SUMMARY
A systematic review of the epidemiology of gastro-oesophageal reflux disease (GORD) has been performed, applying strict criteria for quality of studies and the disease definition used. The prevalence and incidence of GORD was estimated from 15 studies which defined GORD as at least weekly heartburn and/or acid regurgitation and met criteria concerning sample size, response rate, and recall period. Data on factors associated with GORD were also evaluated.
An approximate prevalence of 10–20% was identified for GORD, defined by at least weekly heartburn and/or acid regurgitation in the Western world while in Asia this was lower, at less than 5%. The incidence in the Western world was approximately 5 per 1000 person years. A number of potential risk factors (for example, an immediate family history and obesity) and comorbidities (for example, respiratory diseases and chest pain) associated with GORD were identified.
Data reported in this systematic review can be interpreted with confidence as reflecting the epidemiology of “true” GORD. The disease is more common in the Western world than in Asia, and the low rate of incidence relative to prevalence reflects its chronicity. The small number of studies eligible for inclusion in this review highlights the need for global consensus on a symptom based definition of GORD.
INTRODUCTION
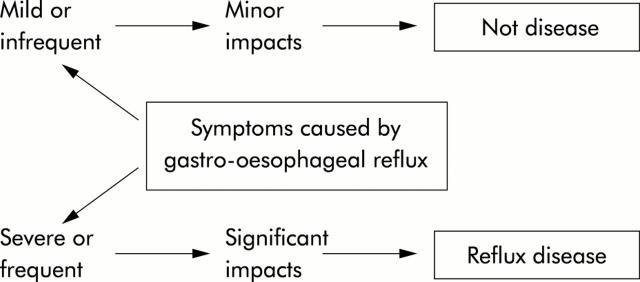
The study of the epidemiology of GORD is restricted by the lack of consensus over the basic definition of the disease. To review the global epidemiology of GORD is currently problematic as there is no internationally applied definition, although the need for this has been recognised.1 Gastro-oesophageal reflux manifests as a continuum of symptom frequency and/or severity in the general population. Occasional symptoms are experienced by a large proportion of the population but GORD results from frequent or severe symptoms which are sufficient to impair the individual’s health related quality of life (fig 1 ▶).
Figure 1.
Schema illustrating the need to distinguish between individuals who report minor reflux induced symptoms and those in whom these symptoms have significant impacts. The latter group makes up a minority of those who report reflux induced symptoms in population surveys.
To establish the true prevalence of GORD, a symptom threshold must be defined which adequately selects for patients whose quality of life is impaired as a result of their disease. Experience of heartburn at least twice weekly is thought to be sufficient to result in impaired quality of life.1 However, in keeping with the general lack of consensus in this area, epidemiological studies reporting the prevalence of symptoms of GORD rarely refer to those occurring twice weekly. More commonly, studies refer to weekly or at least weekly symptoms of gastro-oesophageal reflux.
The aim of this paper is to systematically review the findings of studies which report the prevalence or incidence of GORD, defined as symptoms of gastro-oesophageal reflux at least weekly or by physician diagnosis. A realistic estimate of the prevalence of GORD can be made on review of these studies. Data from these studies on the association of GORD with a range of risk factors, comorbidities, and complications can then be interpreted with confidence.
STUDY SELECTION
Studies to be covered in the review were primarily identified via three Medline searches whose terms reflected the symptomatic basis used for defining GORD. The search terms were selected to identify studies describing the prevalence or incidence of GORD, and used “reflux” or “heartburn”, together with “prevalence”, “incidence”, and “risk factor”. As additional sources of potential studies for inclusion, reviews of the epidemiology of GORD2,3 and our existing database were also examined for appropriate publications.
The initial searches identified 36 studies which were suitable for further analysis. These publications were then analysed according to predefined criteria. Eligible study samples were to be selected from the general population or general practice, and either form part of a population based study or serve as a control group, representative of the general population, in a case control study. GORD was to be diagnosed through a symptom questionnaire or by a clinician. Surveys were required to state the prevalence of at least weekly heartburn, acid regurgitation, or a combination of the two.
In order to ensure that included data were representative of the general population, a sample size had to be reported and the minimum sample size was set at 200. Sample size was defined as the number of eligible individuals who were approached for inclusion in the study. In order to minimise response bias, studies were required to report a response rate of at least 50%. Similarly, to reduce the chance of recall bias, the recall period was required to be given as 12 months or less.
Fifteen studies (originating from North America, Europe, and Asia) met the criteria for inclusion in this review (tables 1 ▶ and 2 ▶). A further 21 studies did not meet the inclusion criteria (table 3 ▶).
Table 1.
