Abstract
Background: Proinflammatory cytokines, especially tumour necrosis factor α (TNF-α), play a prominent role in the pathogenesis of cancer cachexia. Thalidomide, which is an inhibitor of TNF-α synthesis, may represent a novel and rational approach to the treatment of cancer cachexia.
Aims: To assess the safety and efficacy of thalidomide in attenuating weight loss in patients with cachexia secondary to advanced pancreatic cancer.
Methods: Fifty patients with advanced pancreatic cancer who had lost at least 10% of their body weight were randomised to receive thalidomide 200 mg daily or placebo for 24 weeks in a single centre, double blind, randomised controlled trial. The primary outcome was change in weight and nutritional status.
Results: Thirty three patients (16 control, 17 thalidomide) were evaluated at four weeks, and 20 patients (eight control, 12 thalidomide) at eight weeks. At four weeks, patients who received thalidomide had gained on average 0.37 kg in weight and 1.0 cm3 in arm muscle mass (AMA) compared with a loss of 2.21 kg (absolute difference −2.59 kg (95% confidence interval (CI) −4.3 to −0.8); p = 0.005) and 4.46 cm3 (absolute difference −5.6 cm3 (95% CI −8.9 to −2.2); p = 0.002) in the placebo group. At eight weeks, patients in the thalidomide group had lost 0.06 kg in weight and 0.5 cm3 in AMA compared with a loss of 3.62 kg (absolute difference −3.57 kg (95% CI −6.8 to −0.3); p = 0.034) and 8.4 cm3 (absolute difference −7.9 cm3 (95% CI −14.0 to −1.8); p = 0.014) in the placebo group. Improvement in physical functioning correlated positively with weight gain (r = 0.56, p = 0.001).
Conclusion: Thalidomide was well tolerated and effective at attenuating loss of weight and lean body mass in patients with cachexia due to advanced pancreatic cancer.
Keywords: thalidomide, cachexia, pancreatic cancer, weight loss, randomised controlled trial
Cancer cachexia is a major cause of morbidity and mortality, occurring in up to 80% of patients with advanced cancer and contributing directly to death in 20% of cases.1,2 Patients show profound wasting from both fat and skeletal muscle compartments with death usually occurring when weight loss reaches 30% of premorbid levels. The complex metabolic disturbances in cachexia result in a block in the accretion of lean body mass that nutritional supplementation and appetite stimulants alone are unable to reverse.2,3 This appears to be mediated through a combination of the proinflammatory cytokine response of the host, and the production of specific cytokines and catabolic factors by the tumour. The cytokines tumour necrosis factor α (TNF-α), interleukin 6 (IL-6), and interferon γ (IFN-γ) have all been implicated in the pathogenesis of cachexia, and in cachectic tumour bearing murine models treatment with anti-TNF-α, anti-IL-6, and anti-IFN-γ antibodies can attenuate the disease process.4,5–10 More recently, specific catabolic factors such as lipid mobilising factor and proteolysis inducing factor (PIF) which directly stimulate tissue breakdown have also been identified in cancer patients that are losing weight.11,12
There is also some evidence that cytokines play a role in the pathogenesis of anorexia, which is commonly associated with cachexia.13 It has been suggested that by mimicking the hypothalamic effect of excessive negative feedback signalling from leptin by persistent stimulation of anorexigenic peptides such as corticotrophin releasing factor, or by inhibition of the neuropeptide Y pathway, cytokines could induce anorexia.14 Thus modulating cytokine expression in cancer patients may also affect cancer associated anorexia.
Thalidomide has complex immunomodulatory and anti-inflammatory properties. It has been shown to downregulate the production of TNF-α and other proinflammatory cytokines, inhibit the transcription factor nuclear factor κB (NFκB), downregulate cyclooxygenase 2, and inhibit angiogenesis.15,16 In clinical trials it is effective in ameliorating human immunodeficiency virus associated wasting and the weight loss seen in subjects with active pulmonary tuberculosis.17,18 We therefore hypothesised that thalidomide would be effective in attenuating or reversing the weight loss seen in patients with cancer cachexia. During the course of our trial, an open label pilot study of thalidomide in the treatment of cachexia in 11 patients with inoperable oesophageal cancer has been reported. In this study, thalidomide reversed weight loss over the two weeks of the trial and this was associated with an increase in lean body mass.19 To date, no other trial evaluating the effect of thalidomide on cancer cachexia has been undertaken. Thus the aim of our present study was to assess the safety and efficacy of thalidomide in attenuating weight loss in patients with cachexia secondary to advanced pancreatic cancer.
