (IgG) to several H pylori antigens, including NapA and AphC. Immunodepletion and cross reactivity studies indicated that the IgG response was H pylori directed as seroreactivity could only be substantially eliminated by adsorption with H pylori but not with C jejuni, E coli, E aerogenes, S typhimurium, or Y pseudotuberculosis. Specific polyclonal antisera (rabbit) to H pylori only reacted weakly with C jejuni and E coli. Similarly, others have demonstrated little cross reactivity with anti-H pylori serum and other prokaryotes (Streptococcus sanguis, Salmonella typhimurium, Campylobacter fetus, Nesseria meningitidis, Haemophilius influenzae, Staphylococcus aureus, and Yersinia enterocolitica).17,38 It is not possible however to exclude cross reactivity with other gastrointestinal commensals given the many hundreds of such organisms inhabiting the gastrointestinal tract. However, the H pylori directed specificity of the anti-NapA and anti-AphC IgG response is supported by the inability of other bacteria, including C jejuni, to immunodeplete NapA and AphC antibodies from uninfected patient sera, even though homologues of AphC and NapA from C jejuni exhibit the highest degree of identity to H pylori AphC and NapA, at 67% and 38%, respectively.
As prospective data were not available for this study there is an inherent uncertainty in unequivocally ascribing the observed antibody responses to H pylori to a high incidence of prior exposure to the bacterium. Although spontaneous eradication/transient colonisation of H pylori infection has been documented in paediatric populations14,16,19,39–42 it is thought to be a less frequent event in adults, yet a significant rate (7.7%) of IgG seroreversion was found in a young and middle aged Danish population.8 Others too have detected anti-H pylori antibodies in uninfected individuals by immunoblotting (for example, see Nilsson and colleagues43). One possible explanation for seroreversion in the absence of therapeutic intervention is the widespread use of antimicrobials for other infections with secondary clearance of H pylori. However, there is evidence to suggest that this is unlikely to account for all cases of apparent spontaneous eradication.44,45 Strain variation, host genetic factors, and gastric atrophy have also been proposed to account for some cases of seroreversion.46–49
In addition to antibody responses, both the proliferative and cytokine responses of NapA and AphC stimulated PBMC and LPL were influenced by the infection status of the individuals. Both the gastric and peripheral lymphoproliferative responses of the uninfected seropositive group were significantly greater than those observed for infected individuals in response to NapA, AphC, HPS, but not rUreB. Furthermore, NapA, AphC, and HPS activated but not rUreB treated PBMC and LPL from uninfected subjects secreted significantly more IFN-γ than infected subjects, observations that are similar to previous studies with various preparations and extracts of H pylori.26,50,51 Others too have shown that gastric biopsy samples from uninfected dyspeptic patients have more IFN-γ secreting T cells than infected samples, suggesting that IFN-γ type responses might be protective.52 A number of groups have also reported suppressed lymphocyte responses from infected subjects compared with negative controls,26,50,51 possibly due to an altered T cell response secondary to infection or, alternatively, the production of immunosupressive factor(s) by the pathogen. This is in agreement with our present data showing preferential IL-10 secretion by infected individuals.
Finally, as the cytokine profile during infection is documented to play a regulatory role with respect to immunoglobulin production, including subclass and isotype switching, it was of interest to determine the NapA and AphC specific IgG subclass pattern in H pylori infected and uninfected subjects. Both NapA and AphC preferentially, but not exclusively, elicited a stronger IgG3 response in uninfected subjects and a significantly stronger IgG4 response in H pylori positive individuals. Secretion of IgG4 by human PBMC is known to be suppressed by IFN-γ in vitro53 but enhanced by IL-10,54 a major regulatory cytokine. Studies on various infections including Lyme disease,55 rubella56,57 and mumps58 indicate a close association between IFN-γ and IgG3 production. Furthermore, the potent opsonising and complement fixing properties of IgG3 have prompted speculation that IgG3 positivity may play a role in disease resolution, particularly in the case of Lyme borreliosis. In this regard it is interesting to note in this study that H pylori negative subjects displayed a predominantly T helper 1-like cytokine and antibody response to NapA and AphC. However, it appears likely that the IgG subclass distribution will be influenced by the biochemical/antigenic properties of the molecules based on the antigen specific cytokine profiles observed in this present and other studies.