Population studies of the prevalence of gastro-oesophageal reflux disease (defined as at least weekly heartburn and/or acid regurgitation or diagnosed by a physician) included in the analysis
| Reference (first author) | Country | Population | Age &;group (y) | Method of data &;collection | Sample &;size | Response &;rate (%) | Recall &;period | Prevalence &;of heartburn &;(%) | Prevalence &;of acid &;regurgitation &;(%) | Prevalence of heartburn and/or acid regurgitation (%) |
| Locke (1997)4 | USA | Olmsted County | 25–74 | Postal questionnaire | 2073 | 73 | 1 year | 17.8 | 6.3 | 19.8 |
| Locke (1999)5 | USA | Olmsted County | 25–74 | Postal questionnaire | 2118 | 72 | 1 year | 17.4* | 6.6* | 20 |
| Talley (1992)6 | USA | Olmsted County | 30–64 | Postal questionnaire | 1021 | 82 | 1 year | 13.2 | 6.5 | |
| El-Serag (2004)7 | USA | Employees | 18–75 | Postal questionnaire | 915 | 57 | 1 year | Blacks: 27, &;Whites: 23 | Blacks: 16, &;Whites: 15 | Blacks: 29, &;Whites: 28 |
| Mohammed (2003)8 | UK | Twin Registry | 19–81 | Postal questionnaire | 8960 | 56 | 1 year | – | – | 18 |
| Thompson (1982)9 | UK | Employees and elderly residents | 17–91 | Physician interview | 315* | 95.6 | 1 year | 10.3 | – | |
| Isolauri (1995)10 | Finland | National population | >20 | Postal questionnaire | 2500 | 68 | 1 week | 15 | 15 | – |
| Terry (2000)11 | Sweden | National population | (Median 68) | Face to face interview | 1123* &;controls in &;case control &;study | 73 | 1 year | – | – | 16.7 |
| Valle (1999)12 | Italy | Employees | 21–68 | Physician interview | 768 | 91 | 1 year | 7.7 | 6.6 | – |
| Diaz-Rubio (2004)13 | Spain | National population | 40–79 | Telephone interview | 8686 | 71.2 | 1 year | – | – | 9.8 |
| Hu (2002)14 | China | National population | >18 | Telephone interview | 2640 | 62 | 1 year | – | – | 4.8 |
| Wong (2003)15 | China | Ethnic population | >18 | Telephone interview | 3605 | 61.3 | 1 year | – | – | 2.5 |
| Pan (2000)16 | China | Population of 2 regions | 18–70 | Assisted self &;completed &;questionnaire | 5000 | 99.8 | 1 year | 3.1 | – |
*Calculated from raw data.
Table 2.
Population studies of the incidence of gastro-oesophageal reflux disease (defined as at least weekly heartburn and/or acid regurgitation or diagnosed by a physician) included in the analysis
Table 3.
Identified population prevalence studies on symptoms of gastro-oesophageal reflux not meeting the inclusion criteria for analysis (in reverse date order)
| Reference (first author) | Sample size &;reported | Sample size &;⩾200 | Response &;rate reported | Response &;rate ⩾50% | Recall period &;reported | Recall period &;⩽1 year | At least weekly &;symptoms &;reported |
| Rajendra (2004)36 | No | No | |||||
| Wong (2004)37 | No | ||||||
| Fujimoto (2003)38 | No | No | No | No | |||
| Khoshbaten (2003)39 | No | No | No | ||||
| Nilsson (2003)22 | No | ||||||
| Watanabe (2003)40 | No | ||||||
| Cameron (2002)41 | No | ||||||
| Conio (2002)42 | No | No | |||||
| Louis (2002)43 | No | No | |||||
| Agreus (2001)44 | No | ||||||
| Avidan (2001)45 | No | No | No | ||||
| Haque (2000)46 | No | ||||||
| Kennedy (2000)47 | No | ||||||
| Jasani (1999)48 | No | ||||||
| Lagergren (1999)49 | No | ||||||
| Oliveria (1999)50 | No | ||||||
| Kennedy (1998)51 | No | ||||||
| Corder (1996)52 | No | ||||||
| Mold (1991)53 | No | ||||||
| Ruth (1991)54 | No | No | |||||
| Nebel (1976)55 | No |
PREVALENCE OF GORD
North America
Some of the most comprehensive data on the epidemiology of GORD are from the population of Olmsted County, Minnesota, USA. Two published papers identified comorbidities and risk factors,4,5 while a third, older study, reported only the prevalence of GORD.6
Locke et al used the validated gastro-oesophageal reflux questionnaire, distributed by post, to assess the prevalence of GORD in a sample of Olmsted County residents between the ages of 25 and 74 years, who were stratified according to age and sex. The prevalence of at least weekly heartburn and/or acid regurgitation reported in the 1997 and 1999 publications was 19.8%4 and 20%,5 respectively. The prevalence of at least weekly heartburn alone over the previous year in these two studies was 17.8% and 17.4%.4,5 Acid regurgitation was less common at 6.3% and 6.6%.4,5 Nearly half (42%) of those subjects with at least weekly heartburn and/or acid regurgitation had experienced their symptoms for more than 10 years, and a further fifth (19%) for between 5 and 10 years.4
An older North American study was also conducted in the population of Olmsted County. The validated bowel disease questionnaire was sent to 1021 eligible subjects between the ages of 30 and 64 years. Recalling their symptoms over the past year, 13.2% of individuals reported at least weekly heartburn and 6.5% reported at least weekly acid regurgitation.6
Although these population based studies have provided valuable data on the prevalence of GORD, the population of Olmsted County does not accurately reflect that of the USA as a whole, as it is disproportionately Caucasian: over 95% of this population is White.4–6 The most recently published survey of the prevalence of GORD in the USA focused specifically on ethnic variation in the prevalence of GORD, by recruiting subjects from the employees of the Houston Veterans’ Affairs Medical Center, of whom 55% are black. Using the gastro-oesophageal reflux questionnaire, at least weekly heartburn was reported by 27% of Black and 23% of White subjects, and at least weekly acid regurgitation by 16% of Black and 15% of White subjects.7 These data are valuable in that they highlight the fact that ethnicity is not a significant predictor of either symptom. However, it should be noted that this was in an employed study population, which may not be representative of the population as a whole.
Europe
Six of the studies which met the inclusion criteria originated from Europe and, of these, two were carried out in the UK. One, a study of genetic influences on GORD, recruited subjects from the St. Thomas’ Adult UK Twin Registry. A validated questionnaire was sent to all pairs of twins in this registry. Of the 1960 evaluable twin pairs who responded, 18% reported having experienced heartburn and/or acid regurgitation at least weekly over the previous year.8
The other UK study is considerably older. Thompson and Heaton invited 327 apparently healthy British individuals to be interviewed by a consultant gastroenterologist. These subjects were all from the UK city of Bristol and were selected from medical staff and students, a randomly selected population undergoing screening for coronary artery disease, and elderly residents of self catering flats. Eligible subjects who agreed to be interviewed were aged 17–91 years and reported experiencing heartburn at least weekly over the previous year with a prevalence of 10.3%.9 The representativeness of this study sample is open to question as subjects originated from a relatively limited geographical area and were all either employed or care home residents.