PATIENTS AND METHODS
Patients
Between April 1999 and April 2003, we undertook a prospective, randomised, double blind, placebo controlled study at a single centre (Queen Alexandra Hospital, Portsmouth, UK) in patients with cachexia due to inoperable pancreatic cancer. Inclusion criteria were: diagnosis of pancreatic cancer made on the basis of typical clinical and radiological findings, operative appearance, and/or histological diagnosis; patient deemed inoperable either on the basis of tumour anatomy, inability to survive major surgery, or patient preference; greater than 10% weight loss over the preceding six months; and likely life expectancy of at least six weeks based on clinical judgment. Exclusion criteria were: any form of treatment for pancreatic cancer in the preceding six weeks; weight less than 40 kg; concomitant use of corticosteroids, anabolic drugs, hormonal agents, or other appetite stimulants; age less than 18 years; subjective or objective evidence of peripheral neuropathy; severe constipation, vertigo, or vestibular disease; or severe comorbidity. Women of child bearing potential were required to have a negative pregnancy test prior to starting the trial and use two methods of contraception throughout the trial and for one month afterwards. The trial was approved by the local ethics committee and all patients gave written informed consent. All procedures were carried out in accordance with the declaration of Helsinki and the CONSORT guidelines.
Methods
At baseline, patients underwent a detailed medical history and examination. Premorbid weight and duration of weight loss were reported by the patient. Height (cm), weight (kg), mid upper arm circumference (MAC, cm), triceps skinfold thickness (TSF, mm), quality of life, full blood count, serum biochemistry, liver function tests, C reactive protein, and erythrocyte sedimentation rate were all recorded. All participants were weighed without shoes in their underclothes on the same set of spring balanced scales (SSEC, Germany). MAC was measured using stretch resistant tape and TSF using Harpenden skinfold callipers, as previously described.20 Grip strength was measured from the non-dominant hand using a digital hand grip dynamometer (Twinbird, Japan). Quality of life was determined using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 form, along with additional disease specific pancreatic cancer module PAN26.21,22
Participants were subsequently assessed every four weeks for six months with the same measurements and blood tests recorded at each visit. All measurements were undertaken by the same investigator (TJ).
At enrolment, participants were randomised to receive either thalidomide 200 mg daily or identical placebo. Randomisation was undertaken in blocks of four using a sequential series of sealed envelopes containing a computer generated code. Randomisation envelopes were opened by a third party who dispensed the trial drug in a double blind fashion.
Thalidomide was obtained from Penn Pharmaceuticals Ltd (Gwent, UK) as 100 mg tablets, and prescribed in accordance with the published guidelines for the clinical use and dispensing of thalidomide.23 The dose of thalidomide was reduced to 100 mg in patients who suffered from unacceptable daytime somnolence, constipation, or developed a rash. Patients in whom symptoms did not subsequently resolve were then withdrawn from the trial. Compliance was assessed by participant self reporting and tablet count at each visit.
Outcome measures
The primary outcome measure was change in weight at four weeks. Secondary outcome measures were change in bone free muscle mass, grip strength, quality of life, and survival. Bone free arm muscle area (AMA), a validated marker of lean muscle mass, was calculated from MAC and TSF using the formula (MAC − πTSF)2/4π minus a correction factor of 10 for male sex or 6.5 for female sex.20
Adverse event information was obtained at every clinic visit by recording spontaneously reported complaints from patients and by asking specifically about recognised side effects.
Statistical analysis
Analysis was performed on an intention to treat basis. Data are presented as means (SEM) and absolute differences (95% confidence limits (CI)) when appropriate. Continuous variables were analysed using a two sample t test, Mann-Whitney U test, or the Pearson correlation test, as appropriate. Categorical data was analysed using Fisher’s exact test as appropriate. Survival curves were plotted using Kaplan-Meier survival estimates from the date of study enrolment. Results were considered statistically significant if the p value was less than 0.05.
Sample size calculations were undertaken based on assumptions from published studies of weight loss in patients with cancer cachexia.24–26 To detect a mean difference in weight of 2.5 kg at four weeks with 80% power and a 5% significance level, 17 patients were required in each group. This was inflated to 25 in each group to account for an attrition rate of 25% at four weeks.
Statistical calculations were performed using SPSS version 11.5 software (SPSS, Chicago, USA).
