Abstract
Background and aims: Anal sphincter weakness and rectal sensory disturbances contribute to faecal incontinence (FI). Our aims were to investigate the relationship between symptoms, risk factors, and disordered anorectal and pelvic floor functions in FI.
Methods: In 52 women with “idiopathic” FI and 21 age matched asymptomatic women, we assessed symptoms by standardised questionnaire, anal pressures by manometry, anal sphincter appearance by endoanal ultrasound and magnetic resonance imaging (MRI), pelvic floor motion by dynamic MRI, and rectal compliance and sensation by a barostat.
Results: The prevalence of anal sphincter injury (by imaging), reduced anal resting pressure (35% of FI), and reduced squeeze pressures (73% of FI) was higher in FI compared with controls. Puborectalis atrophy (by MRI) was associated (p<0.05) with FI and with impaired anorectal motion during pelvic floor contraction. Volume and pressure thresholds for the desire to defecate were lower, indicating rectal hypersensitivity, in FI. The rectal volume at maximum tolerated pressure (that is, rectal capacity) was reduced in 25% of FI; this volume was associated with the symptom of urge FI (p<0.01) and rectal hypersensitivity (p = 0.02). A combination of predictors (age, body mass index, symptoms, obstetric history, and anal sphincter appearance) explained a substantial proportion of the interindividual variation in anal squeeze pressure (45%) and rectal capacity (35%).
Conclusions: Idiopathic FI in women is a multifactorial disorder resulting from one or more of the following: a disordered pelvic barrier (anal sphincters and puborectalis), or rectal capacity or sensation.
Keywords: faecal incontinence, pathophysiology, pelvic floor, rectal capacity
Faecal incontinence (FI) is relatively common and substantially impairs quality of life.1–4 The factor most commonly implicated in idiopathic FI in women is intrapartum anal sphincter injury.5,6 However, most women who sustain anal sphincter injury during vaginal delivery do not develop FI.6 Moreover, it is unclear why women who generally sustain obstetric trauma in the second to third decade develop FI several decades thereafter.7 These observations and objective assessments of anorectal functions suggest that other risk factors and/or disturbances of anorectal functions contribute to the pathophysiology of this disorder. Thus patients with idiopathic FI may also have exaggerated or reduced rectal sensation, and exaggerated anal sphincter relaxation.8
The role of puborectalis dysfunction in FI is poorly understood.7 The puborectalis is a U-shaped component of the levator ani that maintains a relatively acute anorectal angle at rest and contracts further when continence is threatened. Puborectalis function measured by a dynamometer was reduced in FI.9 However, it is unclear if impaired puborectalis function is due to muscle injury or a disorder of muscle function.
Previous studies suggesting that patients with FI frequently have a pudendal neuropathy have been questioned following the recognition that delayed pudendal nerve latencies are not an accurate marker for pudendal neuropathy.10 Previous studies have reported either normal or reduced rectal compliance in idiopathic FI.8,11–13 In these studies, interpretation of rectal compliance measurements is suspect because compliance was analysed by techniques (for example, a latex balloon) which are subject to limitations.10,14
Thus there are several gaps in our understanding of the pathophysiology of idiopathic FI.7 While different studies have generally appraised one or two mechanisms, the relationship between symptoms and disturbances of recto-anal and pelvic floor dysfunction in FI is unclear. The clinical subtypes of “urge” and “passive” FI are associated with more pronounced weakness of the external and internal sphincters, respectively.15 However, it is also conceivable that these symptom subtypes may reflect other pathophysiological disturbances (for example, rectal compliance or sensation, or pelvic floor dysfunction).
The general aim of this study was to comprehensively evaluate symptoms, risk factors, structure, and continence mechanisms in “idiopathic” FI. Specifically, we wished to elucidate the risk factors for disordered continence mechanisms, assess the relationship between symptoms and objective disturbances, and also the relationship between disordered structure and function. Our hypotheses were that: (i) anal sphincter and pelvic floor injury, including external sphincter and puborectalis atrophy, occur more frequently in women with FI compared with age matched asymptomatic women; and (ii) the symptom of rectal urgency is associated with reduced rectal compliance and increased sensitivity to rectal distension while lack of awareness of FI (that is, “passive” FI) reflects diminished recto-anal sensation.
METHODS
Participants
Between June 2000 and February 2003, 52 consecutive female patients (mean age 63.4 (SEM 3.9) years) with FI and 21 healthy asymptomatic women (aged 61.5 (2.4) years) consented to participate in this study, which was approved by the Institutional Review Board of the Mayo Clinic. A clinical interview and physical examination were performed in all participants. Healthy controls were recruited by public advertisement. Exclusion criteria for controls included significant cardiovascular, respiratory, neurological, psychiatric, or endocrine disease, irritable bowel syndrome as assessed by a validated bowel disease questionnaire,16 medications (with the exception of oral contraceptives or thyroid supplementation), and abdominal surgery (other than appendectomy or cholecystectomy). In addition, healthy subjects who had any previous anorectal operations, including haemorrhoid procedures, or had sustained anorectal trauma during delivery (that is, grade 3 or 4 laceration), as documented by obstetric records, were excluded. Patients with a neurological disorder (for example, diabetes mellitus with neuropathy) or connective tissue disease (for example, scleroderma), or previous major anorectal surgery (for example, for rectal prolapse or anal sphincter defects) known to be associated with FI were excluded. The principal investigator also assessed six other patients who were eligible but declined to participate in the study.
Design
In addition to a clinical assessment, anal pressures, rectal compliance, rectal sensation, anal sphincter structure (by endoanal ultrasound and magnetic resonance imaging (MRI)) and pelvic floor motion (by MRI) were assessed in all healthy subjects and incontinent patients. The pelvic floor muscles were also evaluated by concentric needle electromyography (EMG) in incontinent patients. All assessments were completed over a 72 hour period.
Clinical assessment
In addition to evaluation by a gastroenterologist, all patients completed a validated questionnaire pertaining to bowel symptoms, abdominal discomfort, as well as severity and circumstances surrounding FI. The severity of FI was graded by a validated scale incorporating the type and frequency of incontinence, presence and severity of urgency, and use of sanitary devices for incontinence (table 1 ▶).17 FI was characterised as urge, passive, combined (that is, urge and passive), or neither, based on patient responses to the questionnaire. Those patients who reported they were “often” or “usually” incontinent because they had “great urgency and could not reach the toilet on time” were considered to have urge incontinence. Those patients who reported they were “often” or “usually” “unaware when the leakage was actually happening” were considered to have “passive” incontinence.
Table 1.