In summary, these findings may have implications with regard to protective immunity. In animal studies NapA has been identified as a protective vaccine59 and it will be of interest to determine whether AphC demonstrates similar properties.. However, identification of different immune responses to H pylori antigens in H pylori negative and positive populations suggests that the nature of the immune response to H pylori exposure may have an influence on patient/disease outcome.
Acknowledgments
The authors acknowledge Professor D Weir for the biopsy material, Dr A Moran and John Ferris for C jejuni and Dr Ken Whelan for various samples of gut flora. This work was supported by a Health Research Board grant (YSA).
Abbreviations
IFN-γ, interferon γ
IL-10, interleukin 10
LPL, lamina propria lymphocytes
NapA, neutrophil activating protein
PBMC, peripheral blood mononuclear cells
rUreB, recombinant urease B subunit
AphC, alkyl hydroperoxide reductase
PBS, phosphate buffered saline
SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis
PCR, polymerase chain reaction
HPS, H pylori sonicate
REFERENCES
- 1.Marshall BJ. Unidentified curved bacilli on the gastric epithelium in active chronic gastritis. Lancet 1983;I:1273–5. [PubMed] [Google Scholar]
- 2.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984;I:1311–15. [DOI] [PubMed] [Google Scholar]
- 3.Bayerdorffer E , Neubauer A, Rudolph B, et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet 1995;345:1591–4. [DOI] [PubMed] [Google Scholar]
- 4.Blaser MJ, Parsonnet J. Parasitism by the “slow” bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J Clin Invest 1994;94:4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ernst PB, Pecquet S. Interactions between Helicobacter pylori and the local mucosal immune system. Scand J Gastroenterol 1991;187:56–64. [PubMed] [Google Scholar]
- 6.Rathbone BJ, Wyatt JI, Worsley BW, et al. Systemic and local antibody responses to gastric Campylobacter pyloridis in non-ulcer dyspepsia. Gut 1986;27:642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen LP, Rosenstock SJ, Bonnevie O, et al. Seroprevalence of immunoglobulin G, M and A antibodies to H. pylori in an unselected Danish population. Am J Epidemiol 1996;143:1157–64. [DOI] [PubMed] [Google Scholar]
- 8.Rosenstock S , Jorgensen T, Andersen L, et al. Seroconversion and seroreversion in IgG antibodies to H.pylori: a serology based prospective cohort study. J Epidemiol Community Health 2000;54:444–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell HM, Bohane TD, Berkowicz J, et al. Antibody to Campylobacter pylori in families of index children with gastrointestinal illness due to C pylori. Lancet 1987;2:681–2. [DOI] [PubMed] [Google Scholar]
- 10.Von Wulffen H , Grote HJ Enzyme linked immunosorbent assay for detection of immunoglobulins A and G antibodies to Campylobacter pylori. Eur J Clin Microbiol Infect Dis 1988;7:559–65. [DOI] [PubMed] [Google Scholar]
- 11.Glupczynski Y , Burette A, Goosens H, et al. Effects of antimicrobial therapy on the specific serological response to Helicobacter pylori infection. Eur J Clin Microbiol Infect Dis 1992;11:583–8. [DOI] [PubMed] [Google Scholar]
- 12.Langenberg W , Rauws EA, Houthoff HJ, et al. Follow-up study of individuals with untreated Campylobacter pylori-associated gastritis and of noninfected persons with non-ulcer dyspepsia. J Infect Dis 1988;157:1245–9. [DOI] [PubMed] [Google Scholar]
- 13.Granstrom M , Tindberg Y, Blennow M. Seroepidemiology of Helicobacter pylori infection in a cohort of children monitored from 6 months to 11 years of age. J Clin Microbiol 1997;35:468–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perri F , Pastore M, Clemente R, et al. Helicobacter pylori infection may undergo spontaneous eradication in children: a 2-year follow-up study. J Pediatr Gastroenterol Nutr 1998;27:181–3. [DOI] [PubMed] [Google Scholar]
- 15.Xia HH, Talley NJ. Natural acquisition and spontaneous elimination of Helicobacter pylori infection: clinical implications. Am J Gastroenterol 1997;92:1780–7. [PubMed] [Google Scholar]
- 16.Klein PD, Gilman RH, Leon-Barua R, et al. The epidemiology of Helicobacter pylori in Peruvian children between 6 and 30 months of age. Am J Gastroenterol 1994;89:2196–200. [PubMed] [Google Scholar]