RESULTS
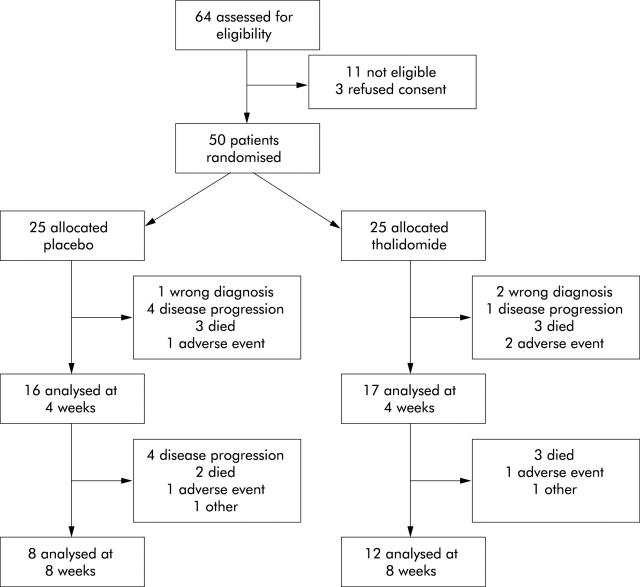
A total of 50 patients were randomised and enrolled into the study. One subject in the placebo arm and two in the thalidomide arm were wrongly diagnosed and subsequently excluded from further analysis. Both patient groups were well matched at baseline with no significant difference between any of the variables (p>0.05) (table 1 ▶). Concomitant drug use was similar between the two groups: in the placebo group, 4/24 patients were taking Creon, 2/24 cholestyramine, 9/24 opiate analgesics, 7/24 other analgesics, 4/24 antidepressants, 8/24 cardiovascular medications, and 4/24 acid suppressants compared with 5/23, 3/23, 7/23, 10/23, 6/23, 11/23, and 6/23, respectively, in the treatment group. Patients were also well matched for global health score and physical functioning, as measured by the EORTC QLQ-C30 questionnaire. There were 33 patients available for assessment at four weeks and 20 at eight weeks. The predominant reason from withdrawal from the trial was death or disease progression (fig 1 ▶). The high attrition rate meant that after eight weeks there were too few patients for meaningful comparison.
Table 1.
Baseline patient characteristics
| Placebo group (n = 24) | Thalidomide group (n = 23) | |
| Sex (M:F) | 13:11 | 12:11 |
| Age (y) | 71(1.7) | 69 (1.3) |
| Premorbid weight (kg) | 73.0 (2.3) | 77.1 (3.3) |
| Baseline weight (kg) | 63.3 (2.2) | 67.4 (2.7) |
| %Weight loss at baseline | 13.2 (1.4) | 12.2 (1.3) |
| Baseline MAC (mm) | 27.1 (0.9) | 27.6 (1.0) |
| Baseline Hb (g/dl) | 12.1 (0.4) | 12.4 (0.3) |
| Baseline Alb (g/dl) | 35.0 (1.0) | 37.1 (0.9) |
| Baseline ESR (mm/h) | 62.0 (7.9) (n = 22) | 54.7 (6.7) (n = 20) |
| Baseline C reactive protein (mg/l) | 21.5 (4.8) | 30.0 (9.5) (n = 22) |
| EORTC QLQ-C30 global health score* | 50 (4.6) | 56 (4.7) |
| EORTC QLQ-C30 physical functioning* | 72 (5.1) | 75 (5.1) |
Values are mean (SEM).
MAC, mean arm circumference; Hb, haemoglobin; Alb, albumin; ESR, erythrocyte sedimentation rate.
*EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire. High score represents high level of functioning (range 0–100).
There were no significant differences between the two groups.
Figure 1.
Flow diagram of progress through the trial.
Weight and nutritional status
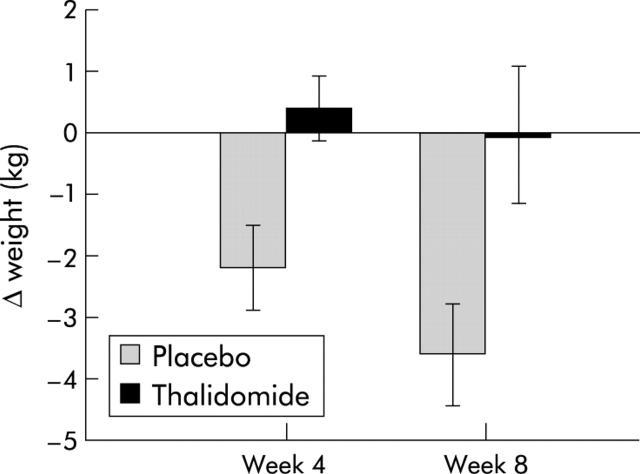
At the primary end point of four weeks there was a significant difference in weight change, with patients in the treatment group gaining a mean of 0.37 kg while those in the placebo group lost a mean of 2.21 kg (absolute difference −2.59 kg (−4.3 to −0.8); p = 0.005). At week 8 there was still a significant difference between the two groups, with patients in the treatment group losing 0.06 kg compared with 3.62 kg (absolute difference −3.57 kg (−-6.8 to −0.3); p = 0.034) in the placebo group (fig 2 ▶).
Figure 2.
Change in weight in pancreatic cancer patients randomised to either thalidomide (n = 17, week 4; n = 12, week 8) or placebo (n = 16, week 4; n = 8, week 8). Differences between groups: p = 0.005 at four weeks and p = 0.034 at eight weeks.