A population based study in Finland was based on a random sample of 2500 people aged 20 years or above, which was obtained from the national population registry. A postal questionnaire recorded heartburn and regurgitation, among other symptoms, experienced on the day of response or during the preceding week, month, and year. The study population reported an equal prevalence of heartburn and acid regurgitation (15%) during the previous week.10
A case control study of risk factors for oesophageal adenocarcinoma carried out by Terry et al randomly selected subjects for its control group from the Swedish population register, who were frequency matched to resemble the age and sex distribution among the cases for this study. It is important to note that, in this case, frequency matching limits the representativeness of the sample, as oesophageal adenocarcinoma patients are generally elderly. The response rate of 73% in this group yielded a total 1123 subjects, who were interviewed face to face by a professional interviewer, aided by computer. Recalling their symptoms over the previous year, 16.7% of the control group had experienced at least weekly heartburn and/or acid regurgitation.11
A study in Italy used physician interview to diagnose GORD in staff of the San Matteo Hospital and the Military Factory of Pavia. The 700 subjects who completed interviews were aged 21–68 years. At least weekly heartburn was reported by 7.7%, and regurgitation of the same frequency by 6.6%.12 As this study population included only employed individuals, these results may not be representative of the population as a whole.
In a Spanish study by Diaz-Rubio et al, a random sample of the general population of Spain was obtained from the national telephone directory in computerised format. A validated questionnaire was completed via telephone interview and a response rate of 71% was sufficient to generate the target sample of 2500 individuals. Recalling their symptoms over the previous year, 9.8% of subjects reported at least weekly heartburn or acid regurgitation. Similarly to the Olmsted County study, 41.8% of those with weekly symptoms reported having experienced them for 10 years or more.13
Asia
The three eligible Asian studies were all carried out in Hong Kong or China. Hu et al carried out a study in which ethnic Chinese households in Hong Kong were contacted by telephone, their telephone numbers having been randomly generated by computer. A Chinese version of a previously validated bowel symptom questionnaire was completed by 1649 subjects. These individuals, who were all at least 18 years old, reported at least weekly heartburn and/or acid regurgitation with a prevalence of 4.8%.14
A second Chinese study also used a validated questionnaire, completed over the telephone, to assess symptoms of gastro-oesophageal reflux. Ethnic Chinese households were contacted, following random selection by computer from the telephone directory, and the questionnaire was completed by 2209 subjects. These individuals, who were all over the age of 18 years, reported at least weekly heartburn and/or acid regurgitation with a prevalence of 2.5%.15 It should be noted that the use of a telephone survey in a developing country may bias the study population towards the urban, and possibly more wealthy, population.
The third of the Chinese studies was conducted by Pan et al. Subjects were randomly selected from the adult population of Beijing and Shanghai, and asked to complete a questionnaire while assisted by trained doctors or medical students. Five thousand residents of these two regions, aged 18–70 years, provided data, with 3.1% reporting at least weekly heartburn over the previous year.16
Inter-study comparisons
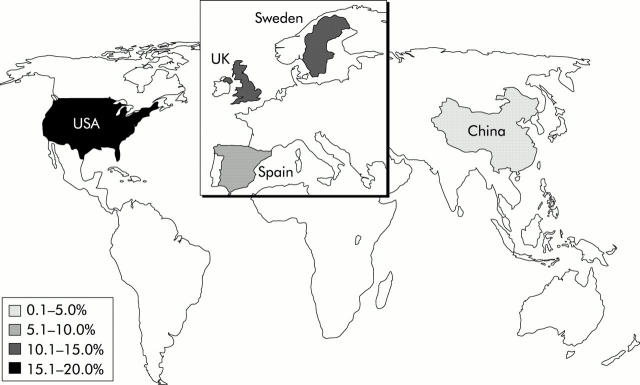
On the basis of inter-study comparisons, certain regional and/or ethnic variations in the prevalence of GORD can be identified (figs 2 ▶, 3 ▶). There is little difference between the prevalence of GORD (as defined by heartburn and/or acid regurgitation at least weekly) in North America (19.8–20%, n = 2) and in Europe (9.8–18%, n = 3). There is some indication that the prevalence may be lower in Southern than Northern Europe. There is a definite trend, however, towards a lower prevalence of GORD in Asia (2.5–4.8%, n = 2).
Figure 2.
Global variation in the prevalence of gastro-oesophageal reflux disease, defined as at least weekly heartburn and/or acid regurgitation.
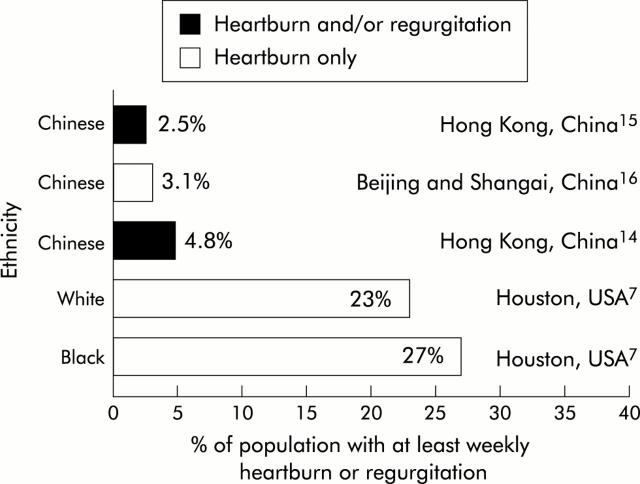
Figure 3.