- 17.Meyer B , Werth B, Beglinger C, et al. Helicobacter pylori infection in healthy people: a dynamic process? Gut 1991;32:347–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres J , Perez-Peres G, Goodman KJ, et al. A comprehensive review of the natural history of Helicobacter pylori infection in children. Arch Med Res 2000;31:431–69. [DOI] [PubMed] [Google Scholar]
- 19.Malaty HM, El-Kasabany A, Graham DT, et al. Age at acquisition of H. pylori infection: a follow-up study from infancy to adulthood, Lancet 2002;359:931–5. [DOI] [PubMed] [Google Scholar]
- 20.Marshall BJ, Armstrong JA, McGechie DB, et al. Attempt to fulfil Koch’s postulates for pyloric Campylobacter. Med J Aust 1985;142:436–9. [DOI] [PubMed] [Google Scholar]
- 21.Morris A , Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am J Gastroenterol 1987;82:192–9. [PubMed] [Google Scholar]
- 22.Towbin H , Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 1979;76:4350–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–5. [DOI] [PubMed] [Google Scholar]
- 24.Silhavy TJ, Berman ML, Enquist LW. Experiments with gene fusions. Cold Spring Harbour: Cold Spring Harbour Laboratory Press, 1984.
- 25.Don RH, Cox PT, Wainwright BJ, et al. ‘Touchdown’ PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res 1989;19:4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan XJ, Chua A, Shahi CN, et al. 1994. Gastric T lymphocyte responses to Helicobacter pylori in patients with H pylori colonisation. Gut 1994;35:1379–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cockburn M , Cow B. The effect of measurement error on the determination of H. pylori prevalence. Epidemiology 1997;8:205–9. [DOI] [PubMed] [Google Scholar]
- 28.Pearce DC, Peach HG, Farish SJ. H. pylori antibody titres in serum, plasma, and successively thawed specimens: implications for epidemiological studies,, J Clin Pathol 1996;49:1017–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feldman RA, Deeks JJ, Evans SJW, the H. pylori Serology Study Group. Multi-laboratory comparison of eight commercially available H. pylori serology kits. Eur J Microbiol Infect Dis 1995;14:428–33. [DOI] [PubMed] [Google Scholar]
- 30.Durrant I , Fowler S. Chemiluminescent detection systems for protein blotting. In: Dunbar BS, ed. Protein blotting: A practical approach. New York: IRL Press, 1994:141–52.
- 31.Nilsson I , Ljungh A, Aleljung P, et al. Immunoblot assay for serodiagnosis of Helicobacter pylori infections. J Clin Microbiol 1997;35:427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nilsson I , Lindkvist P, Wretlind B, et al. Immunoblots are superior to EIA for anti-H. pylori antibody determinations in young children from both high and low endemic areas. Gut 1999;45 (suppl 111) :A97. [Google Scholar]
- 33.Rocha GA, Oliveira AM, Queiroz DM, et al. Immunoblot analysis of humoral immune response to Helicobacter pylori in children with and without duodenal ulcer. J Clin Microbiol 2000;38:1777–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell HM, Hazell SL, Kolesnikow T, et al. Antigen recognition during progression from acute to chronic infection with a cagA-positive strain of Helicobacter pylori. Infect Immun 1996;64:1166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westblom TU, Madan E, Gudipati S, et al. Diagnosis of Helicobacter pylori infection in adult and pediatric patients by using Pyloriset, a rapid latex agglutination test. J Clin Microbiol 1992;30:96–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chmiela M , Lawnik M, Czkwianianc E, et al. Systemic humoral responses to H. pylori in children and adults. Arch Immunol Ther Exp 1998;46:161–7. [PubMed] [Google Scholar]
- 37.Yamaguchi H , Osaki T, Kai M, et al. Immune response against a cross-reactive epitope on the heat shock protein 60 homologue of Helicobacter pylori. Infect Immun 2000;68:3448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Espersen F . Immunoglobulin G antibodies to Helicobacter pylori in patients with dyspeptic symptoms investigated by the western immunoblot technique. J Clin Microbiol 1992;30:1743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahalanabis D , Rahman MM, Sarker SA, et al. 1996. H. pylori infection in the young in Bangladesh: prevalence, socio-economic and nutritional aspects., Int J Epidemiology 1996;25:894–8. [DOI] [PubMed] [Google Scholar]