Scale for grading severity of faecal incontinence
| Grade 1 | Grade 2 | Grade 3 | |
| Frequency of incontinence | Up to once/month (n = 7) | <1/week (n = 14) | ⩾1/week (n = 31) |
| Usual type of bowel incontinence | Gas only/only enough to stain underwear (size of a quarter) (n = 11) | Small amount of stool (n = 31) | Moderate or large amount of stool (n = 10) |
| No of protective pads changed/day | None (n = 12) | One (n = 22) | >1 (n = 16) |
| Urgency | Never (n = 9) | Sometimes (n = 11) | Often/usually (n = 32) |
Maximum total score = 12. Scores of 1–4, 5–8, and 9–12 were categorised as mild, moderate, and severe faecal incontinence, respectively.
n = number of patients in each category.
Anorectal manometry
Procedure
Anal sphincter pressures were measured by a pneumohydraulic manometric perfusion system incorporating four water perfused transducers evenly distributed around the catheter circumference at the same level along the longitudinal axis. A station pull through technique was employed, recording resting and squeeze pressures three times at 1 cm intervals in the anal canal. Subjects were encouraged to maintain squeeze for 30 seconds; a rest period of 45 seconds separated sequential squeeze measurements.
Data analysis
By convention, the average resting pressure over 30 seconds and the maximum squeeze pressure during 30 seconds were analysed. At every level, pressures were averaged across all four transducers. Average resting and squeeze pressures were the highest circumferential pressures recorded at rest and during squeeze, respectively, at any level in the anal canal, averaged across three manoeuvres. We have recently demonstrated that anal pressures measured by these methods are reproducible.18
Rectal compliance and sensation
Procedure
After two magnesium citrate enemas (Fleets; CB Fleet, Lynchburg, Virginia, USA), rectal compliance and sensation were recorded by an “infinitely” compliant 7 cm long balloon with a maximum volume of 500 ml (Hefty Baggies; Mobil Chemical Co., Pittsford, New York, USA) linked to an electronic rigid piston barostat (Mayo Clinic, Rochester, Minnesota, USA) as previously described.18,19 An initial or conditioning distension was performed to reduce variability in rectal sensory thresholds and compliance thereafter.20 Then, a rectal staircase distension (0–32 mm Hg in 4 mm Hg steps at one minute intervals) was conducted. Rectal compliance and sensory thresholds for first sensation, desire to defecate (DD), and urgency were recorded during the staircase distension; the threshold was the first sensation of each symptom.
Data analysis
As previously described, rectal pressure-volume relationships were analysed by averaging balloon volume over the second 30 second segment at each pressure.19,21 Thereafter, each compliance curve was summarised using a power exponential model as previously described using the NLIN procedure in the SAS software package.19,22 Estimated κ and β for each subject were used to calculate the pressure corresponding to half maximum volume (Prhalf), a measure of rectal compliance. Maximum volume during the compliance curve reflected rectal capacity.
Data for sensory thresholds that were not recorded were imputed using a “censored” data approach. When the first sensation threshold was not perceived, this threshold pressure was imputed using the perceived threshold for DD or urgency, whichever came earlier. The threshold for DD was imputed using the urgency threshold or the highest pressure during the pressure-volume curve, whichever came first. As 68/73 subjects experienced DD during rectal distension, this threshold was used in subsequent analyses.
Anal ultrasound
Procedure
Anal ultrasound was performed with patients in the left lateral decubitus position with a rotating probe (B&K Medical, Gentofte, Denmark) providing a 360° view with a 7 MHz or 10 MHz transducer inserted into the rectum. A single radiologist, who was blinded to the results of pelvic MRI imaging, conducted and interpreted every ultrasound examination in a standardised manner, identifying abnormal internal and external sphincter morphology. The endoanal probe was introduced as far as the puborectalis muscle and then slowly withdrawn, with images taken at the level of the puborectalis, and at the level of the deep, superficial, and subcutaneous external sphincter. The internal sphincter was measured anteriorly and laterally on the left and right sides, at the mid canal level.
Data analysis
Findings were characterised as normal, mild focal thinning, marked focal thinning, defect, or atrophy. A focal full thickness hypoechoic defect in the external sphincter was considered to be a scar or defect, generally secondary to a prior tear. In contrast with MRI, no attempt was made to discriminate between a tear and a scar by ultrasound. Internal sphincter atrophy was identified by diffuse thinning of this sphincter (that is, measured diameter ⩽ 1 mm).23 For the external sphincter, atrophy was defined by a diffuse reduction in muscle bulk on serial images below the level of the puborectalis, such that the muscle was identifiable with difficulty. Abnormalities were further characterised by their location in the cross sectional plane and the longitudinal axis of the anal canal; the latter was summarised on a four point scale, extending from the most superficial portion of the subcutaneous external sphincter to the anorectal junction.
Pelvic MRI imaging
Procedure
All controls and 51/52 patients had a pelvic MRI examination; one patient had claustrophobia precluding an MRI. Using a previously described technique, the anal sphincters were imaged by a disposable endorectal colon coil (MRInnervu; Medrad, Inc., Indianola, Pennsylvania, USA) prior to dynamic MRI proctography.24 The endoanal coil was placed within a rigid lexan sheath to eliminate variability in the shape of the colon coil and provide a fixed circular geometry at cross sectional imaging.
After removing the disposable endoanal coil, 120 ml of ultrasound gel were instilled into the rectum and a four element phased array coil placed around the pelvis. An interactive single shot fast spin echo imaging technique24,25 was then employed for dynamic MRI proctography. Images were acquired in the supine position with an FOV of 24–32 cm, slice thickness of 5 mm, TR of 1400–2000 ms, TE of 90 ms, and a matrix size of 256×160 (NEX 0.5). An oblique sagittal plane bisecting the anorectum was defined by selecting three points from axial images during real time imaging. Images were then acquired every 1.4–2 seconds during rest, squeeze, and defecation. Using real time image reconstruction, we could monitor the examination, ensure performance of desired manoeuvres, and instruct or encourage patients.
Data analysis
A single radiologist, blinded to clinical history, physical examination, and the results of other imaging studies, analysed all examinations. Anal sphincter abnormalities from endoanal magnetic resonance were characterised by type, as described above for endoanal ultrasound with one exception—that is, for MRI a distinction was made between tears and scars in the external sphincter. Complete disruption in the sphincter was defined as a tear. A focal heterogeneous signal in the anal sphincter without complete disruption was characterised as a scar.
