Abstract
Background: Enteropathy in coeliac disease (CD) is sustained by a gliadin specific Th1 response. Interleukin (IL)-10 can downregulate Th1 immune responses.
Aim: We investigated the ability of recombinant human (rh) IL-10 to suppress gliadin induced Th1 response.
Patients and methods: IL-10 RNA transcripts were analysed by competitive reverse transcription-polymerase chain reaction in duodenal biopsies from untreated and treated CD patients, non-coeliac enteropathies (NCE), and controls. CD biopsies were cultured with a peptic-tryptic digest of gliadin with or without rhIL-10. The proportion of CD80+ and CD25+ cells in the lamina propria, epithelial expression of Fas, intraepithelial infiltration of CD3+ cells, as well as cytokine synthesis (interferon γ (IFN-γ) and IL-2) were measured. Short term T cell lines (TCLs) obtained from treated CD biopsies cultured with gliadin with or without rhIL-10 were analysed by ELISPOT for gliadin specific production of IFN-γ.
Results: In untreated CD and NCE, IL-10 RNA transcripts were significantly upregulated. In ex vivo organ cultures, rhIL-10 downregulated gliadin induced cytokine synthesis, inhibited intraepithelial migration of CD3+ cells, and reduced the proportion of lamina propria CD25+ and CD80+ cells whereas it did not interfere with epithelial Fas expression. In short term TCLs, rhIL-10 abrogated the IFN-γ response to gliadin.
Conclusions: rhIL-10 suppresses gliadin specific T cell activation. It may interfere with the antigen presenting capacity of lamina propria mononuclear cells as it reduces the expression of CD80. Interestingly, rhIL-10 also induces a long term hyporesponsiveness of gliadin specific mucosal T cells. These results offer new perspectives for therapeutic strategies in coeliac patients based on immune modulation by IL-10.
Keywords: interleukin 10, coeliac disease, immune suppression, organ culture, gliadin specific T cell lines
Coeliac disease (CD) is a lifelong intolerance to wheat gliadin and related prolamines (rye, barley) leading to enteropathy in genetically susceptible individuals. The features of biopsies from the small intestine suggest that the mucosal damage is due to a delayed-type hypersensitivity reaction to gliadin.1 Indeed, a Th1 response dominated by high levels of interferon γ (IFN-γ) has been reported in the small intestine of untreated coeliac patients and in the mucosa from treated patients following culture in vitro with gliadin.2
Interleukin (IL)-10 is an important immunoregulatory cytokine which acts on antigen presenting cells via inhibition of cytokine synthesis and expression of costimulatory and MHC class II molecules.3–6 In addition, IL-10 directly interferes with T cell proliferation and differentiation.7,8 Activation of human CD4+ T cells with allogeneic antigen presenting cells in the presence of IL-10 results in a long lasting antigen specific unresponsiveness of T cells.9 Furthermore, IL-10 induces differentiation of type 1 T regulatory (Tr1) cells which are able to suppress the Th1 immune response in vivo and in vitro through secretion of IL-10 and transforming growth factor β (TGF-β).10,11 Dysregulation of Tr1 cells seem to be relevant in the pathogenesis of autoimmune diseases in humans.12
IL-10 plays an important role in the regulation of the inflammatory cascade in the intestinal mucosa. Mice genetically deficient for IL-10 develop chronic enterocolitis caused by an unregulated Th1 response to endogenous bacterial flora.13 Similarly, expression of the IL-10 gene under the control of the IL-2 promoter in pathogenic CD4+ CD45RBhigh splenic T cells prevents severe colitis when these cells are adoptively transferred into recipient mice with severe combined immunodeficiency disease (SCID).14
The role of IL-10 in the gut in humans has been addressed in a few studies.15–17 Although under healthy conditions gut lamina propria T cells (LPT) spontaneously secrete high levels of IFN-γ,15 it has been shown that LPT can also release high amounts of IL-10 in vitro.16 In explant cultures of human fetal gut, human recombinant (rh)IL-10 downregulates mucosal T cell activation, metalloproteinase production, and prevents loss of extracellular matrix and mucosal damage induced by pokeweed mitogen or Staphylococcalenterotoxin B.17
Topical administration of rhIL-10 to three patients with ulcerative colitis reduced mucosal inflammation.18 Furthermore, a multicentre placebo controlled study has shown that subcutaneous administration of rhIL-10 daily for 28 days to patients with mild to moderately active Crohn’s disease is safe and well tolerated, and results in clinical and endoscopic improvement.19
In CD, the role of IL-10 has not yet been fully clarified.2,20,21 Previous studies failed to detect IL-10 RNA in both untreated and treated coeliac duodenal biopsies in vitro challenged with gliadin.2 Recently, increased levels of IL-10 and IFN-γ have been reported in intraepithelial lymphocytes isolated from untreated coeliac mucosa,20 not confirming previous observations where IL-10 producing cells were mainly distributed in the lamina propria.21
In order to define the immunosuppressive function of IL-10 in CD, we measured the ratio of IL-10/IFN-γ RNA transcripts in whole duodenal coeliac biopsies and explored the effect of exogenous rhIL-10 on gliadin induced T cell activation in ex vivo cultured treated and untreated coeliac intestinal mucosa. Furthermore, to investigate the long term effect of rhIL-10 on mucosal T cells, we measured the IFN-γ response of gliadin specific short term T cell lines (TCL) generated from IL-10 treated coeliac explants.
PATIENTS AND METHODS
Patients
For cytokine analysis, duodenal biopsies were obtained during gastroduodenal endoscopy and snap frozen at −70°C. Forty three patients with untreated CD (median age 3 years; range 0.8–18), 11 patients with treated CD (36.5 years; 20–58), nine patients with non-coeliac enteropathy (NCE) (3.6 years; 0.6–16), and 19 controls (8.7 years; 2.5–16.9) were analysed. The diagnosis of CD was based on ESPGHAN criteria.22 Controls included age matched patients with a normal duodenal mucosa. Patients with NCE had low to moderate grade atrophy, an increased number of intraepithelial lymphocytes (IELs), and/or lamina propria CD25+ cells. They were affected by duodenitis (5/9), IgA deficiency (1/9), cow’s milk intolerance (2/9), and multiple food intolerance (1/9).
For ex vivo challenge studies, 11 untreated CD patients (median age 13 years; range 8–14), 12 treated CD patients (31 years; 15–58) on a gluten free diet for more than one year, and 10 normal controls (36 years; 19–45) were enrolled. Patients were recruited after appropriate local ethics committee approval and informed consent was obtained. To obtain TCL, biopsies from two treated coeliac patients were used. Several biopsy specimens from the duodenum were obtained by gastroduodenal endoscopy, placed immediately in ice chilled saline solution, and processed for ex vivo organ culture.
Ex vivo organ cultures
Culture was performed as reported previously.23 Biopsies were challenged with a peptic-tryptic digest (PT) of gliadin (1 mg/ml) in the presence or absence of rhIL-10 (50 ng/ml).24 For reverse transcription-polymerase chain reaction (RT-PCR) analysis, biopsies at different time points (0, 1, 2, 4, 6, 8, 12, and 24 hours) were snap frozen at −70°C. For immunostaining, biopsies were embedded after 24 hours in OCT compound and snap frozen for cryosectioning.
