Abstract
Background: Wireless capsule endoscopy (WCE) offers endoscopic access to the small bowel and may therefore change diagnostic and therapeutic strategies in small bowel diseases.
Aim: The aim of this prospective study was to validate the gain in information and therapeutic impact of WCE in patients with Crohn’s disease.
Methods: Fifty six consecutive patients with Crohn’s disease underwent computed tomography (CT) enteroclysis, and if stenoses <10 mm were excluded, WCE was carried out.
Results: In 15 patients (27%), WCE could not be performed due to strictures detected by CT enteroclysis. From the other 41 patients, jejunal or ileal lesions were found in 25 patients by WCE compared with 12 by CT enteroclysis (p = 0.004). This gain in information was mainly due to detection of small mucosal lesions such as villous denudation, aphthoid ulcerations, or erosions. Both methods were not significantly different in the detection of lesions in the terminal/neoterminal ileum (WCE 24 patients, CT enteroclysis 20 patients). Therapy was changed due to WCE findings in 10 patients. Consecutively, all of them improved clinically.
Conclusions: Capsule endoscopy improves the diagnosis of small bowel Crohn’s disease. This may have significant therapeutic impact.
Keywords: wireless capsule endoscopy, computed tomography enteroclysis, Crohn’s disease
Crohn’s disease is a chronic recurrent inflammatory disease that may affect all segments of the gastrointestinal tract. Large reviews state that approximately two thirds of patients have ileocaecal disease, 20% colonic involvement, and 10–30% small bowel involvement.1,2 Based on these data, it was concluded that up to 80% of patients with Crohn’s disease need topical therapy to be released into the terminal ileum and colon. Accordingly, specific slow release preparations were developed.3 These conclusions were based on the fact that the upper and lower gastrointestinal tract were easily accessible for endoscopy while the small intestine could only be diagnosed by radiological methods.4 Recently, wireless capsule endoscopy (WCE) has been introduced. First results demonstrate capsule endoscopy to be superior to barium follow through in the diagnosis of small intestinal bleeding and in different types of small bowel diseases.5–8 Three studies in patients with suspected Crohn’s disease8–10 showed a high diagnostic yield. However, their results may have been hampered by the incompleteness of ileocolonoscopy8 or by the fact that capsule findings were mostly located in the terminal ileum which could also be seen by ileocolonoscopy.9,10 A more recent study examined a larger number of patients and included those with known Crohn’s disease. However, it too was not prospective.11
To adequately investigate the value of WCE in Crohn’s disease, we conducted a prospective study comparing capsule endoscopy with computed tomography (CT) enteroclysis, as well as standard endoscopic methods (oesophagogastroduodenoscopy (OGD), ileocolonoscopy) in 56 patients with Crohn’s disease.
PATIENTS AND METHODS
Study design
Fifty six consecutive patients (35.8 (1.6) years; 55% women) with Crohn’s disease participated in this prospective trial, conducted from August 2001 to November 2003 at the Department of Gastroenterology, Charité University Hospital, Berlin. Fourteen of the 56 patients had undergone previous iliocaecal resection and another two patients had segmental small intestinal resection. In five patients the diagnosis was newly established. Patients with strictures <1 cm in diameter by CT enteroclysis were excluded from the study. Clinical and epidemiological data of the 41 patients who received WCE are given in table 1 ▶. Data from 10 patients reported previously were included in the analysis.12 None of the patients was receiving non-steroidal anti-inflammatory drugs.
Table 1.
Patient data at study entry (n = 41)
| Sex (M/F) | 18/23 |
| Age (y) | 35.9 (1.8)* |
| Duration of symptoms (months) | 72.2(12.3)* |
| CDAI | 255.0 (20.4)* |
| CDEIS | 6.9 (1.1)* |
| Previous surgery | 15 |
*Data are mean (SEM).
CDAI, Crohn’s disease activity index; CDEIS, Crohn’s disease endoscopic index of severity (according to Mary and Modigliani13).
All eligible patients underwent OGD, ileocolonoscopy, CT enteroclysis, and WCE within two weeks. Each technique was evaluated by one investigator (capsule—WV, radiology—PR, and endoscopy—GS), who was blinded to the results of the other investigators. Only the study coordinator (JB) had access to all of the data. Evaluation of endoscopic and radiological examinations was performed according to previously defined criteria.
Ethical guidelines
The protocol was approved by the local ethics committee and written informed consent was obtained from each patient.