The appearance of the puborectalis muscle was characterised by MRI as symmetric and normal, unilateral atrophy, or bilateral atrophy. Agreement between endoanal ultrasound and magnetic resonance was rated as outlined in table 2 ▶.
Table 2.
Criteria for measuring agreement between endoanal ultrasound and magnetic resonance imaging (MRI) for the internal and external anal sphincters
| Level of agreement | Criteria |
| Complete agreement | Normal appearance versus mild focal thinning |
| Similar findings* within two hours on the clock face | |
| Similar findings* located at the same or within one craniocaudal level | |
| Acceptable agreement | Marked focal thinning versus tear |
| Marked focal thinning versus scar | |
| Atrophy alone versus atrophy with tear versus atrophy with scar | |
| Similar findings* separated by ⩾ one craniocaudal level | |
| Similar findings* separated by > 2 hours on the clock face | |
| Disagreement | Any other combination not listed above |
*For external sphincter, similar findings indicates tear or scar by MRI versus defect by ultrasound.
For dynamic images, we evaluated the absolute value of, and changes in the anorectal angle and perineal descent of, the anorectal junction at rest and during rectal evacuation using established methods.26,27 The anorectal angle was the angle between the central axis of the anal canal and the tangent to the posterior wall of the rectum. Descent of the anorectal junction during defecation was measured relative to the pubococcygeal line (in cm); descent inferior to the line was represented as a positive value.
Electromyography (EMG)
Procedure
The external sphincter (all four quadrants), puborectalis, and ischiocavernosus muscles were examined by needle EMG (Nicolet Biomedical Inc, Madison, Wisconsin, USA) in 51, 44, and 40 patients, respectively, by standard techniques, and normal values developed in the Mayo EMG laboratory.24,28,29
Data analysis
Insertional activity at rest and motor unit potential amplitude, duration, percent polyphasia, and recruitment following mild to moderate voluntary muscle contraction were assessed in a standardised semiquantitative manner that has been demonstrated to have minimal intraobserver and interobserver variability.28,29
Statistical analysis
All measured parameters were considered normal or abnormal based on the 5th–95th percentile range for controls. Associations were assessed by χ2 or Fisher’s exact test. The associations we evaluated were between subject status (that is, control or FI) versus baseline clinical characteristics (for example, obstetric history, bowel habits) and anal sphincter appearance by ultrasound/MRI. In addition, the associations between symptom subtype with rectal compliance and sensation, and between anal sphincter appearance and function were assessed. A proportional hazards regression model assessed the association between sensation threshold for DD and rectal compliance, capacity, and group status. In this model, sensory thresholds were censored as described above for five of 73 subjects (that is, two patients and three controls) who did not perceive DD during rectal distension.
Receiver operating characteristic (ROC) curves evaluated the sensitivity and specificity of anorectal assessments for discriminating between health and FI. A logistic regression model analysed whether demographic variables (that is, age, body mass index (BMI)), obstetric-gynaecological history (that is, number of forceps deliveries, episiotomies, and hysterectomy status), anal pressures (categorised as normal or reduced), anal sphincter appearance and pelvic floor motion by MRI, and rectal compliance could discriminate between controls and FI.
A multiple linear regression model analysed the extent to which variation in objective anorectal function parameters could be explained by predictor variables, including obstetric history and anal sphincter appearance by MRI. Obstetric history and anal sphincter appearance (by MRI) were each collapsed into two categories. Thus for obstetric history, the analysis evaluated the predictive utility of “mild” (that is, subjects with four or more vaginal deliveries but no known episiotomy or forceps delivery) and “severe” injury (that is, subjects with a previous episiotomy or forceps delivery) relative to a reference group without known risk factors (that is, <4 vaginal deliveries with no episiotomy or forceps delivery). For anal sphincter injury, the analysis evaluated the predictive utility of internal and/or external sphincter tears only, and sphincter atrophy with or without tears, relative to normal appearing anal sphincters.
RESULTS
Clinical characteristics
Following a questionnaire based evaluation of symptoms, 19 (37%) patients had urge, eight (15%) passive, and 13 (25%) combined incontinence. Twelve (23%) patients had neither urge nor passive incontinence. Based on the scoring system (table 1 ▶), one (2%) patient had mild (that is, score 1–4), 23 (44%) moderate (score 5–8), and 28 (54%) severe (score 9–12) FI. Thirty three patients (67%) had functional bowel disorders; 13 had diarrhoea predominant irritable bowel syndrome, eight had functional constipation, seven had functional diarrhoea only, and five patients had diarrhoea predominant irritable bowel syndrome and functional constipation. Demographic features are detailed in table 3 ▶. Forceps assisted deliveries (p<0.05), deliveries associated with perineal stitches (p<0.05), and hysterectomy status (p<0.05) were all separately associated with FI. In addition to forceps deliveries, eight (15%) additional patients had other risk factors for anal sphincter injury—that is, definite obstetric trauma or anorectal surgical procedures.
Table 3.
Clinical characteristics
| Characteristic | Asymptomatic subjects | Faecal incontinence |
| Age (y) | 61.5 (2.4) | 61.2 (2) |
| BMI (kg/m2) | 26.5 (0.9) | 28.6 (0.9) |
| No of vaginal deliveries | 1.8 (0.3) | 2.9 (0.3) |
| Subjects with any forceps deliveries | 5 (24%) | 26 (50%) |
| No of deliveries requiring perineal stitches | ||
| None | 11 (52%) | 9 (16)* |
| 1–3 | 10 (48%) | 36 (69%) |
| ⩾3 | 0 | 5 (10%) |
| Hysterectomy | 7 (33%) | 30 (58%) |
Values are mean (SEM).
*Unknown = 2.
Disordered anal sphincter structure and function
FI was associated (p<0.01) with an abnormal appearance (that is, marked focal thinning, scars, defects, or atrophy) of the internal and/or external sphincters by MRI (table 4 ▶; see figs 2 ▶–3 ▶). No control and three patients had atrophy of the internal sphincter. One of 21 (5%) controls and 13 of 51 (25%) patients (p = 0.05 v controls) had atrophy of the external sphincter.
Table 4.
Internal and external anal sphincter appearance by magnetic resonance imaging in faecal incontinence (FI)
| Internal anal sphincter | External anal sphincter | |||||
| Normal/mild focal thinning | Marked focal thinning, scar, or defect | Atrophy with or without scar/defect | ||||
| Controls | FI | Controls | FI | Controls | FI | |
| Normal/mild focal thinning | 17 | 10 | 1 | 4 | 0 | 8 |
| Marked focal thinning, scar, or defect | 0 | 7 | 2 | 15 | 1 | 4 |
| Atrophy with or without scar/defect | 0 | 1 | 0 | 1 | 0 | 1 |
Values are number of patients in each category.