RNA extraction and analysis of mRNA expression by quantitative RT-PCR
The plasmid pHCQ1 encoding for IFN-γ and IL-2 was kindly provided by Dr MF Kagnoff (Department of Medicine, University of California, San Diego, California, USA). To generate RNA to be used as the standard, plasmids were linearised with HindIII and transcribed in vitro using T7 RNA polymerase according to the manufacturer’s instructions (Promega, Biotech, Milan, Italy). Competitive RT-PCR was performed as described previously.25 Total tissue RNA was extracted from whole mucosal biopsies using Trizol (Gibco Life Technologies, Milan, Italy). The integrity of the RNA was checked by agarose gel electrophoresis. A constant amount of total RNA (0.5–1 μg) and serial 10-fold dilutions of standard RNA molecules (1 pg to 0.1 fg) were cotranscribed into cDNA according to the manufacturer’s instructions (Gibco Life Technologies). Primers and amplification conditions used for PCR have been described elsewhere.25 PCR products were visualised on a 2% agarose gel using Vistra-Green dye (Amersham International, Milan, Italy). Densitometric analysis of fluorescent bands was carried out on a STORM-860 analyser using ImageQuant software (Molecular Dynamics, Sunnyvale, California, USA). The ratio of standard/target RNA was plotted against the starting number of standard RNA molecules on a double logarithmic scale to determine the copy number in each sample. Data are presented as number of transcripts/μg total RNA.
Semiquantitative RT-PCR and Southern blot analysis
Semiquantitative RT-PCR was performed as described previously.26,27 RT-PCR reactions were performed with 0.7 μg of total RNA. In preliminary experiments, the optimal number of cycles to obtain a PCR product within the linear phase of the amplification was established. cDNA samples were amplified with β-actin primers for 18 cycles and with IFN-γ primers for 26 cycles. The following primers were used: β-actin sense 5′-CGA GGC CCA GAG CAA GAG A-3′ and antisense 5′-CAC AGC TTC TCC TTA ATG TCA CG-3′ (annealing temperature: 55°C); IFN-γ sense 5′-AAT GCA GGT CAT TCA GAT G-3′ and antisense 5′-TTG GAC ATT CAA GTC AGT T-3′ (annealing temperature: 51°C). To exclude the amplification of genomic DNA contaminating the samples, experiments were also performed using RNA as substrate for the PCR assay. cDNA probes were DNA fragments encoding the full length PCR product. Southern blotting was performed by a chemiluminescence kit according to the manufacturer’s instructions (Amersham International).
Detection of CD25, CD3, CD80, and Fas positive cells in CD mucosa by immunohistochemistry
Cryostat sections (5 μm) were fixed in acetone and stained with the following monoclonal antibodies (mAbs): anti-CD3 (Dako; 1:200), anti-CD25 (Dako, Milan, Italy; 1:25), anti-CD80 (Becton Dickinson, Buccinasco, Milan, Italy; 1:25), and anti-CD95 (PharMingen, San Diego, California, USA; 1:100). Peroxidase-antiperoxidase immunodetection was used for CD3 and Fas and alkaline phosphatase/antialkaline phosphatase for CD25 and CD80.
Costaining experiments were set up to detect activated T cells by immunofluorescence and confocal microscopy (Leica TCS SP, Heidelberg, Germany). Cryosections were fixed in acetone and incubated with mouse mAbs anti-CD25 (Dako; 1:25) and rabbit polyclonal anti-CD3 (Dako; 1:100), followed by horse antimouse FITC conjugate (Vector, Peterborough, UK; 1:200) and swine antirabbit TRITC conjugate (1:300). Sections were counterstained with ToPro-3 (Molecular Probes, San Giuliano Milanese, Milan, Italy) and mounted in phosphate buffered saline (PBS):glycerol (1:1).
The density of cells expressing CD3 in the intraepithelial compartment was expressed as the number of stained cells on 100 enterocytes; the number of cells expressing CD25 and CD80 in the lamina propria was evaluated within a total area of 1 mm2 of lamina propria. Expression of CD25 by mucosal T cells was calculated as a percentage of CD3+ lamina propria lymphocytes. Staining of epithelial cells expressing Fas was graded as weak (+) = 1, moderate (++) = 2, or strong (+++) = 3. Slides were analysed by two observers who were blinded.
Generation of short term gliadin specific T cell lines from treated coeliac biopsies
For generation of gliadin specific TCLs, PT gliadin was treated with tissue transglutaminase (PT-gliadin-tTG) according to a protocol previously described.28 Mucosal explants from two treated CD patients were cultured for 24 hours with PT-gliadin (1 mg/ml) in the presence or absence of rhIL-10 50 ng/ml (R&D systems, Minneapolis, Minnesota, USA). Then, explants were washed in RPMI (containing antibiotics and 2% heat inactivated human serum) and digested with collagenase A 1 mg/ml for one hour at 37°C, as described elsewhere 29. Cells were plated in 24 well plates at 2–3×105/ml and stimulated with 1×106 irradiated autologous peripheral blood mononuclear cells (PBMCs) and 50 μg/ml PT-gliadin-tTG in the absence of rh IL-10. On day 7, TCLs were restimulated with irradiated autologous PBMCs and PT-gliadin-tTG, as described above. TCLs were fed with fresh medium and IL-15 at 10 ng/ml the day after restimulation and every three days. Ten days after the second stimulation, TCLs were assayed for gliadin specificity by cytokine-ELISPOT.
To evaluate the capacity of TCLs to proliferate in response to polyclonal activation, 0.5×105 cells were stimulated in the presence or absence of IL-2 (100 U/ml). After 72 hours, cells were pulsed for 16 hours with 3H-thymidine (1 μC/well) and counted with a beta counter.
IFN-γ and IL-10 ELISPOT assays for the detection of gliadin specific TCLs
T cells (0.5×105) were plated in the presence of 1×105 irradiated autologous PBMCs pulsed or not overnight with 100 μg/ml PT-gliadin-tTG in 96 well nitrocellulose backed plates (MAHAS4510; Millipore, Bedford, Massachusetts, USA) coated with 10 μg/ml of antihuman interferon-γ or IL-10 mAbs (PharMingen) according to a protocol described elsewhere.30 After 20 hours of incubation at 37°C and 5% CO2, plates were extensively washed with PBS/0.05% Tween 20 and incubated with a biotinylated secondary antibody at 2 μg/ml for two hours. Finally, plates were washed and incubated with 50 μl of streptavidin horseradish peroxidase solution (PharMingen) for one hour. Spots were developed by adding aminoethyl carbazole solution (Sigma Chemical Co, St Louis, Missouri, USA) and counted at the Immunospot Image Analyser (AELVIS 3.0; Hannover, Germany).
Statistical analysis
Statistical analysis was performed using non parametric tests: Mann-Whitney U for two independent samples and the Wilcoxon signed rank test for paired samples.