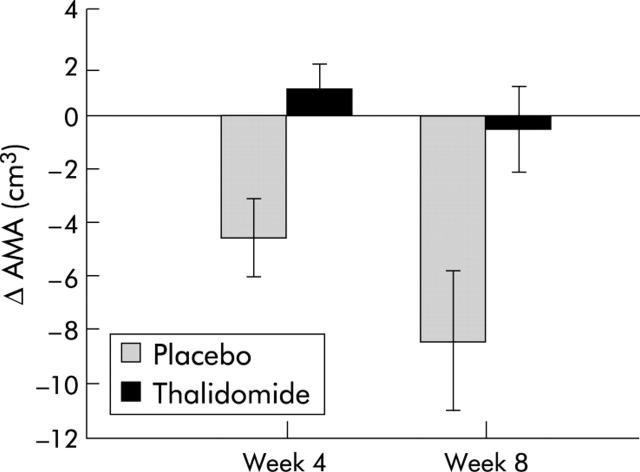
At week 4 there was a significant difference in change in bone free AMA between the two groups. Patients in the treatment group had gained an average of 1 cm3 in bone free AMA while those in the placebo group lost an average of 4.6 cm3 (absolute difference −5.6 cm3 (−8.9 to −2.2); p = 0.002). This remained significant at week 8, with patients in the treatment group having lost an average of 0.5 cm3 compared with 8.4 cm3 (absolute difference −7.9 cm3 (−14.0 to −1.8); p = 0.014) in the placebo group (fig 3 ▶).
Figure 3.
Change in bone free arm muscle area (AMA) in pancreatic cancer patients randomised to either thalidomide (n = 17, week 4; n = 12, week 8) or placebo (n = 16, week 4; n = 8, week 8). Differences between groups: p = 0.002 at four weeks and 0.014 at eight weeks.
There was no significant difference in grip strength between the two groups at any time point. Additionally, grip strength did not differ significantly from baseline at any time in either group (table 2 ▶).
Table 2.
Change in patient weight and nutritional status at weeks 4 and 8
| Placebo group | Thalidomide group | Absolute difference (95% CI for difference) | Significance | |
| Weight change (kg) | ||||
| Week 4 | −2.21 | 0.37 | −2.59 (−4.3 to−0.8) | p = 0.005, t = 3.05 |
| Week 8 | −3.62 | −0.06 | −3.57 (−6.8 to−0.3) | p = 0.034, t = 2.30 |
| Change in AMA (cm3) | ||||
| Week 4 | −4.6 | 1.0 | −5.6 (−8.9 to−2.2) | p = 0.002, t = −3.39 |
| Week 8 cm3 | −8.4 | −0.5 | −7.9 (−14.0 to−1.8) | p = 0.014, t = −2.72 |
| Change in grip strength | ||||
| Week 4 | −0.88 | −1.00 | 0.125 (−2.10 to 2.35) | p = 0.909, t = 0.12 |
| Week 8 | −1.00 | −2.50 | 1.50 (−1.87 to 4.87) | p = 0.363, t = 0.93 |
AMA, bone free arm muscle mass.
Quality of life measurements
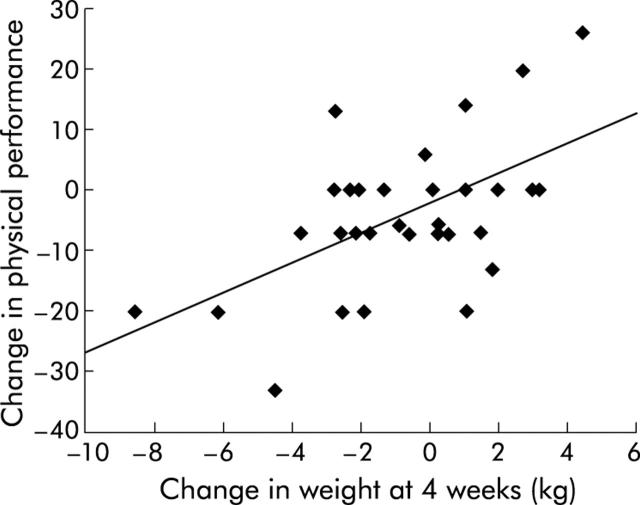
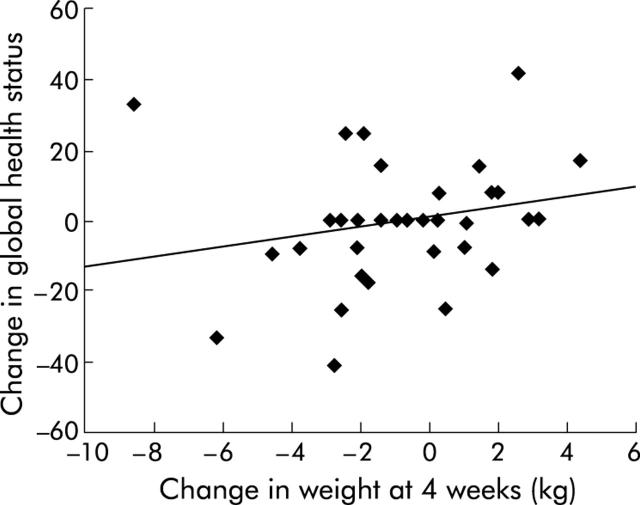
There was no significant difference in global health score or physical functioning between the two groups or from baseline in either group. However, change in physical functioning correlated positively with change in weight (r = 0.56, p = 0.001) and there was a trend suggesting change in global health score correlated positively with change in weight, although this was not significant (r = 0.22, p = 0.221) (figs 4 ▶, 5 ▶)
Figure 4.