Figure 2.
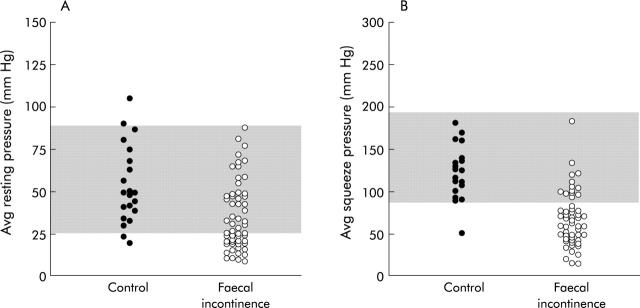
Average anal resting (A) and squeeze (B) pressures in controls and faecal incontinence. The shaded area reflects the 5th–95th percentile range of values for controls; 35% and 73% of patients had reduced resting and squeeze anal pressures, respectively.
Figure 3.
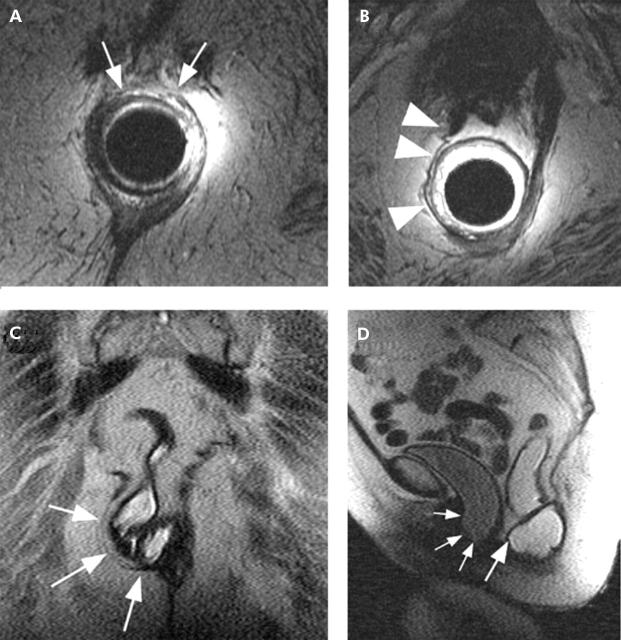
Endoanal magnetic resonance (MR) images (A, B) in an 88 year old incontinent patient demonstrated a small tear in the anterior external anal sphincter (white arrows) and tear and atrophy of the right puborectalis (white arrowheads). Dynamic MR proctography images obtained during defecation in the coronal (C) and mid sagittal planes (D) demonstrated an anterior and lateral rectocele (large white arrows), corresponding to the sphincter and puborectalis abnormalities, in addition to a large cystocele (small white arrows).
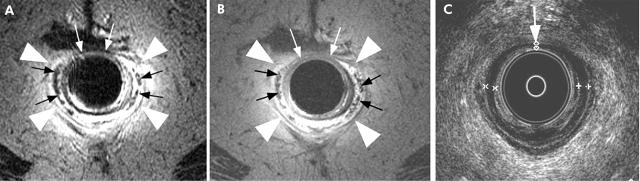
Agreement between endoanal ultrasound and MRI for the appearance of the internal sphincter was complete or acceptable (as defined in table 2 ▶) in 95% of controls and 81% of incontinent patients. For the external sphincter, agreement was complete or acceptable in 95% of controls and 77% of incontinent patients. For the internal sphincter, tears identified by ultrasound, but not by MRI, constituted the primary source for disagreement, accounting for 46% of the discrepancies between these two tests. For the external sphincter, atrophy was visualised by endoanal MRI only, accounting for 59% of the disagreements between ultrasound and MRI (fig 1 ▶).
Figure 1.
Endoanal fast spin echo T2 weighted (A) and spin echo T1 weighted (B) magnetic resonance (MR) images demonstrated marked atrophy of the external anal sphincter (arrowheads) in a 75 year old incontinent patient, making the internal anal longitudinal muscle prominent (black arrows). Corresponding endoanal ultrasound images (C) identified patchy thinning of the internal sphincter, also seen on the MR images (white arrows), but not external sphincter atrophy.
Average anal resting and squeeze pressures in controls were mean 53 (SEM 5) and 128 (8) mm Hg, respectively. Average resting pressure was reduced in 18 (35%) patients and average squeeze pressure was reduced in 38 (73%) patients with FI (fig 2 ▶); both resting and squeeze pressures were normal in only nine (17%) patients. External sphincter tears or atrophy were associated (p = 0.02) with lower average squeeze pressures (that is, less than the 5th percentile value for controls).
Puborectalis structure and function
The puborectalis muscle appeared normal in 20/21 (95%) controls and in 43/51 (84%) patients with FI who had an MRI. One control and five patients had asymmetric puborectalis atrophy (fig 3 ▶); three patients had bilateral (that is, symmetric) atrophy. Puborectalis atrophy was associated (p<0.05) with FI. During squeeze, the anorectal angle declined by a mean of 25° (SEM 2°) in subjects without, and by 14° (2°) in subjects with puborectalis atrophy. Fourteen of 63 (22%) subjects with a normal appearing and 5/9 (56%) subjects with an atrophic puborectalis muscle had impaired puborectalis function during squeeze (that is, anorectal angle declined by <11° from rest to squeeze—5th percentile value for controls); unilateral or bilateral atrophy were associated (p<0.05) with impaired function. All patients with puborectalis atrophy had ⩾4 vaginal deliveries and/or a forceps assisted delivery.
Rectal evacuation
Voluntary rectal evacuation was generally associated with perineal descent and a more obtuse (that is, greater) anorectal angle (table 5 ▶). In nine (18%) patients, the anorectal angle declined (instead of increasing) during evacuation, likely reflecting an evacuation disorder. Conversely, four patients had increased perineal descent during rectal evacuation.
Table 5.