RESULTS
In vivo IL-10 mRNA levels in untreated coeliac mucosa
IL-10 mRNA transcripts were measured by quantitative RT-PCR in 29 untreated and six treated coeliac patients, in five patients with NCE, and in 14 normal controls. IFN-γ mRNA transcripts were measured in 37 untreated and 10 treated coeliac patients, in eight patients with NCE, and in 15 normal controls. IL-10 and IFN-γ mRNA transcripts were both significantly upregulated in CD and NCE in comparison with control or treated CD mucosa (fig 1 ▶, table 1 ▶). However, the ratio IL-10/IFN-γ transcripts was markedly reduced (p<0.05) in CD mucosa in comparison with inflamed non-coeliac, control, and treated coeliac mucosa (table 1 ▶), suggesting that although IL-10 is produced in vivo there is a significant skewing towards a Th1 immune response in CD mucosa (fig 1C ▶).
Figure 1.
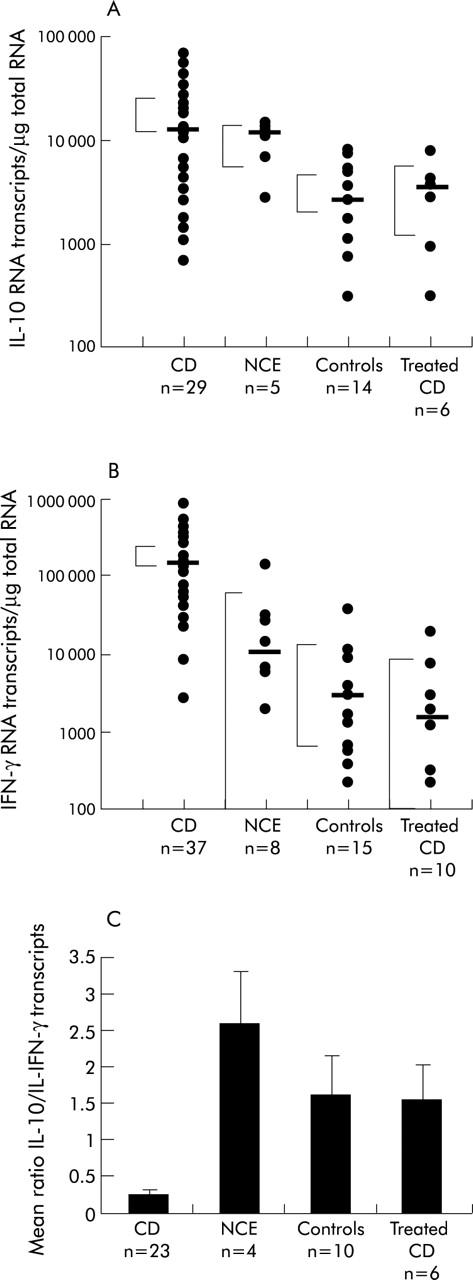
(A) Interleukin 10 (IL-10) mRNA transcripts in whole duodenal biopsies from patients with coeliac disease (CD), non-coeliac enteropathy (NCE), normal controls, and treated CD patients. The bars represent the median with 95% confidence limits. Mann-Whitney test for unpaired samples: CD versus controls, p<0.002; CD versus treated CD, p<0.02; CD versus NCE, p = 0.7. (B) Interferon γ (IFN-γ) mRNA transcripts in whole duodenal biopsies from patients with CD, NCE, normal controls, and treated CD. Bars represent the median with 95% confidence limits. Mann-Whitney for unpaired samples: CD versus controls, p<0.0001; CD versus treated CD, p<0.0001; CD versus NCE, p<0.0001. (C) Ratio between IL-10 and IFN-γ mRNA transcripts measured in whole duodenal biopsies. Values are mean (SD). IL-10/IFN-γ ratio was markedly reduced (p<0.05) in CD enteropathy in comparison with inflamed non-coeliac, control, and treated CD mucosa.
Table 1.
Interleukin 10 (IL-10) and interferon γ (IFN-γ) RNA transcripts in untreated and treated coeliac disease (CD), non-coeliac enteropathy (NCE), and control biopsies.
| IL-10 | IFN-γ | Ratio IL-10/IFN-γ (mean (SEM)) | |||
| Median | Range | Median | Range | ||
| Untreated CD | 12 246 | 1097–67 370 | 138 626 | 2605–450 688 | 0.24 (0.07) |
| Treated CD | 3385 | 311–7979 | 1569 | 222–19 913 | 1.53 (0.48) |
| NCE | 11 237 | 2773–14 371 | 10 114 | 1883–135 986 | 2.58 (0.71) |
| Controls | 2558 | 100–7982 | 2849 | 227–37 598 | 1.6 (0.52) |
Effect of rhIL-10 on PT-gliadin induced IFN-γ mRNA expression in untreated CD biopsies
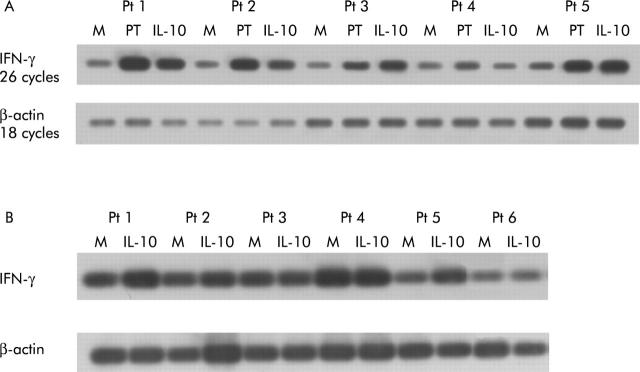
To define whether rhIL-10 downregulates in vitro activation of Th1 cells in active CD mucosa, biopsies from five untreated CD patients were cultured with PT-gliadin in the presence or absence of rhIL-10 (50 ng/ml) for 24 hours. IFN-γ mRNA expression was analysed semiquantitatively by Southern blotting of RT-PCR products. In all patients, IFN-γ mRNA expression was upregulated following activation by PT-gliadin; addition of rhIL-10 reduced gliadin induced IFN-γ production in 3/5 patients (fig 2A ▶). However, if biopsies were cultured with only rhIL-10, in the absence of PT-gliadin, we could not observe downregulation of IFN-γ RNA expression (fig 2B ▶). It appears therefore that IL-10-mediated immune suppression in CD mucosa is driven by the antigen.
Figure 2.
(A) Southern blot analysis of transcripts for interferon γ (IFN-γ) (upper blot) and β-actin (lower blot) in duodenal mucosa from five untreated coeliac disease (CD) patients cultured in vitro with medium alone (M) and with peptic-tryptic (PT)-gliadin in the absence (PT) or presence of human recombinant interleukin 10 (rhIL-10 50 ng/ml) (PT+IL-10) for 24 hours. Total RNA (700 ng) was used for cDNA preparation. An equivalent amount of cDNA per sample was amplified using specific primers for IFN-γ (26 cycles) and β-actin (18 cycles). Polymerase chain reaction products were then separated on a 1% agarose gel, blotted, and hybridised with oligonucleotide probes specific for IFN-γ and β-actin. In the presence of rhIL-10, gliadin induced IFN-γ mRNA production was reduced in 3/5 patients. (B) Southern blot analysis of transcripts for IFN-γ (upper blot) and β-actin (lower blot) in duodenal mucosa from six untreated CD patients cultured in vitro with medium alone (M) or in the presence of rhIL-10 (50 ng/ml) for 24 hours. Southern blot was performed as in (A). IFN-γ levels were not downregulated in biopsies cultured in the presence of rhIL-10 if compared with biopsies cultured in medium alone; paradoxically, in 4/6 patients there was upregulation of IFN-γ transcripts in the presence of rhIL-10.