CT enteroclysis
At CT enteroclysis, the proximal and distal jejunum, ileum, and proximal and terminal ileum were evaluated separately with respect to the lumen, contrast enhancement of the mucosa and the other bowel wall layers, increased density of the peri-intestinal fat representing inflammatory changes and increased vascularity, separation of bowel loops, and possible lymphadenopathy. The length and location of stenotic areas were noted as well as the presence of fistulae, ulcerations, pseudo-diverticulae, and polypous changes of the mucosa. In addition to the primary evaluation of the small intestine, changes involving the large bowel, stomach, and all other abdominal organs were reported, when diagnosed. CT diagnosis was given without any clinical information. As the stomach and proximal duodenum are generally not sufficiently depicted by CT enteroclysis,14 these segments were excluded for comparative analysis with capsule endoscopy.
Wireless capsule endoscopy
Wireless capsule endoscopy was performed using the M2A capsule system (GivenImaging, Yoqneam, Israel), as previously described,15 with the following modifications.
To improve the quality of the pictures specifically in the lower ileum, all patients were prepared with a laxative (sennoside) and successive bowel cleaning using up to 4 litres of PEG solution. Approximately 15 minutes before swallowing the capsules, 10 mg of metoclopramide were administered orally. The capsules were swallowed with a glass of water containing simethicon. Patients were allowed to start drinking two hours and to have a meal four hours after capsule ingestion. Evaluation of capsule endoscopy took approximately 1.5 h/patient. The diagnosis of a stenosis less than 1 cm in diameter on CT enteroclysis was considered a contraindication of WCE.
A standardised evaluation form was completed immediately after each study by the investigator. Duodenum, jejunum/proximal ileum, and terminal/neoterminal ileum were evaluated separately and the presence or absence of small lesions (aphthoid ulcerations, villous denudation, patchy erythema) and large lesions (such as cobblestone pattern, deep/fissural ulcerations) were noted.
Oesophagogastroduodenoscopy and ileocolonoscopy
OGD and ileocolonoscopy were performed using standard procedures. Additionally, a standardised evaluation form was completed immediately after each study by the respective investigator. At OGD, the oesophagus, gastric fundus, corpus, and antrum, and proximal and distal duodenum were evaluated separately, and the presence or absence of small lesions (aphthoid ulcerations, villous denudation, patchy erythema) and large lesions (cobblestone pattern, deep/fissural ulcerations) were noted. Similarly, at ileocolonoscopy, small and large lesions were evaluated in the terminal ileum, caecum, ascending, transverse, descending, and sigmoid colon, and the rectum.
RESULTS
CT enteroclysis
Involvement of the small bowel (jejunum and proximal ileum) was found by CT enteroclysis in 18 (32%) patients and ileocaecal/neoterminal ileal involvement in 33 (59%) patients. Fifteen of these patients had stenoses of <1 cm in diameter and were not investigated further.
Comparison of WCE versus CT enteroclysis
WCE and comparison with CT enteroclysis was performed in 41 patients who had no relevant stenosis on CT enteroclysis. Of these patients, 33 had active (CDAI>150) and eight quiescent disease.
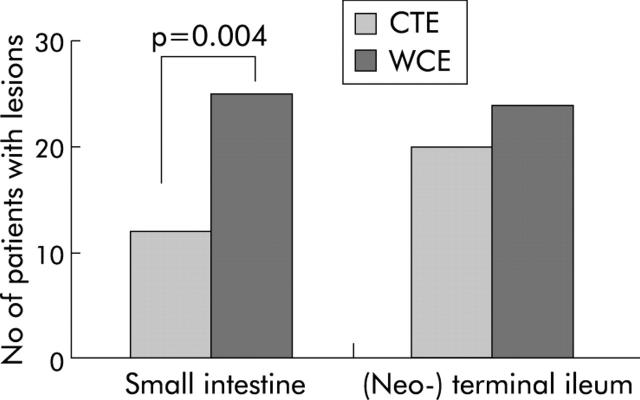
Morphological findings of wireless capsule included very small and superficial lesions, such as patchy erythema along with villous denudation or aphthoid ulcerations (fig 1A ▶), or larger lesions such as ulcerations (fig 1B ▶), cobblestoning, or stenosis (fig 1C ▶). We did not see fistula formation in our patients. Small intestinal involvement was found by WCE in 25 (61%) patients. Ileocaecal/neoterminal ileal involvement was found in 24 (43%) patients.
Figure 1.