Dynamic magnetic resonance imaging assessment of anorectal and pelvic floor motion
| Parameter | Controls | Faecal incontinence | No of patients with abnormal values (<5%tile, >95%tile) |
| Anorectal angle at rest (°) | 104 (4) | 115 (2) | (0, 9) |
| Anorectal angle during squeeze (°) | 69 (4) | 95 (3) | (0, 21) |
| Anorectal angle during evacuation (°) | 126 (3) | 127 (3) | (9, 10) |
| Anorectal angle change (squeeze−rest) (°) | −35 (4) | −20 (2) | (18, 0) |
| Anorectal angle change (evacuation−rest) (°) | 22 (4) | 13 (3) | (1, 10) |
| Location of anorectal junction relative to pubococcygeal line at rest (cm) | 2.8 (0.2) | 2.9 (0.2) | (3, 5) |
| Anorectal junction motion from rest to squeeze (cm) | |||
| Vertical | −1.5 (0.2) | −1.4 (0.1) | (6, 0) |
| AP | −0.3 (0.2) | −0.2 (0.1) | (6, 2) |
| Anorectal junction motion from rest to evacuation (cm) | |||
| Vertical | 3.4 (0.3) | 2.3 (0.2) | (22, 2) |
| AP | 0.2 (0.2) | 0.5 (0.2) | (2, 4) |
All values are mean (SEM).
For vertical motion, negative and positive values reflect upward and downward motion, respectively.
For AP (anterio-posterior) motion, negative and positive values reflect anterior and posterior AP motion, respectively.
EMG evaluation of the pelvic floor
EMG of the external sphincter disclosed neurogenic or mixed (that is, neurogenic and myogenic) injury in 33 of 51 (65%) patients examined (table 6 ▶). EMG examination of the ischiocavernosus in 26 of these 33 patients was either normal (15 patients) or revealed changes comparable with the external sphincter (seven patients) or changes that differed from the external sphincter (four patients).
Table 6.
Electromyography findings in faecal incontinence
| Anal sphincter | Pubo rectalis | Ischio cavernosus | |
| No of patients evaluated | 51 | 44 | 40 |
| Exam attempted but unsuccessful | 1 | 2 | |
| Normal | 14 | 20 | 26 |
| Neurogenic disturbances | 20 | 9 | 8 |
| Myogenic disturbances | 3 | 2 | 1 |
| Mixed disturbances | 13 | 8 | |
| Abnormal insertional activity* | 3 | 1 | |
| Reduced activation* | 1 | 2 | 4 |
*Only patients with isolated disturbances of insertional activity and/or activation are listed in these rows.
Examination of the puborectalis disclosed neurogenic, myogenic, or mixed injury in 19 of 44 (43%) patients examined. EMG of the external sphincter disclosed the same injury pattern in 18 of these 19 patients. However, EMG disturbances of the puborectalis were not associated with puborectalis function evaluated by MRI.
Rectal pressure-volume relationships
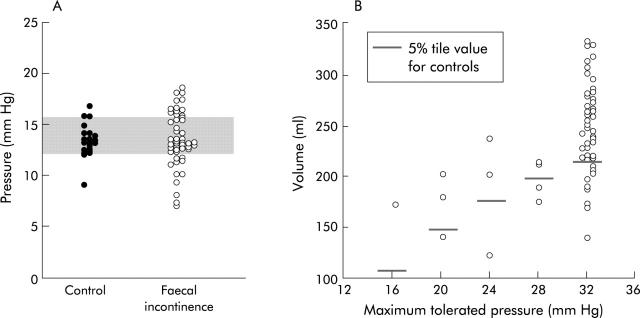
In FI, rectal compliance measured by Prhalf was normal, increased, or reduced (fig 4 ▶). However, Prhalf was not associated with symptoms of urge FI or reduced thresholds for DD and/or urgency. In 25% of incontinent patients, rectal capacity at maximum tolerated pressure during the staircase distension was <5th percentile value for controls, suggesting reduced rectal capacity.
Figure 4.
Rectal compliance (pressure at half maximal volume, A) and rectal capacity (maximum volume during compliance curve, B) in faecal incontinence (FI). Compared with normal values (that is, 5th–95th confidence interval) depicted by the shaded area, rectal compliance was normal, reduced (that is, high Prhalf), or increased (that is, low Prhalf) in FI. Rectal capacity, as measured by balloon volume at maximum tolerated pressure during the rectal compliance curve, was also reduced in 25% of incontinent patients.
Rectal sensation
Forty one (85%), 50 (96%), and 43 (83%) patients reported “first sensation”, DD, and urgency during rectal distension. To ascertain the prevalence of sensory disturbances in FI, we compared threshold volumes and pressures for the most frequently reported sensation (that is, DD) between controls and FI. Rectal sensory thresholds for DD, expressed as pressure (median threshold = 12 mm Hg (FI) v 16 mm Hg (controls); p<0.01) or volume (median threshold = 87 ml (FI) v 162 ml (controls); p<0.001) were lower in FI, indicating hypersensitivity. The pressure threshold for DD was lower (p<0.01) in FI relative to controls, even after adjusting for rectal compliance (that is, Prhalf). Similarly, the volume threshold for DD was lower (p = 0.02) in FI even after adjusting for differences in rectal capacity between controls and FI.
Reduced rectal capacity was associated with the symptom of urgency (p = 0.04), and with rectal hypersensitivity (that is, threshold volume for DD or urgency was lower than the 5th percentile values for controls) (p<0.01) (fig 5 ▶). On the other hand, reduced rectal sensitivity manifested by the lack of awareness of incontinence urgency during rectal distension up to 32 mm Hg was not associated with lack of awareness during an episode of FI (data not shown).
Figure 5.
Relationship between symptoms and rectal compliance (A) and rectal compliance versus hypersensitivity (B) in faecal incontinence (FI). Reduced rectal capacity was associated with urge FI and with rectal hypersensitivity during rectal balloon distension.
Integrated assessment of anorectal and pelvic floor mechanisms in FI
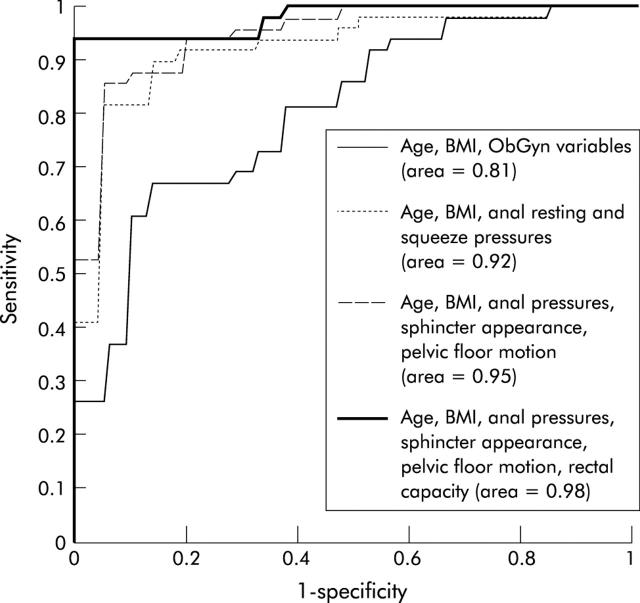
The logistic regression model incorporating multiple variables resulted in an area under the ROC curve of 0.98 (the maximum possible area is 1) (fig 6 ▶). Age and BMI alone were not useful for discriminating between FI and controls (area under curve = 0.57). Anal pressures, pelvic MRI findings (anal sphincter morphology and pelvic floor motion), and rectal capacity, added in sequential order, enhanced the utility of the clinical variables (that is, age, BMI, obstetric history, and hysterectomy status) for discriminating between controls and FI. The perceived utility of obstetric-gynaecological variables for discriminating between controls and FI is not surprising as we excluded controls who had significant risk factors for anal sphincter injury from participating in the study. At a specificity of 90%, clinical variables and anal pressures were 82% sensitive for discriminating between controls and FI. After adding pelvic MRI findings and rectal capacity, sensitivity improved to 94%. Puborectalis dysfunction was associated (p = 0.006) with clinical severity of FI (table 7 ▶). The prevalence of other dysfunctions (for example, reduced rectal capacity) were not significantly associated with the clinical severity of FI.