Effect of rhIL-10 on PT-gliadin induced IFN-γ and IL-2 mRNA expression in treated CD biopsies
To analyse whether rhIL-10 prevented gliadin induced Th1 cell activation in treated coeliac mucosa, duodenal biopsies from six treated coeliac patients and six controls were cultured in vitro with PT-gliadin in the presence or absence of rhIL-10. After eight hours the presence of Th1 cytokines, IFN-γ and IL-2, was assessed by quantitative RT-PCR. Preliminary time course experiments established that eight hours was the optimal time point for cytokine expression (data not shown). The number of transcripts/μg total RNA for IFN-γ in treated coeliac mucosa cultured with medium alone was significantly enhanced by PT-gliadin (mean (SEM) 17 201 (8029) v 93 524 (45 875); p<0.03). Addition of rhIL-10 suppressed the IFN-γ response to gliadin (17 568 (32 702); p<0.03) (fig 3A ▶). Similar results were observed for IL-2 mRNA transcripts. The number of IL-2 mRNA transcripts in treated coeliac mucosa cultured with medium alone was enhanced by PT-gliadin (mean (SEM) 87 677 (23 701) v 162 383 (84 989); p<0.03). Again, addition of rhIL-10 downregulated this response (114 336 (60 325); p<0.03) (fig 3B ▶). No modifications for IFN-γ and IL-2 mRNA transcripts were observed in control biopsies (fig 3A ▶, B).
Figure 3.
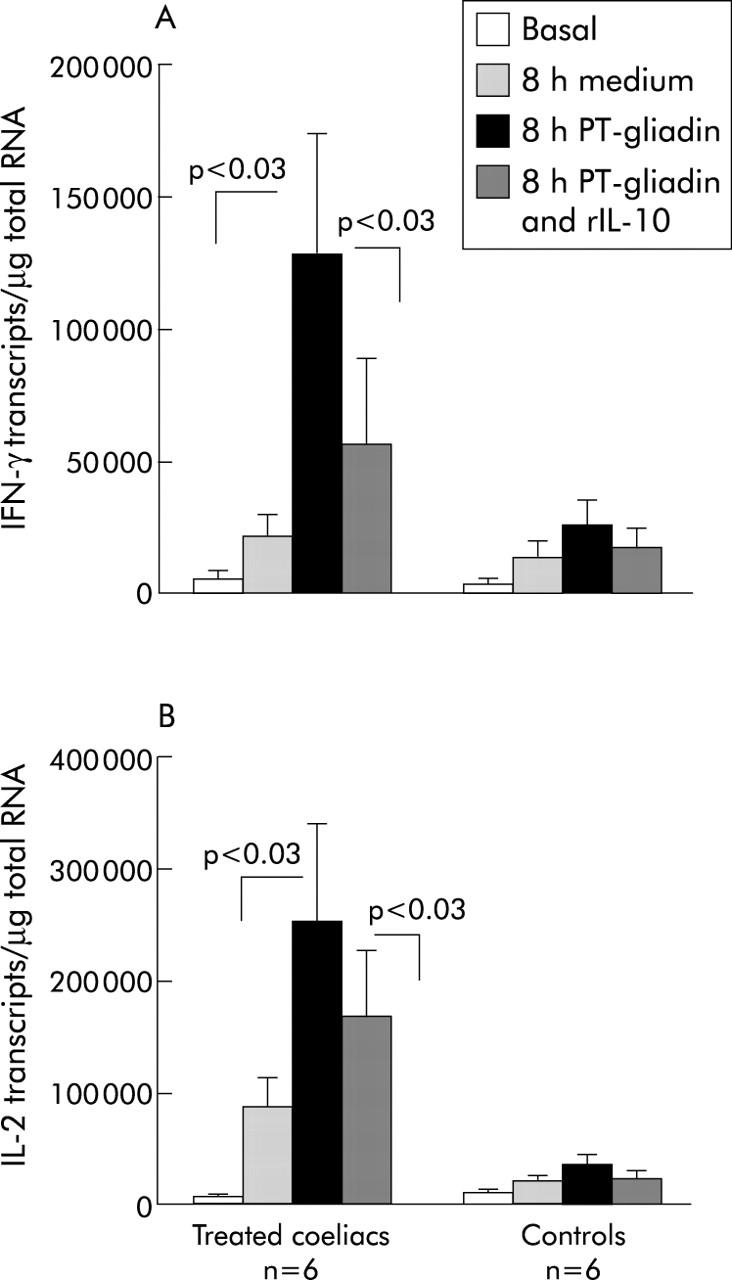
mRNA transcripts for interferon γ (IFN-γ) (A) and interleukin 2 (IL-2) (B) were analysed by quantitative reverse transcription-polymerase chain reaction in treated coeliac disease and control duodenal biopsies after challenge for eight hours in medium alone or with peptic-tryptic (PT)-gliadin in the presence or absence of human recombinant IL-10 (rhIL-10 50 ng/ml). Values are mean (SEM) of the number of transcripts/μg total RNA. A significant increase in IFN-γ and IL-2 was observed in CD mucosa but not in controls in the presence of PT-gliadin (p<0.03). Addition of rhIL-10 downregulated Th1 cytokine synthesis in CD mucosa (p<0.03).
Effect of rhIL-10 on PT-gliadin induced expression of CD25 and CD80 on lamina propria cells in treated CD biopsies
Biopsies from 11 treated coeliac patients were analysed by immunohistochemistry for expression of CD25 and CD80 in the lamina propria after 24 hours of organ culture with gliadin in the presence or absence of rhIL-10. The number of CD25+ cells in treated coeliac mucosa cultured in medium alone was significantly increased after stimulation with gliadin (mean (SEM) 21 (7) v 98 (15); p<0.003). Addition of rhIL-10 to the organ culture at the same time as gliadin inhibited CD25 expression (53 (14); p<0.008) (fig 4A ▶). To define whether this inhibition affected T cells, costaining experiments with anti-CD3 and anti-CD25 mAbs were performed. CD3+CD25+ cells were counted as a percentage of the total lamina propria CD3+ T cells. After in vitro gluten challenge, the proportion of LPT expressing CD25 significantly increased (mean (SEM) 9 (0.8)) in comparison with fragments cultured with only medium (1 (0.3)). Addition of rhIL-10 decreased the number of LPT expressing CD25 (5 (0.4)) (fig 5 ▶).
Figure 4.