- 40.Granstrom M , Tindberg Y, Blennow M. Seroepidemiology of H. pylori infection in a cohort of children monitored from 6 months to 11 years of age. J Clin Microbiol 1997;35:468–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas JE, Dale A, Harding M, et al. H. pylori colonization in early life. Pediatr Res 1995;45:218–23. [DOI] [PubMed] [Google Scholar]
- 42.Perez-Perez GI, Sack RB, Reid R, et al. Transient and persistent H. pylori colonization in native American children. J Clin Microbiol 2003;41:2401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nilsson N , Ljungh A, Aleljung P, et al. Immunoblot assay for serodiagnosis of H. pylori infections. J Clin Microbiol 1997;35:427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rothenbacher D , Bode G, Adler G, et al. Use of commonly prescribed antibiotics is not associated with prevalence of H. pylori infections in adults. Scand J Gastroenterol 1997;32:1096–9. [DOI] [PubMed] [Google Scholar]
- 45.Leung W-K, Hung L, Kwok C, et al. Follow up of serial urea breath test results in patients after consumption of antibiotics for non gastic infections. World J Gastroenterol 2002;8:703–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuipers EJ, Klinkenberg-Knol EC, Vandenbroucke-Grauls CMEJ, et al. Role of H. pylori in the pathogenesis of atrophic gastritis. Scand J Gastroenterol 1997;32 (suppl 223) :28–34. [PubMed] [Google Scholar]
- 47.Kuipers EJ, Uyterlinde AM, Pena AS, et al. Long term sequelae of H. pylori gastritis. Lancet 1995;345:1525–8. [DOI] [PubMed] [Google Scholar]
- 48.Valle J , Kekki M, Sipponen P, et al. Long term course and consequences of H. pylori gastritis. Scand J Gastroenterol 1996;31:546–50. [DOI] [PubMed] [Google Scholar]
- 49.Kokkola A , Kosunen TU, Puolakkainen P, et al. Spontaneous disappearance of H. pylori antibodies in patients with advanced atrophic gastritis. APMIS 2003;111:619–24. [DOI] [PubMed] [Google Scholar]
- 50.Karttunen R . Blood lymphocyte proliferation, cytokine secretion and appearance of T cells with activation surface markers in cultures with Helicobacter pylori. Comparison of the responses of subjects with and without antibodies to H. pylori. Clin Exp Immunol 1991;83:396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duchmann R , Scherer H, Neurath M, et al. Normal interleukin-12 production in individuals with antibodies to Helicobacter pylori. APMIS 1997;105:824–30. [DOI] [PubMed] [Google Scholar]
- 52.Kartunnen R , Kartunnen T, Ekre HP, et al. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in H. pylori positive and negative gastritis. Gut 1995;36:341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sutherland M , Blaser K, Pene J. Effects of interleukin-4 and interferon-gamma on the secretion of IgG4 from human peripheral blood mononuclear cells. Allergy 1993;48:504–10. [DOI] [PubMed] [Google Scholar]
- 54.Jeannin P , Lecoanet S, Delneste Y, et al. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol 1998;160:3555–61. [PubMed] [Google Scholar]
- 55.Widhe M , Ekerfelt C, Forsberg P, et al. IgG subclasses in Lyme borreliosis: a study of specific IgG subclass distribution in an interferon-gamma-predominated disease. Scand J Immunol 1998;47:575–81. [PubMed] [Google Scholar]
- 56.Linde GA. Subclass distribution of rubella virus-specific immunoglobulin G. J Clin Microbiol 1985;21:117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakayama T , Urano T, Osano M, et al. Evaluation of live trivalent vaccine of measles AIK-C strain, mumps Hoshino strain and rubella Takahashi strain, by virus-specific interferon-gamma production and antibody response. Microbiol Immunol 1990;34:497–508. [DOI] [PubMed] [Google Scholar]
- 58.Linde GA, Granstrom M, Orvell C. Immunoglobulin class and immunoglobulin G subclass enzyme-linked immunosorbent assays compared with microneutralization assay for serodiagnosis of mumps infection and determination of immunity. J Clin Microbiol 1987;25:1653–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Satin B , Del Giudice G, Della Bianca V, et al. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J Exp Med 2000;191:1467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]