Figure 6.
Receiver operating characteristic curves demonstrating incremental utility of comprehensive anorectal function assessments for discriminating between controls and faecal incontinence. The proportion of the differences explained by the factors is indicated as a percentage in parentheses. Obstetric-gynaecological (ObGyn) variables included the number of forceps deliveries, number of vaginal deliveries associated with episiotomy, and hysterectomy status. Anal sphincter morphology was graded as normal or abnormal (that is, tear, scar, atrophy, or combination of these abnormalities). Pelvic floor motion was assessed by anorectal angle change during evacuation. BMI, body mass index.
Table 7.
Anorectal sensorimotor dysfunctions by symptom severity
| Severity | Reduced anal resting pressure | Reduced anal squeeze pressure | Puborectalis dysfunction* | Reduced rectal capacity | Rectal hypersensitivity† |
| Moderate (n = 23) | 26% | 65% | 13% | 22% | 13% |
| Severe (n = 28) | 39% | 86% | 50% | 29% | 21% |
*Anorectal angle change from rest to squeeze <5th percentile value for controls.
†Perception threshold for desire to defecate <5th percentile value for controls.
The only patient with mild symptoms of FI had reduced anal resting and squeeze pressures only.
Do symptoms, risk factors, and anal sphincter morphology predict anorectal function disturbances?
The predictive variables explained 23% of intersubject variation in anal resting pressure and 46% of the variation in anal squeeze pressure (table 8 ▶). Anal sphincter injury (by MRI) was the only factor that significantly explained variation in anal resting and squeeze pressures. The symptom of urge incontinence explained a significant proportion of intersubject variation in rectal capacity and impaired pelvic floor motion from rest to squeeze.
Table 8.
Do obstetric-gynaecological risk factors, symptoms, and anal sphincter morphology predict disordered continence mechanisms?
| Predictor variable | Objective parameter | |||||
| Anal resting pressure | Anal squeeze pressure | Rectal capacity | Rectal sensation | Motion rest−squeeze | Motion rest−defecation | |
| Age | 0.004 (−) | 0.002 (−) | 0.014 (−) | <0.001 | 0.022 (−) | 0.033 |
| BMI | 0.001 | 0.005 (−) | 0.001 (−) | <0.001 (−) | 0.020 | 0.017 (−) |
| Obstetric–mild¶ | 0.027 (−) | <0.001 (−) | 0.038 | 0.061* | 0.018 | <0.001 |
| Obstetric–severe¶ | 0.015 (−) | 0.007 | 0.001 (−) | 0.025 | 0.007 | 0.009 |
| Hysterectomy | 0.030 (−) | 0.001 (−) | 0.011 (−) | 0.045 (−) | 0.001 | 0.002 (−) |
| Urge incontinence | <0.001 | 0.052 (−) | 0.061* (−) | 0.026 | 0.112† | 0.013 |
| Passive Incontinence | <0.001 (−) | 0.002 (−) | 0.008 (−) | 0.021 | 0.069* | 0.062* |
| Internal and/or external sphincter tear only | 0.016 (−) | 0.195‡ (−) | 0.047 (−) | 0.007 | 0.001 (−) | 0.005 |
| Internal and/or external sphincter atrophy+tear | 0.063* (−) | 0.263‡ (−) | 0.099† (−) | 0.014 | 0.047 (−) | 0.019 |
| Total variance | 0.23 | 0.45 | 0.35 | 0.23 | 0.18 | 0.25 |
All values except last row are squared partial correlation coefficients.
(−), an inverse correlation between risk factor and objective parameter.
BMI, body mass index.
¶Obstetric: the “mild” category includes subjects with four or more vaginal deliveries but no known episiotomy or forceps delivery. The “severe” category includes subjects with a previous episiotomy or forceps delivery.
Subjects who were incontinent because they “often” or “usually” had great urge and could not reach the toilet on time were defined as having “urge” incontinence. Subjects who were not aware of leakage of stool during an incontinence episode were defined as having “passive” incontinence.
*p<0.05; †p<0.01; ‡p⩽0.005.
DISCUSSION
This study is unique as it comprehensively appraised anorectal and pelvic floor structure and functions maintaining continence, risk factors for disordered anorectal functions, and the relationship between symptoms and disordered functions in women with idiopathic FI. We demonstrated: (i) structural and functional disturbances not only in the anal sphincter but also in the puborectalis in FI; (ii) significant reduction in rectal capacity in 25% of FI patients; reduced rectal capacity was associated with rectal urgency and increased perception of rectal balloon distension; and (iii) that predictive factors (age, BMI, symptoms, obstetric history, and anal sphincter appearance) explained significant portions of the interindividual variation in anal squeeze pressures and rectal capacity in idiopathic FI. The data in this study substantially extend previous observations that idiopathic FI is a multifactorial disorder.8
Only nine FI patients (17%) had normal anal resting and squeeze pressures. Conversely, imaging infrequently revealed significant abnormalities of the anal sphincter and puborectalis in asymptomatic subjects. Consistent with previous studies, internal and external sphincter appearance by MR and ultrasound were generally concordant. Ultrasound was more sensitive in the detection of internal sphincter pathology while MRI was more sensitive for visualising abnormal external sphincter morphology.23,30,31 Anal sphincter atrophy was observed almost exclusively in FI; approximately 25% of FI patients had external sphincter atrophy, demonstrated by MRI only, confirming previous uncontrolled studies with MRI.30–32 It may be important to identify external sphincter atrophy because patients with atrophy do not fare as well as patients without external sphincter atrophy after repair of external sphincter defects.30
One third of FI patients had reduced upward anorectal motion during squeeze, indicating puborectalis dysfunction and supporting a recent study in which puborectalis force was measured by an intrarectal dynamometer.9 Our study is the first to demonstrate puborectalis atrophy in FI and an association between atrophy and puborectalis dysfunction. The aetiology of puborectalis atrophy is unknown but all women with puborectalis atrophy had four or more vaginal deliveries and/or a forceps delivery, supporting previous studies suggesting possible muscle damage during vaginal delivery.33,34 Further studies are necessary to ascertain whether puborectalis atrophy can predict the effect of biofeedback therapy on puborectalis function. Dynamic MRI also revealed paradoxical puborectalis contraction during evacuation in nine patients. It is necessary to identify and address impaired evacuation as retention of stool secondary to impaired evacuation may increase a tendency for incontinence.