Ethnic/regional differences in the prevalence of at least weekly heartburn alone, or at least weekly heartburn and/or acid regurgitation. Data are shown for population studies that satisfied criteria for inclusion in this review which evaluated specific ethnic groups. Data are from the studies indicated.7,14–16
On examining data on the prevalence of at least weekly heartburn, a more defined trend emerges within the Western world. The range of weekly heartburn prevalence in North America (13.2–27%, n = 5) does appear to be higher than that in Europe (7.7–15%, n = 3) although the Asian prevalence (3.1%, n = 1) remains significantly lower than that seen in Western regions. A similar pattern was seen for acid regurgitation alone: 6.3–16% (n = 5) in North America versus 6.6–15% (n = 3) in Europe.
INCIDENCE OF GORD
Two longitudinal studies reporting the incidence of GORD were identified, both based on records of GORD cases, as diagnosed by physicians. The first study was carried out in the UK General Practice Research Database.17 The authors examined a source population of patients aged 2–79 years who were enrolled with a participating general practitioner for at least two years prior to 1996. They then identified those patients with a first diagnosis of GORD during 1996. The overall incidence of GORD was determined to be 4.5 per 1000 person years (95% confidence interval (CI) 4.4–4.7).
The second study was a longitudinal review of claims data from Georgia Medicaid investigating the use of non-steroidal anti-inflammatory drugs (NSAIDs) as a predisposing factor for the development of GORD. This involved interrogation of the records of all patients aged over 25 years and continuously eligible for 1996, 1997, and 1998.18 Patients were excluded if they had received a GORD diagnosis during 1996 or 1997, and were classified into GORD and control cohorts according to GORD diagnoses in 1998. From the data provided, it can be calculated that the incidence of GORD within the whole study population (control and NSAID cohorts) was 0.0054, or 5.4 per 1000 person years.
The diagnostic criteria in these studies are less clear than the definitions used in the prevalence studies. Furthermore, the data are probably influenced by consulting behaviour and it is not clear what proportion of incident GORD cases consulted a physician. However, these data relate to the clinical understanding of the term GORD and are likely to be relevant to everyday practice.
POTENTIAL RISK FACTORS AND COMORBIDITIES ASSOCIATED WITH GORD
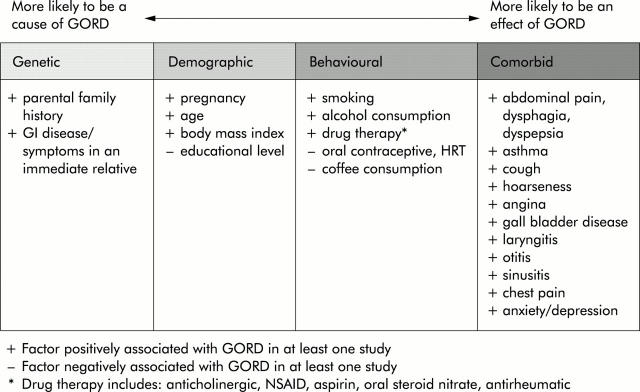
Cross sectional and longitudinal studies included in this review also report the association of GORD with a number of putative risk factors and possible, but not well accepted, complications. These are presented in the section below in four categories—namely, genetic, demographic, behavioural, and comorbid associations. No prejudgement has been made as to the causal nature of each individual relationship but the four categories are discussed in order of their a priori likelihood of being influenced by GORD (fig 4 ▶).
Figure 4.
Factors associated with gastro-oesophageal reflux disease (GORD), suggesting their a priori likelihood of being influenced by the disease.
Genetic factors
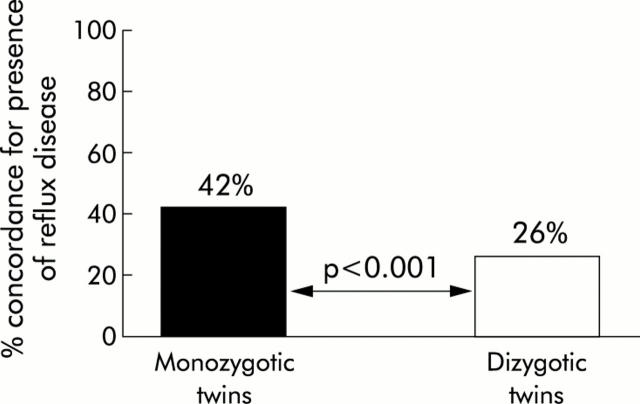
There is sparse, but positive, data on the association between GORD and genetic factors in the studies included in this review.5,7,8 For example, a study of the St Thomas’ Adult UK Twin Registry demonstrated that GORD was significantly associated with a parental family history of reflux disease (odds ratio (OR) 1.46 (95% CI 1.22–1.74)).8 It also highlighted the higher concordance in prevalence of GORD in monozygotic over dizygotic twin pairs (pairwise concordance: 27% v 15%, p = 0.001; casewise concordance: 42% v 26%, p<0.001) (fig 5 ▶). The second Olmsted County study demonstrated a significant association between symptoms of GORD and significant heartburn or disease of the oesophagus or stomach in an immediate relative (OR 2.6 (95% CI 1.8–3.7)).5 The authors found no significant association however between the presence of GORD and a positive history in the subject’s spouse (OR 1.1 (95% CI 0.7–1.7)). These data imply that a genetic component exists in the development of GORD, exerting influence beyond that of any shared familial environmental factors.
Figure 5.
Data from a twin study8 which show evidence of a substantial contribution of genetic factors to occurrence of reflux disease, based on a high concordance for the presence of this problem in monozygotic compared with dizygotic twins.