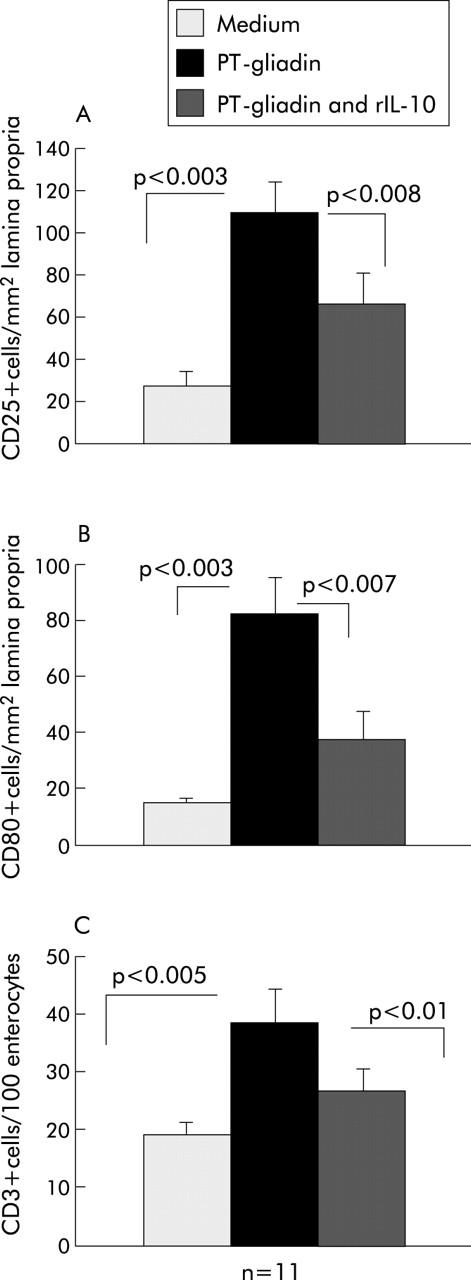
(A) Immunohistochemical staining for CD25+ cells in treated coeliac disease (CD) biopsies challenged with peptic-tryptic (PT)-gliadin in vitro in the presence or absence of human recombinant interleukin 10 (rhIL-10). A significant increase in the number of CD25+ cells/mm2 of lamina propria was observed in CD biopsies cultured in the presence of PT-gliadin in comparison with medium alone (p<0.003). Addition of rhIL-10 together with PT-gliadin resulted in marked inhibition of CD25 expression (p<0.008). Values are mean (SEM). (B) Immunohistochemical staining for CD80+ cells in treated CD biopsies challenged with PT-gliadin in vitro in the presence or absence of rhIL-10. A significant increase in the number of CD80+ cells/mm2 of lamina propria was observed in CD biopsies cultured in the presence of PT-gliadin in comparison with medium alone (p<0.003). Addition of rhIL-10 together with PT-gliadin inhibited CD80 expression (p<0.007). Values are mean (SEM). (C) Immunohistochemical staining for CD3+ intraepithelial lymphocytes in treated CD biopsies challenged with PT-gliadin in vitro in the presence or absence of rhIL-10. Migration of CD3+ T cells into the epithelial compartment was observed on gliadin challenge (p<0.05). Addition of rhIL-10 significantly reduced the number of CD3+ T cells in the epithelial compartment (p<0.01). Values are mean (SEM).
Figure 5.
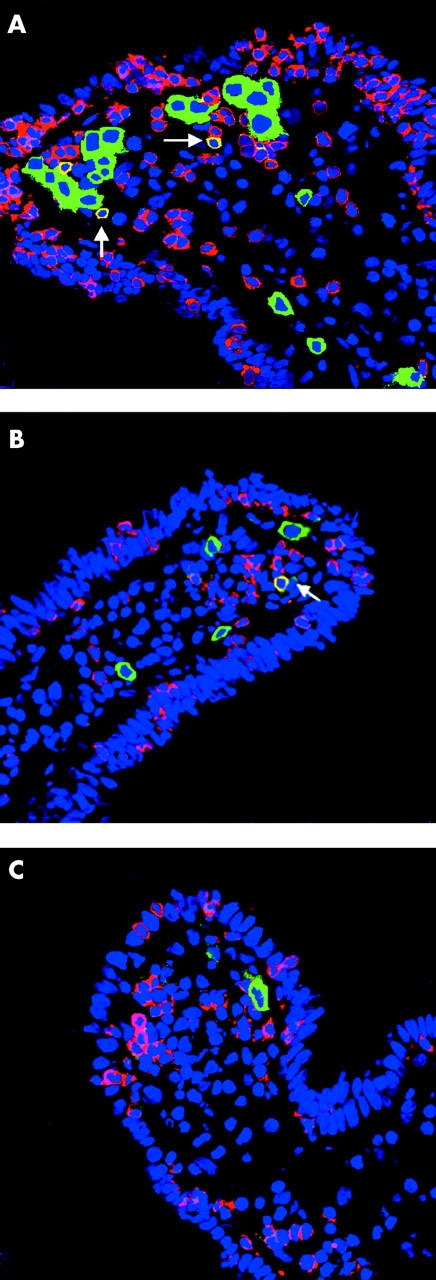
Immunofluorescence staining on duodenal mucosa from a coeliac patient cultured in vitro with peptic-tryptic (PT)-gliadin (A), with PT-gliadin and human recombinant interleukin 10 (rhIL-10) (B), or with medium only (C). In comparison with (C), in (A) there is an increase in CD3+ intraepithelial lymphocytes (in red) and of CD25+ cells (in green), particularly in the subepithelial region. There is also a significant increase in CD3+CD25+ activated T cells (in yellow). In (B), addition of rhIL-10 inhibited T cell activation and intraepithelial lymphocyte infiltration. Original magnification 40×.
Similarly, the number of CD80+ mononuclear cells in the lamina propria cultured in medium alone increased following addition of PT-gliadin (mean (SEM) 12 (2) v 94 (11); p<0.003). Addition of rhIL-10 prevented CD80 expression on lamina propria mononuclear cells (18 (10); p<0.007) (fig 4B ▶).
Effect of rhIL-10 on PT-gliadin induced infiltration of intraepithelial CD3+ lymphocytes and expression of Fas on epithelial cells in treated CD biopsies
Addition of PT-gliadin enhanced infiltration of IELs. The number of CD3+ IELs in treated coeliac mucosa cultured with medium alone was markedly increased following addition of PT-gliadin (mean (SEM) 18 (2) v 41 (6); p<0.005). The presence of rhIL-10 significantly reduced CD3+ IEL density (26 (4); p<0.01) (fig 4C ▶, fig 5 ▶). In contrast, although the intensity of Fas expression on epithelial cells was enhanced by PT-gliadin, it was not modified by the presence of rhIL-10 (fig 6 ▶).
Figure 6.