Relationship between change in physical performance status and change in weight at four weeks in patients with pancreatic cancer (n = 34, p = 0.001).
Figure 5.
Relationship between change in global health status and change in weight at four weeks in patients with pancreatic cancer (n = 34, p = 0.221).
Survival
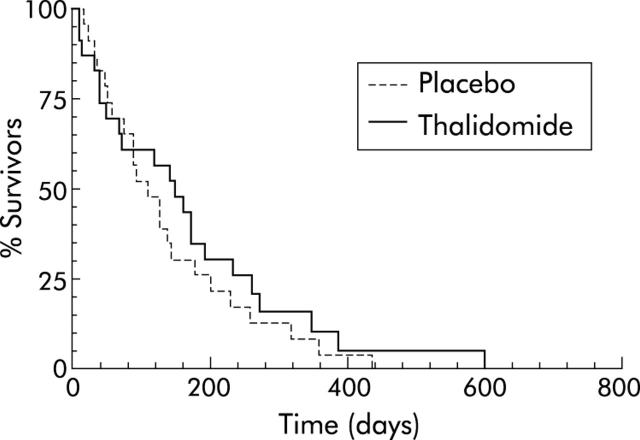
Median duration of survival from entering the study was 148 days in the thalidomide group (95% CI 67–171) compared with 110 days in the placebo group (95% CI 75–136) although this was not statistically significant (p = 0.45). Kaplan-Meier survival curves were not significantly different between the two groups (fig 6 ▶).
Figure 6.
Kaplan-Meier survival curve for patients with pancreatic cancer treated with thalidomide (n = 23) or placebo (n = 24). Median survival 148 days in thalidomide group versus 110 days in the placebo group (p = 0.45).
Safety
Overall, thalidomide appeared to be well tolerated in this study. Two patients (9%) complained of peripheral neuropathy which resolved on stopping the drug, and two patients (9%) developed a rash that necessitated withdrawing from the trial. A further four patients (17%) complained of severe daytime somnolence that required a reduction in drug dosage in two patients and cessation of the drug in the other two. In the symptom scales at four weeks, constipation was significantly more common in the thalidomide group compared with placebo (p = 0.04) and insomnia significantly less common (p = 0.023). There was no significant difference between the two groups in any of the other symptom scales (fatigue, pain, nausea and vomiting, dyspnoea, appetite loss, diarrhoea, or financial difficulties). Other side effects were mild and did not differ significantly from placebo. Two patients in the placebo group and one in the thalidomide group developed deep vein thrombosis and were withdrawn from the study. Thalidomide has previously been associated with an increased risk of thromboembolism in patients with malignant disease27 although this finding was not confirmed in the study.
DISCUSSION
Our study has shown that thalidomide attenuates weight loss in patients with cachexia secondary to pancreatic cancer and that this is associated with a reduction in loss of lean body mass. This is a clinically important finding as it has previously been shown that patients with unresectable pancreatic cancer show inexorable weight loss, as seen in our placebo group, with death occurring when patients have lost approximately 30% of their premorbid weight.24
However, our trial does have some potential limitations. Analysis and interpretation of results from studies involving patients with advanced cancer can be difficult due to the high attrition rate and resultant changing patient population. In our study, only 70% of patients were available for analysis at four weeks and 43% at eight weeks, raising the question that bias may have arisen due to selective attrition as the numbers in both groups were relatively small. Patients in the placebo arm were also on average 4 kg lighter than in the treatment arm, and although this difference was not significant it is possible that this could have contributed to differential weight loss between the two groups. However, the two groups were otherwise well matched for percentage weight loss, performance status, and inflammatory markers at the start of the trial, and the subsequent attrition rate and rate of weight loss in the placebo group was similar to that reported in previous studies, leading us to feel this was unlikely.24–26,28 Lean body mass was estimated indirectly using anthropometric measures which, although they have been shown to correlate well with other indirect measures of lean body mass such as bioelectrical impedance,29 are prone to intra-observer variation and can over or underestimate changes in nutritional status. To keep bias to a minimum, a single trained investigator (TJ) undertook all measurements throughout the study. Furthermore, the change in bone free arm muscle mass in both groups correlated well with weight loss suggesting this was a true effect. This is in keeping with previous trials in human immunodeficiency virus associated wasting where lean body mass was measured by bioelectrical impedance,30,17 and more recently in an open label study of thalidomide in the treatment of cachexia where thalidomide was shown to promote a gain in lean body mass, as measured by DEXA scanning.19
We were unable to demonstrate that the attenuation in loss of body weight led to an improvement in quality of life. This may reflect the fact that global and physical functioning scores in patients with terminal malignancy are not particularly sensitive to weight change. Alternatively, the relatively small sample size may have meant that the study was underpowered to detect small changes in quality of life. It is however clear that overall improvement in physical functioning does correlate strongly with weight gain (p = 0.001) and there was also a trend to a less pronounced but positive correlation between global health score (which encompasses more emotional functioning) and weight gain.