Demographic factors
Four cross sectional studies and one longitudinal study investigated the influence of sex on the prevalence of GORD symptoms.4,5,8,10,18 All five concluded that there was no significant association between sex and GORD. This is despite the well known association between symptoms of GORD and pregnancy, as demonstrated in the Finnish study.10 It is therefore noteworthy that several of the studies excluded pregnant subjects from their analyses.8,10,17
The effect of increasing age on the prevalence of GORD symptoms is unclear, with two European studies reporting a slight but significant association.8,10 This relationship was not seen for heartburn with or without acid regurgitation in Olmsted County.4,5 In the UK GP database study, the incidence of GORD increased with age in both men and women until the age of 69 years, from which point the trend was reversed.17 In the Georgia Medicaid study, a similar trend was observed, although the trend reversed earlier, at 55 years.18 This study reported a marginally significant increase in risk with increasing age (OR 1.1 (95% CI 1.0–1.1)). Such studies of GORD defined on a symptomatic basis are of course unable to evaluate the possibility that objectively demonstrable aspects of GORD, such as reflux oesophagitis, are more prevalent and/or severe in older individuals.
Increasing prevalence of GORD was associated with excess body mass/higher body mass index (BMI), with a reported OR of 2.8 (95% CI 1.7–4.5) in Olmsted County.5,7,8,10 Higher body mass was also significantly associated with the incidence of GORD, with the UK GP database study reporting a small but statistically significant association of GORD diagnosis with BMI >25 (OR 1.3 (95% CI 1.2–1.4)) or BMI >30 (OR 1.3 (95% CI 1.2–1.5)).17 The Georgia Medicaid study also reported a clear positive relationship between GORD diagnosis and obesity (OR 2.8 (95% CI 2.1–3.6)).18
Increasing GORD prevalence was associated with a lower educational level.7,13 GORD was not associated however with handedness (OR 1.06 (95% CI 0.82–1.36)).8
Behavioural factors
A number of behavioural factors are thought to trigger gastro-oesophageal reflux episodes. The most commonly investigated factors in the included studies were cigarette smoking and coffee consumption. Three cross sectional studies demonstrated a significant relationship between GORD symptoms and smoking.5,8,10 Furthermore, the longitudinal studies also investigated the relationship with smoking. The UK GP database study found that there were significantly more ex-smokers (OR 1.2 (95% CI 1.1–1.4)) and slightly more current smokers (OR 1.1 (95% CI 1.0–1.2)) in patients with a new diagnosis of GORD than in the control cohort.17 The Georgia Medicaid study revealed that the odds of a GORD diagnosis were increased significantly by observed tobacco use (OR 2.6 (95% CI 1.9–3.5)).
For coffee consumption, however, there are few data and the picture is less clear. Two cross sectional studies reported that there was no significant association with this behavioural trait5,11 while a third identified an inverse relationship.13 The Swedish study by Terry et al concentrated particularly on behaviour relating to food consumption. In addition to the lack of association with coffee consumption, the authors also demonstrated that there was no association with consumption of trigger foods (total fat, chocolate, mint, coffee, onions, citrus fruits, and tomato), portion size of meals, or time of the last meal of the day.11
The Georgia Medicaid study identified a significant association between a GORD diagnosis and observed alcohol consumption (OR 1.8 (95% CI 1.4–2.4)). This was consistent with the positive association between GORD and alcohol consumption reported in one cross sectional study (OR 1.9 (95% CI 1.1–3.3) for ⩾7 drinks per week). In a second cross sectional study, however, the association between GORD and excess alcohol consumption (>28 units/week for a man, >21 units a week for a woman) did not reach statistical significance (OR 0.99 (95% CI 0.99–1.0)).8 Likewise, alcohol consumption did not significantly affect the risk of a first time diagnosis of GORD in the UK GP database study.17
A number of significant associations were demonstrated with the use of prescription medication. Increased use of anticholinergic drug therapy was significantly associated with the prevalence of GORD (OR 1.52 (95% CI 1.12–2.05)).8 The Olmsted County survey, however, found no significant association between GORD and the use of aspirin (OR 0.8 (95% CI 0.4–1.7)) or NSAIDs (OR 0.9 (95% CI 0.5–1.6)).5 The longitudinal UK GP database study demonstrated significant associations between the incidence of GORD and current use of NSAIDs (OR 1.5 (95% CI 1.3–1.7)) but not of aspirin (OR 1.1 (95% CI 0.9–1.3)). This study also revealed relationships with the current use of nitrates (in the case of patients with ischaemic heart disease) (OR 1.5 (95% CI 1.1–2.0)) and past use of oral steroids (OR 1.3 (95% CI 1.1–1.5)).17
The study in the UK Twin Registry also reported a lack of association of GORD with other drug treatments (including benzodiazepines and calcium antagonists).8 This study did however highlight an interesting inverse association between symptoms of GORD and use of oral contraceptives or hormone replacement therapy (OR 0.76 (95% CI 0.63–0.93)).8
Comorbid factors
A number of comorbidities of GORD were identified by the studies included in this review. Several of the comorbidities identified were gastrointestinal in origin. In the UK GP database study, a history of irritable bowel syndrome (IBS) (OR 1.6 (95% CI 1.2–2.1)) or peptic ulcer disease (OR 2.5 (95% CI 1.7–3.6)) was associated with an increased risk of GORD diagnosis.17 A cross sectional UK study demonstrated that GORD was significantly associated with abdominal pain and symptoms or signs of abdominal IBS.9 In the Olmsted County population, dysphagia and dyspepsia were shown to be significantly associated with GORD whereas the association with globus sensation did not reach statistical significance.4