Rectal compliance and capacity were reduced in 20% of patients with “idiopathic” FI, extending previous studies in ulcerative colitis,35 radiation proctitis,36 and idiopathic FI.12 Reduced rectal compliance was not associated with the symptom of urge FI. In the stepwise logistic regression model, reduced rectal capacity was useful for discriminating between controls and FI, underscoring the importance of reduced rectal capacity to the pathophysiology of FI. Moreover, reduced rectal capacity was associated with the symptom of urgency, and with increased rectal perception. In the linear regression model, anal sphincter atrophy predicted reduced rectal capacity. It seems unlikely that reduced capacity would cause severe anal sphincter injury or vice versa. Shared mechanisms may be responsible for both disturbances and these require further elucidation. Further studies are also necessary to ascertain if reduced rectal capacity is attributable to “active” (for example, increased rectal tone) or “passive” (for example, fibrosis) mechanisms. Rectal capacity and hypersensitivity may improve after combined rectal augmentation using a segment of distal ileum and stimulated gracilis and neosphincter.37 Lastly, the pressure threshold for the desire to defecate was lower in FI, even after correcting for differences in rectal compliance and capacity, suggesting that rectal hypersensitivity cannot be entirely explained by disturbances in biomechanical properties of the rectum.
Previous studies suggesting a relatively high prevalence of pudendal neuropathy in FI were based on delayed pudendal nerve terminal motor latencies, which are subject to methodological limitations.10 In this study, a combined assessment of external sphincter, puborectalis, and ischiocavernosus was used to localise the level of neuromuscular injury in FI. Neurogenic changes isolated to the external sphincter may be caused by injury at any level from motor neurones in the sacral spinal cord to the nerve fascicles entering the anal sphincter. Local trauma, (for example, during vaginal delivery) may damage the nerve fascicles entering the sphincter and/or result in myogenic changes affecting the external sphincter. We inferred that EMG findings suggested pudendal neuropathy only when neurogenic changes affected the anal sphincter and ischiocavernosus muscle. It is unlikely that a neurogenic injury pattern in the external sphincter and ischiocavernosus would reflect selective injury of the pudendal nerve branches that innervate these muscles.
The predictive variables evaluated in this study were reasonably useful as they explained ⩾30% of the interindividual variation in anal squeeze pressure, rectal compliance, and capacity in FI. However, these risk factors explained <30% of the interindividual variation in anal resting pressure and rectal sensation. With the exception of rectal compliance and sensation, obstetric risk factors were not particularly useful in explaining variance in anorectal functions in FI. This may reflect methodological limitations (for example, the need to collapse obstetric risk factors into two categories (mild and severe) given sample size constraints). Alternatively, it is conceivable that while vaginal delivery is a risk factor for anal sphincter injury, its contribution to FI is exceeded by other risk factors (for example, aging), as has been reported for urinary incontinence.38,39
We believe that our group of consecutive patients constitutes a representative sample of patients with “idiopathic” FI seen at a tertiary referral centre; 65% had risk factors which were associated with weakness of the anal sphincters.40 In addition, 67% of patients had one or more functional gastrointestinal disorders. Patients who were recruited had similar demographic and clinical features as those who declined to participate in the study (data not shown). Hence we perceive that the conclusions of our study are applicable to patients in a consultative practice and future studies will need to evaluate the same questions in community FI patients.
Acknowledgments
This work was supported in part by USPHS NIH grants R01 HD38666 (AEB), R01 HD41129 (AEB), and R01 EB00212 (SJR), and General Clinical Research Center grant M01 RR00585. We wish to thank Ms Amy Luedtke for secretarial support.
Abbreviations
FI, faecal incontinence
MRI, magnetic resonance imaging
EMG, electromyography
DD, desire to defecate
ROC, receiver operating characteristic
BMI, body mass index
Conflict of interest: None declared.