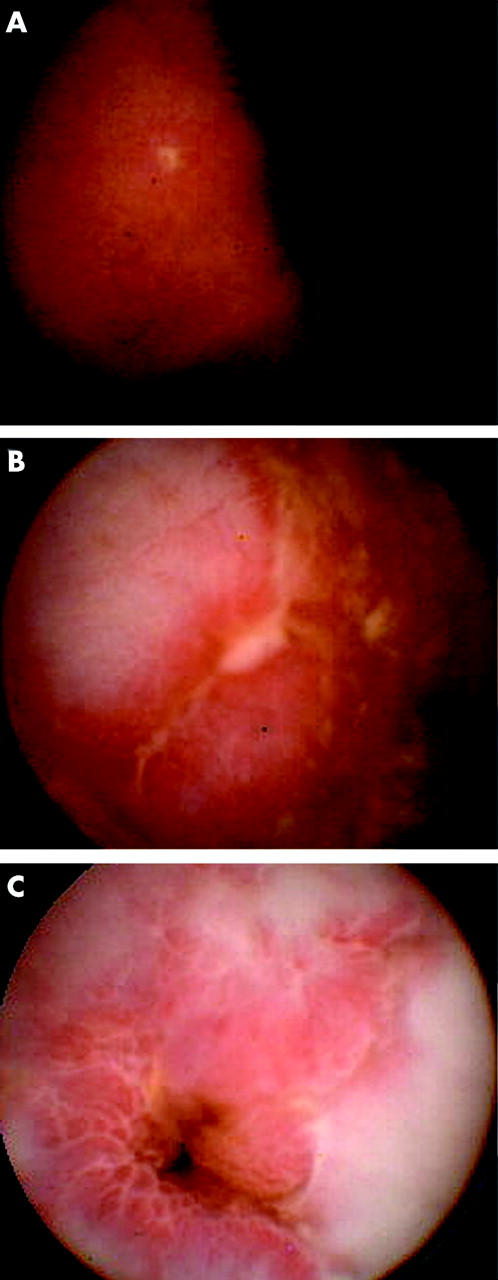
Pathological small intestinal lesions seen by wireless capsule endoscopy. Aphthoid ulceration (A), linear ulceration (B), and jejunal stenosis, resulting in painless capsule retention (C).
In contrast, CT enteroclysis detected inflammatory lesions in only 12 patients in the small intestine and in 20 patients in the terminal/neoterminal ileum. In comparison with CT enteroclysis, this difference was statistically significant for small intestinal involvement (p = 0.004) (fig 2 ▶). This was mainly due to the fact that WCE detected significantly more small lesions in the small intestine than CT enteroclysis (p = 0.007) (table 2 ▶). Furthermore, compared with CT enteroclysis, WCE showed three false negative results in the jejunum and ileum whereas CT enteroclysis did not detect eight lesions seen by capsule endoscopy. However, in 10 investigations, the capsule did not reach the colon during battery lifetime. Therefore, lesions of the terminal/neoterminal ileum shown by CT enteroclysis could not be diagnosed in six patients by WCE. Ileocolonoscopy confirmed all but two lesions seen by WCE in the terminal/neoterminal ileum but detected inflammatory lesions in four additional patients. These four patients had small aphthous lesions or erosions. In two, the capsule did not reach the terminal ileum. The other two patients showed residual food in the terminal ileum that may have hampered visibility of the inflamed segment. In addition, WCE found lesions in the stomach and duodenum in 14 patients. All of these lesions were confirmed by OGD but OGD found other lesions in three more patients. As expected,14 none of these lesions was detected by CT enteroclysis.
Figure 2.
Number of patients with inflammatory changes in the upper gastrointestinal tract and small intestine detected by computed tomography enteroclysis (CTE) and wireless capsule endoscopy (WCE). Statistical comparisons were made according to the McNemar test. As the upper gastrointestinal tract, stomach, and duodenum are generally not sufficiently depicted by CTE, this comparison was excluded from the analysis (see methods section).
Table 2.
Frequency of lesions (n) occurring in Crohn’s disease in 41 patients who underwent the capsule examination
| Small bowel segment | Examination (n) | Patients with small lesions (n) | Patients with large lesions (n) |
| Upper GI tract | OGD (41) | 17 | 0 |
| WCE (41) | 14 | 0 | |
| Small intestine | CTE (41) | 10 | 5 |
| WCE (41) | 23* | 8 | |
| (Neo-)terminal ileum† | Colo (40) | 23 | 13 |
| CTE (41) | 14 | 13 | |
| WCE (32) | 24 | 10 |
Data were stratified with respect to the different examination techniques and the small bowel segments. Small lesions were defined as patchy erythema, villous denudation, and aphthoid ulcerations. Large lesions were defined as large/fissural ulcers, cobblestoning, and stenosis. Small and large lesions can occur in the same patient.