The UK GP database study is also a particularly good source of data on extra oesophageal morbidities which are associated with GORD. In the year following the GORD diagnosis, patients were at increased risk of a first time diagnosis of cough (OR 1.7 (95% CI 1.4–2.1)), angina (OR 3.2 (95% CI 2.1–4.9)), gall bladder disease (OR 3.7 (95% CI 2.1–6.7)), sinusitis (OR 1.6 (95% CI 1.2–2.0)), and chest pain (OR 2.3 (95% CI 1.8–2.8)). Associations with pneumonia, asthma, chronic obstructive pulmonary disease (COPD), laryngitis, hoarseness, and otitis failed to reach statistical significance.17 The longitudinal Georgia Medicaid study reported asthma to be a significantly associated factor. However, asthma was interpreted as a risk factor as it significantly increased the odds of a GORD diagnosis (OR 3.2 (95% CI 2.6–4.0)).18 Similarly, although the UK database study did not identify a significantly increased risk of COPD in the year following a GORD diagnosis, prior COPD did slightly increase the risk of GORD (OR 1.3 (95% CI 1.0–1.8)).17 The significant association of GORD with chest pain was supported by the cross sectional Olmsted County study.4 However, the association between respiratory conditions (bronchitis and hoarseness) and GORD failed to reach statistical significance.4
There is also evidence of the association of GORD with psychosomatic/psychiatric factors. In China, both anxiety and depression were found to be more common in patients with GORD.14 Furthermore, two studies reported a significant association of GORD with a psychosomatic checklist score (a questionnaire based measurement of presence and frequency of psychophysiological symptoms) in Western populations.5,13
CONCLUSIONS
A definition of GORD with a strict symptom frequency threshold has been used in this systematic review in order to allow a realistic assessment of the prevalence and incidence of the disease. True GORD, with symptoms sufficient to impair quality of life, appears to show global variation in its prevalence. When defined as at least weekly heartburn and/or acid regurgitation, the prevalence in the Western world generally ranges between 10% and 20% whereas in Asia the prevalence is reported to be less than 5%. There is a trend for the prevalence in North America to be higher than that in Europe, and a trend is also suggested for a higher prevalence in Northern over Southern Europe. While these prevalence values may represent a slight overestimate of the true prevalence of GORD, given that symptoms at least twice weekly is the definition which is thought to best predict impaired quality of life,1 the same regional trends are also seen in included studies of heartburn alone, and in the Domestic/International Gastroenterology Surveillance Study of upper gastrointestinal symptoms.19
Only two studies included in this review reported the incidence of GORD, and both were based in the Western world. From these limited data, the incidence of GORD can be taken as approximately 5 per 1000 person years. This incidence appears particularly low relative to the prevalence of GORD but is consistent with the recognised chronicity of the disease20; nearly half of affected individuals in the population report experiencing symptoms for more than 10 years.4,13
A number of potential risk factors for GORD have been identified by reviewing the epidemiological data reported in the included studies. However, all of the positive associations have rather small odds ratios, leaving their clinical implications for preventative or therapeutic strategies in doubt. Furthermore, the limitations of cross sectional studies mean that some of these associated factors cannot be described categorically as risk factors or complications. Data from the included longitudinal studies indicate that obesity and possibly increasing age are risk factors for GORD, although sex is not. The relationship with age is difficult to establish, given that older patients may underreport reflux symptoms.21 The relationship with obesity has been confirmed in longitudinal studies of severe gastro-oesophageal reflux symptoms22 and of oesophagitis related hospitalisation,23 and may contribute to the high prevalence of GORD in the USA compared with the rest of the world. Genetics appear to play a role in the epidemiology of GORD but the remarkable geographic differences in prevalence are unlikely to be explained purely on the basis of genetic variation. This means that, as indicated by several recent studies in this area,24–28 the relatively low prevalence of GORD in Asia may not be sustained in the face of ongoing changes in diet and lifestyle.
The lack of association of well accepted behavioural factors with symptoms of GORD in cross sectional studies may reflect behavioural adaptation (an “avoidance pattern”) in response to such symptoms. It is reasonable to expect that, although behaviour such as alcohol consumption, smoking, and diet may contribute to development of the disease, a subject would also be likely to adapt their behaviour in response to the experience of life impairing symptoms. Therefore, behavioural risk factors are particularly difficult to identify in cross sectional studies.
The included longitudinal and cross sectional studies show that respiratory disease and chest pain are among a number of complications of GORD, confirming the findings of other studies of oesophagitis and hospitalisation.29–31 It is notable that potential risk factors and complications tend to be less strongly associated with GORD symptoms than they are with oesophageal complications of GORD, such as erosive oesophagitis and oesophageal adenocarcinoma.32–34
There is a notable lack of epidemiological data describing GORD in children. Adulthood did not form part of the inclusion criteria for this review yet only one of the studies had a sample which included individuals under the age of 18 years.9 Given that symptoms suggestive of GORD are not uncommon in children35 and may give rise to a range of respiratory complications in this age group,30 this is an area which should be further explored. Longitudinal data could prove particularly valuable for the study of the progression of GORD from infancy to adulthood. Further studies are also needed to investigate the influence of Helicobacter pylori infection and temporal trends in GORD prevalence.
In summary, GORD, defined as symptoms likely to impair quality of life, affects up to 20% of the Western population and is associated with a range of risk factors. An understanding of the potential extra oesophageal complications of GORD, primarily of the respiratory system, will aid the physician in diagnosing and managing this chronic disease.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the editorial assistance provided by Dr Becky Clayton and Dr Christopher Winchester in preparing this manuscript.