REFERENCES
- 1.Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci 1993;38:1569–80. [DOI] [PubMed] [Google Scholar]
- 2.Nelson R, Norton N, Cautley E, et al. Community-based prevalence of anal incontinence. JAMA 1995;274:559–61. [PubMed] [Google Scholar]
- 3.Reilly W, Talley N, Pemberton J. Fecal incontinence: prevalence and risk factors in the community. Gastroenterology 1995;108:A32. [Google Scholar]
- 4.Perry S, Shaw C, McGrother C, et al. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut 2002;50:480–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sultan AH, Kamm MA. Faecal incontinence after childbirth. Br J Obstet Gynaecol 1997;104:979–82. [DOI] [PubMed] [Google Scholar]
- 6.Sultan AH, Kamm MA, Hudson CN, et al. Anal-sphincter disruption during vaginal delivery. N Engl J Med 1993;329:1905–11. [DOI] [PubMed] [Google Scholar]
- 7.Rao SS. Pathophysiology of adult fecal incontinence. Gastroenterology 2004;126:S14–22. [DOI] [PubMed] [Google Scholar]
- 8.Sun WM, Donnelly TC, Read NW. Utility of a combined test of anorectal manometry, electromyography, and sensation in determining the mechanism of ‘idiopathic’ faecal incontinence. Gut 1992;33:807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Fraga X, Azpiroz F, Malagelada JR. Significance of pelvic floor muscles in anal incontinence. Gastroenteroogy 2002;123:1441–50. [DOI] [PubMed] [Google Scholar]
- 10.AGA. American Gastroenterological Association Medical Position Statement on Anorectal Testing Techniques. Gastroenterology 1999;116:732–60. [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen OO, Ronholt C, Alstrup N, et al. Anorectal pressure gradient and rectal compliance in fecal incontinence. Int J Colorectal Dis 1998;13:157–9. [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen O, Christensen B, Sorensen M, et al. Rectal compliance in the assessment of patients with fecal incontinence. Dis Colon Rectum 1990;33:650–3. [DOI] [PubMed] [Google Scholar]
- 13.Holmberg A, Graf W, Osterberg A, et al. Anorectal manovolumetry in the diagnosis of fecal incontinence. Dis Colon Rectum 1995;38:502–8. [DOI] [PubMed] [Google Scholar]
- 14.Bharucha AE. Outcome measures for fecal incontinence: anorectal structure and function. Gastroenterology 2004;126:S90–8. [DOI] [PubMed] [Google Scholar]
- 15.Engel AF, Kamm MA, Bartram CI, et al. Relationship of symptoms in faecal incontinence to specific sphincter abnormalities. Int J Colorectal Dis 1995;10:152–5. [DOI] [PubMed] [Google Scholar]
- 16.Talley NJ, Phillips SF, Wiltgen CM, et al. Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc 1990;65:1456–79. [DOI] [PubMed] [Google Scholar]
- 17.Bharucha AE, Locke I, GR, et al. A new questionnaire for constipation and fecal incontinence. Aliment Pharmacol Ther 2004;20:355–64. [DOI] [PubMed] [Google Scholar]
- 18.Bharucha AE, Seide B, Zinsmeister AR. Day-to-day reproducibility of anorectal sensorimotor assessments in healthy subjects. Neurogastroenterol Motil 2004;16:241–50. [DOI] [PubMed] [Google Scholar]
- 19.Law N-M, Bharucha AE, Undale AS, et al. Cholinergic stimulation enhances colonic motor activity, transit and sensation in humans. Am J Physiol Gastrointest Liver Physiol 2001;281:G1228–G37. [DOI] [PubMed] [Google Scholar]
- 20.Hammer HF, Phillips SF, Camilleri M, et al. Rectal tone, distensibility, and perception: reproducibility and response to different distensions. Am J Physiol 1998;274:G584–90. [DOI] [PubMed] [Google Scholar]
- 21.Bharucha AE, Hubmayr RD, Ferber IJ, et al. Viscoelastic properties of the human colon. Am J Physiol Gastrointest Liver Physiol 2001;281:G459–66. [DOI] [PubMed] [Google Scholar]
- 22.Bharucha AE, Camilleri M, Haydock S, et al. Effects of a serotonin 5-HT4 receptor antagonist, SB-207266, on gastrointestinal motor and sensory function in humans. Gut 2000;47:667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beets-Tan RG, Morren GL, Beets GL, et al. Measurement of anal sphincter muscles: endoanal US, endoanal MR imaging, or phased-array MR imaging? A study with healthy volunteers. Radiology 2001;220:81–9. [DOI] [PubMed] [Google Scholar]
- 24.Fletcher JG, Busse RF, Riederer SJ, et al. Magnetic resonance imaging of anatomic and dynamic defects of the pelvic floor in defecatory disorders. Am J Gastroenterol 2003;98:399–411. [DOI] [PubMed] [Google Scholar]
- 25.Busse RF, Riederer SJ, Fletcher JG, et al. Interactive fast spin-echo imaging. Magn Reson Med 2000;44:339–48. [DOI] [PubMed] [Google Scholar]
- 26.Shorvon PJ, McHugh S, Diamant NE, et al. Defecography in normal volunteers: results and implications. Gut 1989;30:1737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goh V, Halligan S, Kaplan G, et al. Dynamic MR imaging of the pelvic floor in asymptomatic subjects. Am J Roentgenol 2000;174:661–6. [DOI] [PubMed] [Google Scholar]
- 28.Daube JR. The description of motor unit potentials in electromyography. Neurology 1978;28:623–5. [DOI] [PubMed] [Google Scholar]
- 29.Daube JR. Assessing the motor unit with needle electromyography. In: Daube J, ed. Clinical neurophysiology contemporary neurology series, vol 46. Philadelphia, PA: Davis, 1996:257–81.
- 30.Briel JW, Stoker J, Rociu E, et al. External anal sphincter atrophy on endoanal magnetic resonance imaging adversely affects continence after sphincteroplasty. Br J Surg 1999;86:1322–7. [DOI] [PubMed] [Google Scholar]
- 31.Rociu E, Stoker J, Eijkemans MJ, et al. Fecal incontinence: endoanal US versus endoanal MR imaging. Radiology 1999;212:453–8. [DOI] [PubMed] [Google Scholar]
- 32.Briel JW, Zimmerman DD, Stoker J, et al. Relationship between sphincter morphology on endoanal MRI and histopathological aspects of the external anal sphincter. Int J Colorectal Dis 2000;15:87–90. [DOI] [PubMed] [Google Scholar]
- 33.Tunn R, DeLancey JO, Howard D, et al. MR imaging of levator ani muscle recovery following vaginal delivery. Int Urogynecol J Pelvic Floor Dysfunct 1999;10:300–7. [DOI] [PubMed] [Google Scholar]
- 34.DeLancey JO, Kearney R, Chou Q, et al. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol 2003;101:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao SS, Read NW, Davison PA, et al. Anorectal sensitivity and responses to rectal distention in patients with ulcerative colitis. Gastroenterology 1987;93:1270–5. [DOI] [PubMed] [Google Scholar]
- 36.Varma JS, Smith AN, Busuttil A. Correlation of clinical and manometric abnormalities of rectal function following chronic radiation injury. Br J Surg 1985;72:875–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams NS, Ogunbiyi OA, Scott SM, et al. Rectal augmentation and stimulated gracilis anal neosphincter: a new approach in the management of fecal urgency and incontinence. Dis Colon Rectum 2001;44:192–8. [DOI] [PubMed] [Google Scholar]
- 38.Rortveit G, Daltveit AK, Hannestad YS, et al. Vaginal delivery parameters and urinary incontinence: the Norwegian EPINCONT study. Am J Obstet Gynecol 2003;189:1268–74. [DOI] [PubMed] [Google Scholar]
- 39.Rortveit G, Hannestad YS, Daltveit AK, et al. Age- and type-dependent effects of parity on urinary incontinence: the Norwegian EPINCONT study. Obstet Gynecol 2001;98:1004–10. [DOI] [PubMed] [Google Scholar]
- 40.Snooks SJ, Henry MM, Swash M. Faecal incontinence due to external anal sphincter division in childbirth is associated with damage to the innervation of the pelvic floor musculature: a double pathology. Br J Obstet Gynaecol 1985;92:824–8. [DOI] [PubMed] [Google Scholar]