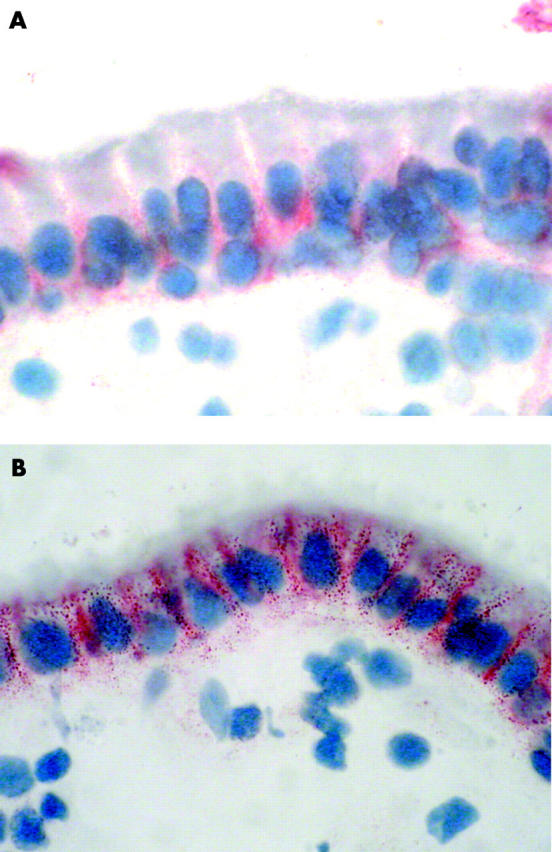
Fas expression in the epithelium of jejunal mucosa from a coeliac patient cultured in vitro with medium only (A) or with peptic-tryptic (PT)-gliadin (B). In (B), intense staining for Fas is detected in almost all epithelial cells. Expression is particularly evident in the basolateral membrane of the enterocytes. A similar pattern was observed culturing the mucosa with PT-gliadin in combination with human recombinant interleukin10 (data not shown). Original magnification 100×.
rhL-10 induces long term suppression of gliadin specific T cell responses in treated CD mucosa
Previous studies reported that rhIL-10 induced long lasting antigen specific unresponsiveness of antigen specific CD4+ T cells.9,10 Based on these findings, we investigated whether rhIL-10 could specifically downregulate Th1 mediated immune responses in treated CD mucosa challenged with PT-gliadin in vitro. Mucosal explants from two treated CD patients were cultured for 24 hours with PT-gliadin in the presence or absence of rhIL10. TCLs were generated from these explants following restimulation with gliadin and autologous APCs, and after three weeks were tested for antigen specificity by IFN-γ and IL-10 ELISPOT assays. TCLs obtained from biopsies cultured in the presence of rhIL-10 showed a significant reduction in the number of gliadin induced IFN-γ spot forming cells (SFC) (mean of net SFC/106 10 (range 3–17)) in comparison with TCLs obtained from biopsies cultured in the absence of rhIL-10 (655 (range 140–1170)). In fig 7A ▶, representative data from TCLs obtained from one of the two treated patients are shown. No significant differences in cell recovery from TCLs obtained from explants cultured in the presence or absence of rhIL-10 were observed (data not shown). Furthermore, the phenotype of control and IL-10 treated TCLs was equivalent (control TCLs 73.3 (3.7)% CD3+, 39 (11.3)% CD3+CD4+, 25.9 (9.7)% CD3+CD8+; IL-10 TCLs 73.1 (20.0)% CD3+, 52.2 (28.6)% CD3+CD4+, 22.8 (2.8)% CD3+CD8+). Despite their hyporesponsiveness to antigen, TCLs from rhIL-10 treated explants retained their ability to proliferate in response to exogenous IL-2 (fig 7B ▶). Importantly, both TCLs obtained from explants cultured in the presence or absence of rhIL-10 produced IL-10 in response to gliadin, with a frequency of IL-10 producing cells significantly higher in TCLs obtained from IL-10 treated biopsies (mean of net SFC/106 1390 (range 1200–1580) v 526 (range 450–603)) (fig 7C ▶).
Figure 7.
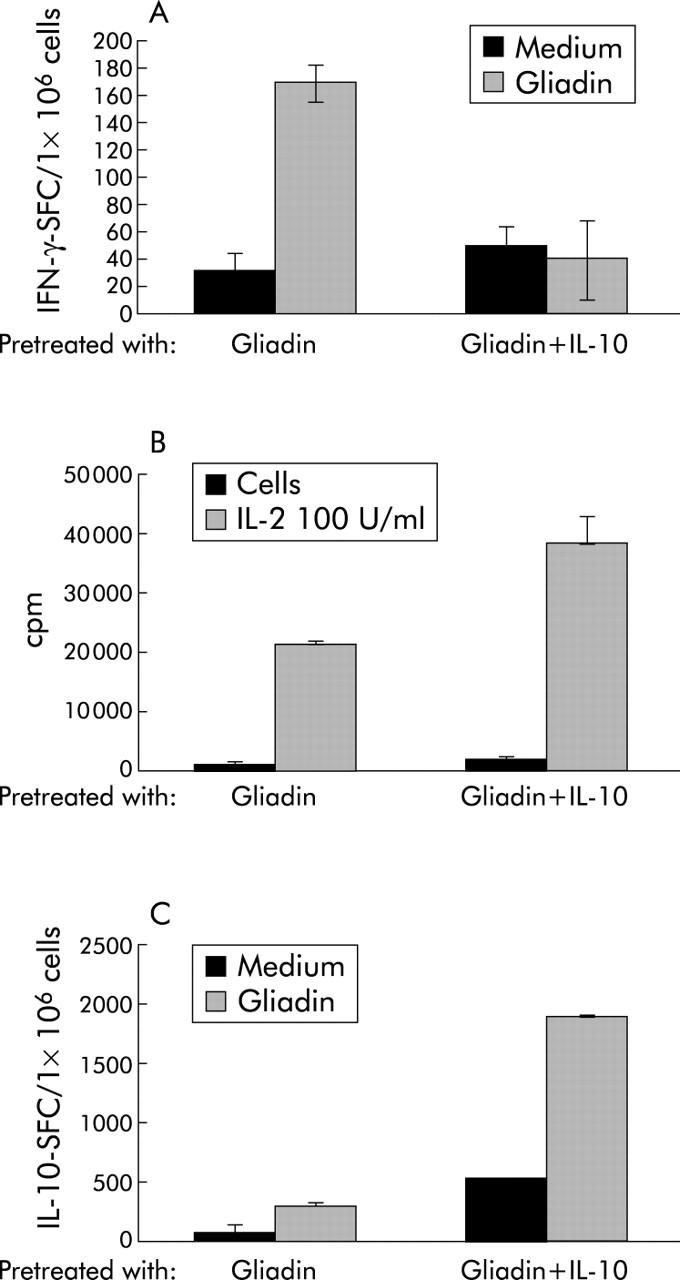
Short term T cell lines (TCLs) were generated from treated CD mucosa challenged for 24 hours with gliadin (gliadin) or gliadin plus human recombinant interleukin 10 (gliadin+IL-10). TCLs were expanded for three weeks by repeated stimulation with APCs, gliadin, and IL-15 and subsequently tested for gliadin specificity by interferon γ (IFN-γ) and IL-10 ELISPOT and for proliferation to exogenously added IL-2. Representative results of TCLs obtained from one of the two CD patients analysed are shown. (A) TCLs from IL-10 treated explants did not produce IFN-γ in response to gliadin. Autologous peripheral blood mononuclear cells pulsed overnight with medium or gliadin (100 μg/ml) were used as APC. (B) IL-10 treated TCLs retained their capacity to proliferate in response to cytokines. No significant differences in proliferative responses to exogenously added IL-2 (100 U/ml) were observed in both control TCLs (gliadin) and IL-10 treated TCLs. (C) Increased frequency of IL-10 producing T cells in response to gliadin stimulation in TCLs obtained from IL-10 challenged biopsies
DISCUSSION
In contrast with most other autoimmune diseases, in CD the trigger (gliadin), the genetic association (HLA DQ2 or DQ8), and the highly specific humoral response (antibodies to tTG) have been well characterised.1 Thus CD represents one of the best candidate autoimmune diseases in which to test novel therapeutic strategies based on manipulation of the immune system with the ultimate goal of re-establishing tolerance. We have shown that IL-10 can potently downregulate Th1 mediated immune responses to gliadin in both treated and untreated coeliac mucosa. IL-10 mediated its effects via downregulation of antigen presentation, reduction of T cell infiltration and activation, and importantly by inducing a long lasting hyporesponsiveness in gliadin specific T cells.