REFERENCES
- 1.Dent J, Brun J, Fendrick AM, et al. An evidence-based appraisal of reflux disease management—the Genval Workshop Report. Gut 1999;44 (suppl 2) :S1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB, Talley NJ. Systematic review: The prevalence and clinical course of functional dyspepsia. Aliment Pharmacol Ther 2004;19:643–54. [DOI] [PubMed] [Google Scholar]
- 3.Heading RC. Prevalence of upper gastrointestinal symptoms in the general population: a systematic review. Scand J Gastroenterol Suppl 1999;231:3–8. [PubMed] [Google Scholar]
- 4.Locke GR III, Talley NJ, Fett SL, et al. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology 1997;112:1448–56. [DOI] [PubMed] [Google Scholar]
- 5.Locke GR III, Talley NJ, Fett SL, et al. Risk factors associated with symptoms of gastroesophageal reflux. Am J Med 1999;106:642–9. [DOI] [PubMed] [Google Scholar]
- 6.Talley NJ, Zinsmeister AR, Schleck CD, et al. Dyspepsia and dyspepsia subgroups: a population-based study. Gastroenterology 1992;102:1259–68. [PubMed] [Google Scholar]
- 7.El-Serag HB, Petersen NJ, Carter J, et al. Gastroesophageal reflux among different racial groups in the United States. Gastroenterology 2004;126:1692–9. [DOI] [PubMed] [Google Scholar]
- 8.Mohammed I, Cherkas LF, Riley SA, et al. Genetic influences in gastro-oesophageal reflux disease: a twin study. Gut 2003;52:1085–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson WG, Heaton KW. Heartburn and globus in apparently healthy people. Can Med Assoc J 1982;126:46–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Isolauri J, Laippala P. Prevalence of symptoms suggestive of gastro-oesophageal reflux disease in an adult population. Ann Med 1995;27:67–70. [DOI] [PubMed] [Google Scholar]
- 11.Terry P, Lagergren J, Wolk A, et al. Reflux-inducing dietary factors and risk of adenocarcinoma of the esophagus and gastric cardia. Nutr Cancer 2000;38:186–91. [DOI] [PubMed] [Google Scholar]
- 12.Valle C, Broglia F, Pistorio A, et al. Prevalence and impact of symptoms suggestive of gastroesophageal reflux disease. Dig Dis Sci 1999;44:1848–52. [DOI] [PubMed] [Google Scholar]
- 13.Diaz-Rubio M, Moreno-Elola-Olaso C, Rey E, et al. Symptoms of gastro-oesophageal reflux: prevalence, severity, duration and associated factors in a Spanish population. Aliment Pharmacol Ther 2004;19:95–105. [DOI] [PubMed] [Google Scholar]
- 14.Hu WH, Wong WM, Lam CL, et al. Anxiety but not depression determines health care-seeking behaviour in Chinese patients with dyspepsia and irritable bowel syndrome: a population-based study. Aliment Pharmacol Ther 2002;16:2081–8. [DOI] [PubMed] [Google Scholar]
- 15.Wong WM, Lai KC, Lam KF, et al. Prevalence, clinical spectrum and health care utilization of gastro-oesophageal reflux disease in a Chinese population: a population-based study. Aliment Pharmacol Ther 2003;18:595–604. [DOI] [PubMed] [Google Scholar]
- 16.Pan G, Xu G, Ke M, et al. Epidemiological study of symptomatic gastroesophageal reflux disease in China: Beijing and Shanghai. Chin J Dig Dis 2000;1:2–8. [Google Scholar]
- 17.Ruigomez A, Wallander MA, Johansson S, et al. Natural history of gastroesophageal reflux disease diagnosed in UK general practice. Aliment Pharmacol Ther 2004;20:751–60. [DOI] [PubMed] [Google Scholar]
- 18.Kotzan J, Wade W, Yu HH. Assessing NSAID prescription use as a predisposing factor for gastroesophageal reflux disease in a Medicaid population. Pharm Res 2001;18:1367–72. [DOI] [PubMed] [Google Scholar]
- 19.Stanghellini V . Three-month prevalence rates of gastrointestinal symptoms and the influence of demographic factors: results from the Domestic/International Gastroenterology Surveillance Study (DIGEST). Scand J Gastroenterol Suppl 1999;231:20–8. [DOI] [PubMed] [Google Scholar]
- 20.Spechler SJ. Epidemiology and natural history of gastro-oesophageal reflux disease. Digestion 1992;51:24–9. [DOI] [PubMed] [Google Scholar]
- 21.Johnson DA, Fennerty MB. Heartburn severity underestimates erosive esophagitis severity in elderly patients with gastroesophageal reflux disease. Gastroenterology 2004;126:660–4. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson M, Johnsen R, Ye W, et al. Obesity and estrogen as risk factors for gastroesophageal reflux symptoms. JAMA 2003;290:66–72. [DOI] [PubMed] [Google Scholar]
- 23.Ruhl CE, Everhart JE. Overweight, but not high dietary fat intake, increases risk of gastroesophageal reflux disease hospitalization: the NHANES I Epidemiologic Followup Study. First National Health and Nutrition Examination Survey. Ann Epidemiol 1999;9:424–35. [DOI] [PubMed] [Google Scholar]
- 24.Wong WM, Lai KC, Lam KF, et al. Onset and disappearance of reflux symptoms in a Chinese population: a 1-year follow-up study. Aliment Pharmacol Ther 2004;20:803–12. [DOI] [PubMed] [Google Scholar]
- 25.Ho KY, Kang JY, Seow A. Prevalence of gastrointestinal symptoms in a multiracial Asian population, with particular reference to reflux-type symptoms. Am J Gastroenterol 1998;93:1816–22. [DOI] [PubMed] [Google Scholar]
- 26.Ho KY, Lim LS, Goh WT, et al. The prevalence of gastrooesophageal reflux has increased in Asia: a longitudinal study in the community. J Gastroenterol Hepatol 2001;16:A132. [Google Scholar]
- 27.Hongo M, Shoji T. Epidemiology of reflux disease and CLE in East Asia. J Gastroenterol 2003;38 (suppl 15) :25–30. [PubMed] [Google Scholar]
- 28.Wong WM, Lim P, Wong BC. Clinical practice pattern of gastroenterologists, primary care physicians, and otolaryngologists for the management of GERD in the Asia-Pacific region: The FAST survey. J Gastroenterol Hepatol 2004;19:S54–S60. [DOI] [PubMed] [Google Scholar]