Finally, although the trial was not powered to investigate survival benefit, it was encouraging to note that the median survival was longer in the thalidomide group than in the placebo group (148 v 110 days) and similar to that seen in recent trials using gemcitabine.31,32
To date, previous trials of nutritional or pharmacological therapy in cachexia have been largely ineffective in increasing lean body mass, and none have demonstrated a survival benefit. Increasing energy intake by means of enteral or parenteral feeding has not been successful in increasing either total weight or lean body mass, and does not improve functional status, quality of life, or survival. Likewise, treatment with appetite stimulants such as corticosteroids and cyprohexidine, and hormonal agents such as megestrol acetate, can lead to a temporary improvement in appetite and sense of well being but any weight gain appears to be due a combination of fat deposition and fluid retention, and are thus only of benefit in the palliation of the end stage symptoms of cachexia.2,3,11,33 Eicosapentaenoic acid (EPA), the major active component of fish oils, has recently attracted considerable attention as a treatment for cachexia due to its potential immunomodulatory properties. However, despite promising early pilot studies in which it appeared to increase lean body mass, two large multicentre trials have recently failed to demonstrate any beneficial effect of EPA over either oral high calorie supplements or megestrol acetate.28,34 The only controlled trial to demonstrate an increase in lean body mass to date used a nutritional supplement containing the three amino acid related nutrients glutamine, arginine, and β-hydroxy-β-methylbutyrate.35 However, at four weeks total weight change was not significantly different between the two groups and lean body mass was only significantly different when measured by bioelectrical impedance analysis and not when measured by air displacement plethysmography.
Although the mechanism by which thalidomide attenuates weight loss is unknown it is likely that it results from modulation of the inflammatory response. One possibility is that its predominant effect is through downregulation of proinflammatory cytokines such as TNF-α. However, in the only previous randomised controlled trial of anti-TNF-α therapy in cachexia, pentoxifylline, a phosphodiesterase inhibitor which inhibits TNF-α production from macrophages, did not promote weight gain.36 In this respect it is interesting that oxpentifylline has also been found to be ineffective in the treatment of Crohn’s disease where TNF-α is known to play a central role.37 These disparate results may be explained by the fact that pentoxifylline is a rather poor inhibitor of TNF-α production compared with thalidomide.
Alternatively, there are several other possible mechanisms through which thalidomide may modulate the immune response in patients with cachexia. Thalidomide has been shown to inhibit NFκB, a ubiquitous intracellular signalling molecule involved in the transcriptional regulation of proinflammatory cytokines. NFκB controls both TNF-α and PIF induced protein catabolism through upregulating the ubiquitin-proteasome pathway.38,39 Thalidomide can inhibit NFκB activity by suppressing inhibitor κB (IκB) kinase activity, inhibiting IκB degradation, and thus NFκB nucleolar translocation.40,41. This may therefore represent a pathway through which thalidomide can downregulate both the proinflammatory host immune response and the activity of tumour derived catabolic factors. Thalidomide has also recently been shown to inhibit lipopolysaccharide mediated induction of cyclooxygenase 2 and prostaglandin E2 which may represent an alternative pathway by which it can promote an anti-inflammatory response.42 It is also feasible that thalidomide could directly affect cancer associated anorexia, in which cytokines are thought to play a key role, and thus influence the development of cachexia. However, in our study we did not find any significant difference in loss of appetite symptom scores between the thalidomide and placebo groups. Finally, it is possible that thalidomide has a direct effect on the pancreatic cancer itself as it has previously been shown to be active against a wide range of blood and solid organ malignancies.
In addition to the immunomodulatory effects of thalidomide, other properties may play a beneficial role in alleviating symptoms in patients with end stage cancer. In an uncontrolled study involving 37 patients with terminal malignancy, the antiemetic, analgesic, and sedative properties of thalidomide were shown to be effective in the palliation of otherwise intractable symptoms.43
In conclusion, we have demonstrated that thalidomide is safe and effective in attenuating severe weight loss in patients with advanced pancreatic cancer, and that this is associated with a reduction in loss of lean body mass. It remains to be seen whether these results can be generalised to all cancers and whether attenuation of weight loss leads to prolonged survival. In the future, combination of thalidomide with nutritional supplements and pharmacological agents may ultimately lead to a better clinical outcome.