Somewhat surprisingly, we observed high levels of IL-10 mRNA transcripts in untreated coeliac mucosa in vivo in comparison with treated CD patients and normal controls. Although we did not precisely define the origin of IL-10 in untreated coeliac mucosa, there was an increase in the number of IL-10 producing mucosal T cells in untreated coeliac mucosa. Recently, increased levels of IL-10 and IFN-γ have been reported in IELs isolated from untreated coeliac duodenal biopsies.20 However, other cells, such as macrophages, epithelial, and dendritic cells may also contribute to increased levels of IL-10. The fact that the ratio between mRNA levels for IL-10 and IFN-γ was significantly lower in untreated and inflamed CD mucosa in comparison with other enteropathies strongly suggests that even these high levels of IL-10, which presumably reflect a compensatory anti-inflammatory pathway, are not sufficient to suppress the overwhelming Th1 mediated response in active CD. This hypothesis is further supported by evidence that IL-10 is also upregulated during chronic inflammation of the gut mucosa due to Helicobacter pylori gastritis,31 ulcerative colitis, and Crohn’s disease.32
To evaluate if addition of exogenous rhIL-10 suppressed the powerful gliadin specific Th1 mediated immune response, we established ex vivo organ cultures of untreated CD biopsies. Indeed, we found that in 3/5 experiments, rhIL-10 was capable of downregulating expression of IFN-γ mRNA in untreated CD mucosa. This effect of rhIL-10 was antigen specific as in the absence of gliadin rhIL-10 did not interfere with T cell activation. We also performed ex vivo organ cultures of non-inflamed biopsies from treated coeliac patients in the presence or absence of rhIL-10. In this case, the effect was more dramatic as we observed that rhIL-10 potently downregulated gliadin induced immune activation in the lamina propria; in fact, a decrease in the number of CD25+ and CD80+ cells, and in expression of IFN-γ and IL-2 mRNA, was evident.
In the epithelial compartment, although rhIL-10 was able to prevent intraepithelial infiltration of CD3+ T cells, it did not affect expression of Fas. These data are similar to those obtained in previous studies with CTLA-4Ig, which also downregulated gliadin specific T cell activation (as judged by expression of CD25, ICAM-1, IFN-γ, and IL-2), but did not interfere with expression of Fas on epithelial cells.33 It is therefore likely that expression of Fas on epithelial cells is independent of the state of activation of LPT cells. Importantly, in contrast with treatment with CTLA-4Ig, rhIL-10 suppressed gliadin induced infiltration of IELs. The factors which control the migration of CD3+ T cells into the intraepithelial area in CD are not clear. However, failure of CTLA-4Ig to control this migration seems to suggest that, similar to expression of Fas, IEL infiltration does not depend on the state of T cell activation in the lamina propria 33. On the other hand, in small intestine explants from human fetal gut, activation of T cells with anti-CD3 mAbs induced a significant increase in the number of IELs.34 These data suggest that in some cases infiltration of CD3+ T cells is linked to activation of T cells in the lamina propria.
IL-10 mediated inhibition of CD80 expression6 in lamina propria mononuclear cells in IL-10 treated biopsies indicates that at least some of the inhibitory effects on T cells were due to downregulation of antigen presentation. Importantly, IL-10 not only inhibited short term T cell responses, it also induced a long term hyporesponsiveness in gliadin specific T cell lines. In fact, TCLs isolated from biopsies cultured for 24 hours in the presence of rhIL-10 and PT-gliadin and expanded in the presence of PT-gliadin-tTG and IL-15 for three weeks did not produce IFN-γ on subsequent rechallenge with gliadin in the absence of cytokines. Classically, in vitro activation of CD4+ T cells in the presence of IL-10, or with IL-10 treated dendritic cells, results in a state of functional unresponsiveness termed anergy. 9,35 Moreover, when anergic T cells from MLRs performed in the presence of IL-10 were isolated at the clonal level, a subset of T cell clones which possessed a unique profile of cytokine production was isolated.10 These T cells produced high levels of IL-10 and TGF-β, and based on their ability to downregulate immune responses in vitro and in vivo via production of IL-10 and TGF-β, were termed Tr1 cells. Thus IL-10-anergised CD4+ T cells contained the precursors of Tr1 cells, and IL-10 was a critical factor for their differentiation.36 Similarly, it is possible that the hyporesponsive TCLs isolated from CD biopsies treated with rhIL-10 and gliadin contain the precursors of Tr1 cells. In this context an interesting finding is the increased frequency of IL-10 producing cells in TCLs obtained from explants cultured in the presence of rhIL-10.
In conclusion, our data provide the first evidence for an immunoregulatory effect of IL-10 on gliadin dependent T cell activation in treated and untreated CD mucosa. IL-10 acts by interfering with antigen presentation and results in induction of hyporesponsive gliadin specific T cells. As Tr1 cells exist naturally in the human gut mucosa and maintain intestinal homeostasis,11 using IL-10 to induce or expand gliadin specific Tr1 cells is a possibility worthy of pursuit as cellular therapy to re-establish tolerance to gluten in patients with CD.
Acknowledgments
This work received financial support from the Commission of the European Communities, specific RTD programme “Quality of Life and Management of Living Resources”, QLK1-CT-1999-00037, Evaluation of the prevalence of coeliac disease and its genetic components in the European population, and by Ministero della Sanita “Nuove terapie per il morbo celiaco”. The technical help of Mr C Meccariello and Dr Cipriano Luigi (Institute of Food Science and Technology, CNR Avellino, Italy) is gratefully acknowledged.