*p = 0.007 v CTE (McNemar test).
†The ileum was reached in only 40 patients (jejunal capsule retention in one patient).
Ten capsules did not reach the colon, implying that the terminal ileum was not reached. In one patient the colonoscope could not be passed into the terminal ileum
OGD, oesophagogastroduodenoscopy, CTE, CT enteroclysis, Colo, ileocolonoscopy, WCE, wireless capsule endoscopy.
In eight patients with quiescent disease (CDAI <150), two patients had duodenal involvement, six had small intestinal involvement, and seven had (neo-) terminal ileal involvement.
Complications of WCE
All capsules were swallowed without major problems, and capsule endoscopy was well tolerated. Two patients felt abdominal pain for approximately 15 minutes while the capsule was passing the inflamed ileal segment. The capsule was impacted in two patients. Although CT enteroclysis had shown inflammatory changes in the terminal ileum of these patients, the diameter of the small bowel lumen was measured as >1 cm so that the capsule could be given. One of these patients had a painful impaction in the lower abdomen for three days. The capsule finally passed after anti-inflammatory treatment (prednisolone 100 mg once daily for three days). The other patient had painless capsule retention before a jejunal stenosis, which was not seen on CT enteroclysis. The capsule was located by fluoroscopy and successfully removed two days after capsule ingestion by push enteroscopy.
Due to prolonged gastric transit, one patient had to be examined twice as the first capsule passed through the pylorus with the meal after four hours thus rendering visualisation of the small intestine impossible. The examination was repeated after two days and the capsule passed the stomach within half an hour.
Besides some stool residuals in the ileum, image quality was excellent in all examinations. The colon was reached within the battery lifetime in all but 10 patients (76%).
Therapeutic impact of WCE findings
Treatment was changed based on the results of WCE in 10 patients. In five patients, a diagnosis of Crohn’s disease was established by WCE with all other diagnostic procedures being negative. In these patients the diagnosis was based on the presence of multiple aphthous or erosive lesions (>10) that were either continuous or segmentally distributed. Mucosal reddening was also seen frequently in these patients but was not considered sufficient to diagnose Crohn’s disease erythematous lesions. Moreover, care was taken that infections were excluded by duodenal biopsy (M Whipple), stool microbiology, or serology (for example, yersinia enterocolica, campylobacter), and that intake of non-steroidal anti-inflammatory drugs was excluded. These patients improved significantly after treatment with glucocorticoids and mesalazine.
In five patients with established Crohn’s disease, therapeutic strategies were changed due to the results of WCE. The capsule detected strictures in the small bowel in two patients. The first stricture was located in the proximal jejunum (fig 1C ▶). The capsule had to be removed by push enteroscopy. Symptoms resolved after surgery in this patient. The other stricture was located in the terminal ileum. It was considered to be an inflammatory stricture. Thus steroid pulse therapy was initiated and the capsule was excreted after three days. The patient improved clinically. Steroid therapy was tapered within three months.
Another three patients had seemingly refractory Crohn’s disease. These patients had little inflammatory changes in the colon which, however, did not adequately reflect clinical activity. The WCE examination revealed previously undetected upper small inflammatory involvement. Patient 1 was receiving prednisolone for three months and had multiple aphthous lesions in the jejunum and ileum. He was changed to azathioprine and responded well. Control capsule examination after six months showed complete healing of the lesions. Patient 2 had a relapse while receiving budesonide and mesalazine. Capsule endoscopy showed two inflamed small intestinal segments presenting with multiple aphthous lesions. He improved considerably with azathioprine although control capsule examination showed unchanged mucosal lesions. Patient 3 was initially treated with budesonide and had a relapse (diarrhoea and bleeding). Again, capsule endoscopy showed multiple aphthes and superficial ulcerations in the small intestine. He was changed to infliximab and improved significantly. Control capsule endoscopy revealed healing of approximately half of the small intestinal lesions. Although change to immunosuppressive therapy would have been possible in these patients without capsule examination, the results of WCE provided us with explanations for the symptoms of patients and gave a rationale for the therapeutic decision.
DISCUSSION
Our data present the first prospective comparison of WCE with CT enteroclysis in patients with established and suspected Crohn’s disease.