Acknowledgments
We thank Mr Bernie Higgins (Senior Lecturer, Department of Statistics, Portsmouth University, Portsmouth) for his help and advice with statistical analysis.
Abbreviations
TNF, tumour necrosis factor
IL, interleukin
IFN, interferon
PIF, proteolysis inducing factor
NFκB, nuclear factor κB
IκB, inhibitor κB
MAC, mid upper arm circumference
TSF, triceps skinfold thickness
AMA, arm muscle area
EPA, eicosapentaenoic acid
EORTC QLQ, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire
Conflict of interest: None declared.
REFERENCES
- 1.Dunlop R. Clinical epidemiology of cancer cachexia. In: Bruera E, Higginson I, eds. Cachexia-anorexia in cancer patients, vol 5. Oxford: Oxford University Press, 1996:76–82.
- 2.Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer 2002;2:862–71. [DOI] [PubMed] [Google Scholar]
- 3.Barber MD, Ross JA, Fearon KC. Cancer cachexia. Surg Oncol 1999;8:133–41. [DOI] [PubMed] [Google Scholar]
- 4.Oliff A, Defeo-Jones D, Boyer M, et al. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell 1987;50:555–63. [DOI] [PubMed] [Google Scholar]
- 5.Langstein HN, Doherty GM, Fraker DL, et al. The roles of gamma-interferon and tumor necrosis factor alpha in an experimental rat model of cancer cachexia. Cancer Res 1991;51:2302–6. [PubMed] [Google Scholar]
- 6.Strassmann G, Fong M, Kenney JS, et al. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest 1992;89:1681–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costelli P, Carbo N, Tessitore L, et al. Tumor necrosis factor-alpha mediates changes in tissue protein turnover in a rat cancer cachexia model. J Clin Invest 1993;92:2783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray S, Schell K, McCarthy DO, et al. Tumor growth, weight loss and cytokines in SCID mice. Cancer Lett 1997;111:111–15. [DOI] [PubMed] [Google Scholar]
- 9.Matthys P, Heremans H, Opdenakker G, et al. Anti-interferon-gamma antibody treatment, growth of Lewis lung tumours in mice and tumour-associated cachexia. Eur J Cancer 1991;27:182–7. [DOI] [PubMed] [Google Scholar]
- 10.Strassmann G, Kambayashi T. Inhibition of experimental cancer cachexia by anti-cytokine and anti-cytokine-receptor therapy. Cytokines Mol Ther 1995;1:107–13. [PubMed] [Google Scholar]
- 11.Tisdale MJ. Cancer anorexia and cachexia. Nutrition 2001;17:438–42. [DOI] [PubMed] [Google Scholar]
- 12.Wigmore SJ, Todorov PT, Barber MD, et al. Characteristics of patients with pancreatic cancer expressing a novel cancer cachectic factor. Br J Surg 2000;87:53–8. [DOI] [PubMed] [Google Scholar]
- 13.Ramos EJ, Suzuki S, Marks D, et al. Cancer anorexia-cachexia syndrome: cytokines and neuropeptides. Curr Opin Clin Nutr Metab Care 2004;7:427–34. [DOI] [PubMed] [Google Scholar]
- 14.Inui A. Cancer anorexia-cachexia syndrome: are neuropeptides the key? Cancer Res 1999;59:4493–501. [PubMed] [Google Scholar]
- 15.Sampaio EP, Sarno EN, Galilly R, et al. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J Exp Med 1991;173:699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon JN, Goggin PM. Thalidomide and its derivatives: emerging from the wilderness. Postgrad Med J 2003;79:127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan G, Thomas S, Fierer DS, et al. Thalidomide for the treatment of AIDS-associated wasting. AIDS Res Hum Retroviruses 2000;16:1345–55. [DOI] [PubMed] [Google Scholar]
- 18.Tramontana JM, Utaipat U, Molloy A, et al. Thalidomide treatment reduces tumor necrosis factor alpha production and enhances weight gain in patients with pulmonary tuberculosis. Mol Med 1995;1:384–97. [PMC free article] [PubMed] [Google Scholar]
- 19.Khan ZH, Simpson EJ, Cole AT, et al. Oesophageal cancer and cachexia: the effect of short-term treatment with thalidomide on weight loss and lean body mass. Aliment Pharmacol Ther 2003;17:677–82. [DOI] [PubMed] [Google Scholar]
- 20.Heymsfield SB, McManus C, Smith J, et al. Anthropometric measurement of muscle mass; revised equations for calculating bone-free arm muscle area. Am J Clin Nutr 1982;36:680–90. [DOI] [PubMed] [Google Scholar]
- 21.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993 Mar 3 85:365–76. [DOI] [PubMed]
- 22.Fitzsimmons D, Johnson CD, George S, et al. Development of a disease specific quality of life (QoL) questionnaire module to supplement the EORTC core cancer QoL questionnaire, the QLQ-C30 in patients with pancreatic cancer. EORTC Study Group on Quality of Life. Eur J Cancer 1999;35:939–41. [DOI] [PubMed] [Google Scholar]