- 29.Ruhl CE, Sonnenberg A, Everhart JE. Hospitalization with respiratory disease following hiatal hernia and reflux esophagitis in a prospective, population-based study. Ann Epidemiol 2001;11:477–83. [DOI] [PubMed] [Google Scholar]
- 30.El-Serag HB, Gilger M, Kuebeler M, et al. Extraesophageal associations of gastroesophageal reflux disease in children without neurologic defects. Gastroenterology 2001;121:1294–9. [DOI] [PubMed] [Google Scholar]
- 31.El-Serag HB, Sonnenberg A. Comorbid occurrence of laryngeal or pulmonary disease with esophagitis in United States military veterans. Gastroenterology 1997;113:755–60. [DOI] [PubMed] [Google Scholar]
- 32.Romero Y, Cameron AJ, Locke GR 3rd, et al. Familial aggregation of gastroesophageal reflux in patients with Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterology 1997;113:1449–56. [DOI] [PubMed] [Google Scholar]
- 33.El-Serag HB, Johanson JF. Risk factors for the severity of erosive esophagitis in Helicobacter pylori-negative patients with gastroesophageal reflux disease. Scand J Gastroenterol 2002;37:899–904. [DOI] [PubMed] [Google Scholar]
- 34.El-Serag HB, Sonnenberg A. Associations between different forms of gastro-oesophageal reflux disease. Gut 1997;41:594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson SP, Chen EH, Syniar GM, et al. Prevalence of symptoms of gastroesophageal reflux during childhood: a pediatric practice-based survey. Pediatric Practice Research Group. Arch Pediatr Adolesc Med 2000;154:150–4. [DOI] [PubMed] [Google Scholar]
- 36.Rajendra S, Alahuddin S. Racial differences in the prevalence of heartburn. Aliment Pharmacol Ther 2004;19:375–6. [DOI] [PubMed] [Google Scholar]
- 37.Wong WM, Lam KF, Cheng C, et al. Population based study of noncardiac chest pain in southern Chinese: Prevalence, psychosocial factors and health care utilization. World J Gastroenterol 2004;10:707–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujimoto K, Iwakiri R, Okamoto K, et al. Characteristics of gastroesophageal reflux disease in Japan: increased prevalence in elderly women. J Gastroenterol 2003;38:3–6. [PubMed] [Google Scholar]
- 39.Khoshbaten M . Gastro-esophageal reflux disease in northwestern Tabriz, Iran. Indian J Gastroenterol 2003;22:138–9. [PubMed] [Google Scholar]
- 40.Watanabe Y, Fujiwara Y, Shiba M, et al. Cigarette smoking and alcohol consumption associated with gastro-oesophageal reflux disease in Japanese men. Scand J Gastroenterol 2003;38:807–11. [DOI] [PubMed] [Google Scholar]
- 41.Cameron AJ, Lagergren J, Henriksson C, et al. Gastroesophageal reflux disease in monozygotic and dizygotic twins. Gastroenterology 2002;122:55–9. [DOI] [PubMed] [Google Scholar]
- 42.Conio M, Filiberti R, Blanchi S, et al. Risk factors for Barrett’s esophagus: a case-control study. Int J Cancer 2002;97:225–9. [DOI] [PubMed] [Google Scholar]
- 43.Louis E, DeLooze D, Deprez P, et al. Heartburn in Belgium: prevalence, impact on daily life, and utilization of medical resources. Eur J Gastroenterol Hepatol 2002;14:279–84. [DOI] [PubMed] [Google Scholar]
- 44.Agreus L, Svardsudd K, Talley NJ, et al. Natural history of gastroesophageal reflux disease and functional abdominal disorders: a population-based study. Am J Gastroenterol 2001;96:2905–14. [DOI] [PubMed] [Google Scholar]
- 45.Avidan B, Sonnenberg A, Giblovich H, et al. Reflux symptoms are associated with psychiatric disease. Aliment Pharmacol Ther 2001;15:1907–12. [DOI] [PubMed] [Google Scholar]
- 46.Haque M, Wyeth JW, Stace NH, et al. Prevalence, severity and associated features of gastro-oesophageal reflux and dyspepsia: a population-based study. N Z Med J 2000;113:178–81. [PubMed] [Google Scholar]
- 47.Kennedy T, Jones R. The prevalence of gastro-oesophageal reflux symptoms in a UK population and the consultation behaviour of patients with these symptoms. Aliment Pharmacol Ther 2000;14:1589–94. [DOI] [PubMed] [Google Scholar]
- 48.Jasani K, Piterman L, McCall L. Gastroesophageal reflux and quality of life. Patient’s knowledge, attitudes and perceptions. Aust Fam Physician 1999;28 (suppl 1) :S15–18. [PubMed] [Google Scholar]
- 49.Lagergren J, Bergstrom R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31. [DOI] [PubMed] [Google Scholar]
- 50.Oliveria SA, Christos PJ, Talley NJ, et al. Heartburn risk factors, knowledge, and prevention strategies: a population-based survey of individuals with heartburn. Arch Intern Med 1999;159:1592–8. [DOI] [PubMed] [Google Scholar]
- 51.Kennedy TM, Jones RH, Hungin AP, et al. Irritable bowel syndrome, gastro-oesophageal reflux, and bronchial hyper-responsiveness in the general population. Gut 1998;43:770–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corder AP, Jones RH, Sadler GH, et al. Heartburn, oesophagitis and Barrett’s oesophagus in self-medicating patients in general practice. Br J Clin Pract 1996;50:245–8. [PubMed] [Google Scholar]
- 53.Mold JW, Reed LE, Davis AB, et al. Prevalence of gastroesophageal reflux in elderly patients in a primary care setting. Am J Gastroenterol 1991;86:965–70. [PubMed] [Google Scholar]
- 54.Ruth M, Mansson I, Sandberg N. The prevalence of symptoms suggestive of esophageal disorders. Scand J Gastroenterol 1991;26:73–81. [DOI] [PubMed] [Google Scholar]
- 55.Nebel OT, Fornes MF, Castell DO. Symptomatic gastroesophageal reflux: incidence and precipitating factors. Am J Dig Dis 1976;21:953–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.