The main result of our study was the increase in diagnostic yield of WCE in comparison with CT enteroclysis. Until now, radiological methods have been the gold standard for investigating the small intestine,16–18 with CT enteroclysis recommended for Crohn’s disease.19,20 Our data clearly showed that WCE was superior to CT enteroclysis in detecting small mucosal abnormalities, such as mucosal reddening or aphthes. These results are not surprising. Before the introduction of gastrointestinal endoscopy,13,21 radiology was also the standard for detecting lesions in the stomach or colon. However, as endoscopy has the ability to directly visualise the gastrointestinal mucosa in colour and in detail, it has almost completely replaced radiological techniques. Thus the small intestine has remained the only part of the gastrointestinal tract that needs radiology as a diagnostic tool. With the advent of WCE, a better alternative may be available with an obvious higher sensitivity for small lesions in the entire small intestine and without the need for radiation exposure.
To date, four studies have reported the diagnostic yield of WCE in patients with suspected Crohn’s disease,8–11 However, all of these studies were limited in their information, either because they were performed retrospectively or had a large time interval between ileocolonoscopy and enteroclysis, or had a high failure rate for intubation of the terminal ileum. Our study was performed prospectively and therefore allows clear conclusions to be drawn concerning the sensitivity of WCE for small bowel lesions.
Our results showed that small intestinal involvement in Crohn’s disease occurs much more frequently than is commonly considered. It is known from older studies that the small intestine is affected by inflammatory changes in up to 30% of case.1,2 These studies were mainly based on radiological data. Our results, based on capsule data, suggest small bowel involvement in approximately 60% of patients with prediagnosed Crohn’s disease.
However, our results do not suggest that radiological imaging is redundant in Crohn’s disease. Due to the risk of narrowing and strictures, extensive Crohn’s enteritis is seen as a relative contraindication to WCE.22 In fact, in our study, 15 patients were excluded from WCE as CT enteroclysis detected a stricture <1 cm, leading to a failed WCE in 27% of cases. Despite this prediagnosis, the capsule retention rate in our study (approximately 5%) was higher than that given by the company (overall capsule retention rate reported as 2%).23 Provided that small bowel radiography is performed in patients with clinical suspicion of relevant strictures, we believe capsule endoscopy is a safe method in patients with Crohn’s disease.
Surprisingly, WCE detected relevant strictures in two patients overlooked by CT enteroclysis. None of these patients had developed obvious small bowel obstruction. However, detection of the stenoses explained clinical symptoms in these patients. One of them was successfully operated on and the other improved after steroid therapy.
Concerning therapeutic impact, our data show that WCE is a very useful tool in Crohn’s disease, offering explanation of clinical symptoms and reasons for therapy failure in a number of patients. Furthermore, using topically pH dependent released drugs (budesonide, 5-ASA) might be inadequate in a number of patients. The lack of therapeutic response in some patients to drugs released into the terminal ileum or colon might be due to yet undiagnosed small bowel disease. Such patients may profit from systemic treatment such as was seen in some of the patients in our study.
Detection of small bowel involvement in Crohn’s disease in patients who were considered to have no inflammatory lesions by all other methods could also explain findings of increased small bowel permeability in such patients.24,25 The hypothesis that disturbances of the intestinal barrier precede inflammatory changes might therefore be incorrect; rather, they may reflect early changes which escaped previous diagnosis.
In summary, our data show that WCE can be a useful tool in detecting small bowel lesions in patients with Crohn’s disease as well as explaining clinical symptoms and improving the selection of therapeutic approaches.
Abbreviations
WCE, wireless capsule endoscopy
CT, computed tomography
OGD, oesophagogastroduodenoscopy
CDAI, Crohn’s disease activity index
Conflict of interest: None declared.