- 23.Powell RJ, Gardner-Medwin JM. Guideline for the clinical use and dispensing of thalidomide. Postgrad Med J 1994;70:901–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wigmore SJ, Plester CE, Richardson RA, et al. Changes in nutritional status associated with unresectable pancreatic cancer. Br J Cancer 1997;75:106–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barber MD, Ross JA, Voss AC, et al. The effect of an oral nutritional supplement enriched with fish oil on weight-loss in patients with pancreatic cancer. Br J Cancer 1999;81:80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMillan DC, Wigmore SJ, Fearon KCH, et al. A prospective randomized study of megesterol acetate and ibuprofen in gastrointestinal cancer patients with weight loss. Br J Cancer 1999;79:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett CL, Schumock GT, Desai AA, et al. Thalidomide-associated deep vein thrombosis and pulmonary embolism. Am J Med 2002;113:603–6. [DOI] [PubMed] [Google Scholar]
- 28.Fearon KC, Von Meyenfeldt MF, Moses AG, et al. Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut 2003;52:1479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kushner RF, Haas A. Estimation of lean body mass by bioelectrical impedance analysis compared to skinfold anthropometry. Eur J Clin Nutr 1988;42:101–6. [PubMed] [Google Scholar]
- 30.Haslett P, Hempstead M, Seidman C, et al. The metabolic and immunologic effects of short-term thalidomide treatment of patients infected with the human immunodeficiency virus. AIDS Res Hum Retroviruses 1997;13:1047–54. [DOI] [PubMed] [Google Scholar]
- 31.Colucci G, Giuliani F, Gebbia V, et al. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic cancer: a prospective, randomized phase III study of the Gruppo Ocologia dell’Italia Meridionale. Cancer. 2002 15 94:902–10. [PubMed]
- 32.Berlin JD, Catalano P, Thomas JP, et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 2002;20:3270–5. [DOI] [PubMed] [Google Scholar]
- 33.Barber MD. Cancer cachexia and its treatment with fish-oil-enriched nutritional supplementation. Nutrition 2001;17:751–5. [DOI] [PubMed] [Google Scholar]
- 34.Jatoi A, Rowland KM, Loprinzi CL, et al. An eicosapentainoic acid (EPA)-enriched supplement versus megestrol acetate (MA) versus both for patients with cancer-associated wasting. A collaborative effort from the North Central Cancer Treatment Group (NCCTG) and the National Cancer Institute of Canada. Proc Am Soc Clin Oncol 2003;22:743. [DOI] [PubMed] [Google Scholar]
- 35.May PE, Barber A, D’Olimpio JT, et al. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am J Surg 2002;183:471–9. [DOI] [PubMed] [Google Scholar]
- 36.Goldberg RM, Loprinzi CL, Mailliard JA, et al. Pentoxifylline for treatment of cancer anorexia and cachexia? A randomized, double-blind, placebo-controlled trial. J Clin Oncol 1995;13:2856–9. [DOI] [PubMed] [Google Scholar]
- 37.Bauditz J, Haemling J, Ortner M, et al. Treatment with tumour necrosis factor inhibitor oxpentifylline does not improve corticosteroid dependent chronic active Crohn’s disease. Gut 1997;40:470–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li YP, Reid MB. NF-kappaB mediates the protein loss induced by TNF-alpha in differentiated skeletal muscle myotubes. Am J Physiol Regul Integr Comp Physiol 2000;279:R1165–70. [DOI] [PubMed] [Google Scholar]
- 39.Whitehouse AS, Tisdale MJ. Increased expression of the ubiquitin-proteasome pathway in murine myotubes by proteolysis-inducing factor (PIF) is associated with activation of the transcription factor NF-kappaB. Br J Cancer 2003;89:1116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keifer JA, Guttridge DC, Ashburner BP, et al. Inhibition of NF-kappa B activity by thalidomide through suppression of IkappaB kinase activity. J Biol Chem 2001;276:22382–7. [DOI] [PubMed] [Google Scholar]
- 41.Jin SH, Kim TI, Hans DS, et al. Thalidomide suppresses the interleukin 1beta-induced NFkappaB signaling pathway in colon cancer cells. Ann N Y Acad Sci 2002;973:414–8. [DOI] [PubMed] [Google Scholar]
- 42.Fujita J, Mestre JR, Zeldis JB, et al. Thalidomide and its analogues inhibit lipopolysaccharide-mediated induction of cyclooxygenase-2. Clin Cancer Res 2001;7:3349–55. [PubMed] [Google Scholar]
- 43.Bruera E, Strasser F, Palmer JL, et al. Effect of fish oil on appetite and other symptoms in patients with advanced cancer and anorexia/cachexia: a double-blind, placebo-controlled study. J Clin Oncol 2003;21:129–34. [DOI] [PubMed] [Google Scholar]