Abbreviations
CD, coeliac disease
IL-10, interleukin 10
rhIL-10, human recombinant IL-10
NCE, non-coeliac enteropathy
RT-PCR, reverse transcription-polymerase chain reaction
TCL, T cell lines
IFN-γ, interferon γ
Tr1 cells, type 1 T regulatory cells
TGF-β, transforming growth factor β
LPT, lamina propria T cells
IELs, intraepithelial lymphocytes
PT, peptic-tryptic
mAb, monoclonal antibody
PBS, phosphate buffered saline
tTG, tissue transglutaminase
PBMCs, peripheral blood mononuclear cells
SFC, spot forming cells
REFERENCES
- 1.Schuppan D . Current concept of celiac disease pathogenesis. Gastroenterology 2000;119:234–42. [DOI] [PubMed] [Google Scholar]
- 2.Nilsen EM, Frode LJ, Lundin KEA, et al. Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology 1998;115:551–63. [DOI] [PubMed] [Google Scholar]
- 3.Fiorentino DF, Zlotnik A, Vieira P, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol 1991;146:3444–51. [PubMed] [Google Scholar]
- 4.Fiorentino DF, Zlotnik A, Mosmann TR, et al. IL-10 inhibits cytokine production by activated macrophages. J Immunol 1991;147:3815–22. [PubMed] [Google Scholar]
- 5.de Waal Malefyt R , Haanen J, Spits H, et al. Interleukin 10 and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med 1991;174:915–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buelens C , Willems F, Delvaux A, et al. Interlukin-10 differentially regulates B7-1 (CD80) and B7-2 (CD86) expression on human peripheral blood dendritic cells. Eur J Immunol 1995;25:2668–72. [DOI] [PubMed] [Google Scholar]
- 7.de Waal Malefyt R , Yssel H, de Vries JE. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inihibition of IL-2 production and proliferation. J Immunol 1993;150:4754–65. [PubMed] [Google Scholar]
- 8.Taga K , Mostowski H, Tosato G. Human interleukin-10 can directly inhibit T-cell mediated growth. Blood 1993;81:2964–71. [PubMed] [Google Scholar]
- 9.Groux H , Bigler M, deVries JE, et al. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+T cells. J Exp Med 1996;184:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groux H , O’Garra A, Bigler M, et al. A CD4+ T cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 1997;389:737–42. [DOI] [PubMed] [Google Scholar]
- 11.Khoo UY, Proctor IE, MacPherson AJ. CD4+ T cell downregulation in human intestinal mucosa: evidence for intestinal tolerance to luminal bacterial antigens. J Immunol 1997;158:3626–34. [PubMed] [Google Scholar]
- 12.Yudoh K , Matsuno H, Nakazawa F, et al. Reduced expression of the regulatory CD4+ T cell subset is related to Th1/Th2 balance and disease severity in rheumatoid arthritis. Arthritis Rheum 2000;43:617–27. [DOI] [PubMed] [Google Scholar]
- 13.Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun 1998;66:5224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagenbaugh A , Sharma S, Dubinett SM, et al. Altered immune responses in interleukin-10 transgenic mice. J Exp Med 1997;185:2101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDonald TT, Pender S. Lamina propria T cells. Chem Immunol Basel Karger 1998;71:103–17. [DOI] [PubMed] [Google Scholar]
- 16.Braunstein J , Qiao L, Autschbach F, et al. T cells of the human intestinal lamina propria are high producers of interleukin-10. Gut 1997;41:215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pender SLF, Breese EJ, Gunther U, et al. Suppression of T cell-mediated injury in human gut by interleukin 10: Role of matrix metalloproteinases. Gastroenterology 1998;115:573–83. [DOI] [PubMed] [Google Scholar]
- 18.Schreiber S , Heinig T, Thiele HG, et al. Immunoregulatory role of Interleukin 10 in patients with inflammatory bowel disease (IBD). Gastroenterology 1995;108:1434–44. [DOI] [PubMed] [Google Scholar]
- 19.Fedorak RF, Gangl A, Elson CO, et al. for the Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn’s disease. Gastroenterology 2000;119:1473–82. [DOI] [PubMed] [Google Scholar]
- 20.Forsberg G , Hernell OL, Melgar S, et al. Paradoxical coexpression of proinflammatory and downregulatory cytokines in intestinal T cells in childhood celiac disease. Gastroenterology 2002;123:667–78. [DOI] [PubMed] [Google Scholar]
- 21.Beckett CG, Dell’Olio D, Kontakou M, et al. Analysis of interleukin-4 and interleukin-10 and their association with the lymhocytic infiltrate in the small intestine of patients with coeliac disease. Gut 1996;39:818–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker-Smith JA, Guandalini S, Schmitz J, et al. Revised criteria of diagnosis of coeliac disease. Arch Dis Child 1990;65:909–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fais S , Maiuri L, Pallone F, et al. Gliadin induced changes in the expression of MHC-class II antigens by human small intestinal epithelium. Organ culture studies celiac disease mucosa. Gut 1992;33:472–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Ritis G , Occorsio P, Auricchio S, et al. Toxicity of wheat flour proteins and protein-derived peptides for in vitro developing intestine from rat fetus. Pediatr Res 1979;13:1255–61. [DOI] [PubMed] [Google Scholar]
- 25.Jung HC, Eckmann L, Yang SK, et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest 1995;95:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monteleone G , Trapasso F, Parrello T, et al. Bioactive IL-18 expression is up-regulated in Crohn’s disease. J Immunol 1999;163:143–47. [PubMed] [Google Scholar]
- 27.Salvati VM, MacDonald TT, Bajaj-Elliott M, et al. Interleukin-18 and associated markers of T-Helper cell type 1 activity in celiac disease. Gut 2002;50:186–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van de Wal Y , Kooy YMC, van Veelen, et al. Cutting edge: selective deamidation by tissue transglutaminase strongly enhances gliadin specific T cell reactivity. J Immunol 1998;161:1585–8. [PubMed] [Google Scholar]
- 29.Troncone R , Gianfrani C, Mazzarella G, et al. The majority of gliadin-specific T cell clones from the coeliac small intestinal mucosa produce both γ-interferon and IL4. Dig Dis Sci 1998;43:156–61. [DOI] [PubMed] [Google Scholar]
- 30.Scognamiglio P , Accapezzato D, Casciaro MA, et al. Presence of effector CD8+ T cells in hepatitis C virus-exposed healthy seronegative donors. J Immunol 1999;162:6681–9. [PubMed] [Google Scholar]
- 31.Bodger K , Wyatt JI, Heatley RV. Gastric mucosal secretion of interleukin-10: relations to histopathology, Helicobacter pylori status, and tumor necrosis factor-alpha secretion. Gut 1997;49:739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Autschbach F , Braunstein J, Heimke B, et al. In situ expression of interleukin-10 in non-inflamed human gut and in inflammatory bowel disease. Am J Pathol 1998;153:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maiuri L , Auricchio S, Coletta S, et al. Blockage of T-cell costimulation inhibits T-cell action in celiac disease. Gastroenterology 1998;115:564–72. [DOI] [PubMed] [Google Scholar]
- 34.Monk T , Spencer J, Cerf-Bensussan N, et al. Stimulation of mucosal T cells in situ with anti-CD3 antibody: location of the activated T cells and their distribution within the mucosal microenvironment. Clin Exp Immunol 1988;74:216–22. [PMC free article] [PubMed] [Google Scholar]
- 35.Steinbrink K , Wolfl M, Jonuleit H, et al. Induction of tolerance by IL-10 treated dendritic cells. J Immunol 1997;159:4772–80. [PubMed] [Google Scholar]
- 36.Roncarolo MG, Bacchetta R, Bordignon C, et al. Type 1 T regulatory cells reviewed in Immunol Rev 2001;182:68–79. [DOI] [PubMed]