REFERENCES
- 1.Mekhjian HS, Switz DM, Melnyk CS, et al. Clinical features and natural history of Crohn’s disease. Gastroenterology 1979;77:898–906. [PubMed] [Google Scholar]
- 2.Steinhardt HJ, Loeschke K, Kasper H, et al. European Cooperative Crohn’s Disease Study (ECCDS): clinical features and natural history. Digestion 1985;31:97–108. [DOI] [PubMed] [Google Scholar]
- 3.Summers RW, Switz DM, Sessions JT, et al. National Cooperative Crohn’s Disease Study: results of drug treatment. Gastroenterology 1979;77:847–69. [PubMed] [Google Scholar]
- 4.Vecchioli A, Brizi MG, Masselli G, et al. Combined diagnostic imaging of Crohn’s disease: an outlook. Rays 2002;27:11–18. [PubMed] [Google Scholar]
- 5.Lewis BS, Swain P. Capsule endoscopy in the evaluation of patients with suspected small intestinal bleeding: Results of a pilot study. Gastrointest Endosc 2002;56:349–53. [DOI] [PubMed] [Google Scholar]
- 6.Costamagna G, Shah SK, Riccioni ME, et al. A prospective trial comparing small bowel radiographs and video capsule endoscopy for suspected small bowel disease. Gastroenterology 2002;123:999–1005. [DOI] [PubMed] [Google Scholar]
- 7.Ell C, Remke S, May A, et al. The first prospective controlled trial comparing wireless capsule endoscopy with push enteroscopy in chronic gastrointestinal bleeding. Endoscopy 2002;34:685–9. [DOI] [PubMed] [Google Scholar]
- 8.Fireman Z, Mahajna E, Broide E, et al. Diagnosing small bowel Crohn’s disease with wireless capsule endoscopy. Gut 2003;52:390–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eliakim R, Fischer D, Suissa A, et al. Wireless capsule video endoscopy is a superior diagnostic tool in comparison to barium follow-through and computerized tomography in patients with suspected Crohn’s disease. Eur J Gastroenterol Hepatol 2003;15:363–7. [DOI] [PubMed] [Google Scholar]
- 10.Herrerias JM, Caunedo A, Rodriguez-Tellez M, et al. Capsule endoscopy in patients with suspected Crohn’s disease and negative endoscopy. Endoscopy 2003;35:564–8. [DOI] [PubMed] [Google Scholar]
- 11.Mow WS, Lo SK, Targan SR, et al. Initial experience with wireless capsule enteroscopy in the diagnosis and maagement of inflammatory bowel disease. Clin Gastroenterol Hepatol 2004;2:31–40. [DOI] [PubMed] [Google Scholar]
- 12.Voderholzer WA, Ortner M, Rogalla P, et al. Diagnostic yield of wireless capsule enteroscopy in comparison with computed tomography enteroclysis. Endoscopy 2003;35:1009–14. [DOI] [PubMed] [Google Scholar]
- 13.Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Therapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut 1989;30:983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wold PB, Fletcher JG, Johnson CD, et al. Assessment of small bowel Crohn disease: noninvasive peroral CT enterography compared with other imaging methods and endoscopy—feasibility study. Radiology 2003;229:275–81. [DOI] [PubMed] [Google Scholar]
- 15.Iddan G, Menon G, Glukhovsky A, et al. Wireless capsule endoscopy. Nature 2000;405:417. [DOI] [PubMed] [Google Scholar]
- 16.Nolan DJ. Small bowel enteroclysis: pros. Abdom Imaging 1996;21:243–4. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt S, Lepori D, Meuwly JY, et al. Prospective comparison of MR enteroclysis with multidetector spiral-CT enteroclysis: interobserver agreement and sensitivity by means of “sign-by-sign” correlation. Eur Radiol 2003;13:1303–11. [DOI] [PubMed] [Google Scholar]
- 18.Bernstein CN, Boult IF, Greenberg HM, et al. A prospective randomized comparison between small bowel enteroclysis and small bowel follow-through in Crohn’s disease. Gastroenterology 1997;113:390–8. [DOI] [PubMed] [Google Scholar]
- 19.Nolan DJ. The true yield of the small-intestinal barium study. Endosopy 1997;29:447–53. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg HI, Caruthens SB, Nelson JA, et al. Radiographic findings of the National Cooperative Crohn’s Disease Study. Gastroenterology 1979;77:925–37. [PubMed] [Google Scholar]
- 21.Hirschowitz BI, Modlin IM. The history of endoscopy. In: Classen M, Tytgat GHJ, Lightdale CJ, eds. Gastroenterological endoscopy. Stuttgart: Thieme, 2002:1–16.
- 22.Faigel DO, Fennerty MB. “Cutting the cord” for capsule endoscopy. Gastroenterology 2002;123:1385–8. [DOI] [PubMed] [Google Scholar]
- 23.Enns R, Mergener K, Brandabur J, et al. Capsule endoscopy (CE): A multicenter, international review and comparison of capsule studies done in three different tertiary-care centers. Gastrointest Endosc 2003;57:AB101. [Google Scholar]
- 24.Wyatt J, Vogelsang H, Hubl W, et al. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet 1993;341:1437–9. [DOI] [PubMed] [Google Scholar]
- 25.Bjarnason I . Intestinal permeability. Gut 1994;35:S18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]