Abstract
Activation of CD40 is essential for thymus-dependent humoral immune responses and rescuing B cells from apoptosis. Many of the effects of CD40 are believed to be achieved through altered gene expression. In addition to Bcl-x, a known CD40-regulated antiapoptotic molecule, we identified a related antiapoptotic molecule, A1/Bfl-1, as a CD40-inducible gene. Inhibition of the NF-κB pathway by overexpression of a dominant-active inhibitor of NF-κB abolished CD40-induced up-regulation of both the Bfl-1 and Bcl-x genes and also eliminated the ability of CD40 to rescue Fas-induced cell death. Within the upstream promoter region of Bcl-x, a potential NF-κB-binding sequence was found to support NF-κB-dependent transcriptional activation. Furthermore, expression of physiological levels of Bcl-x protected B cells from Fas-mediated apoptosis in the absence of NF-κB signaling. Thus, our results suggest that CD40-mediated cell survival proceeds through NF-κB-dependent up-regulation of Bcl-2 family members.
CD40 is a 50-kDa glycoprotein member of the tumor necrosis factor (TNF) receptor (TNFR) superfamily, a family of type I receptors with more than 20 known members, which mediates a diverse array of biological processes (1). Studies with X-linked hyper-IgM syndrome (HIGMX), a genetic defect in the corresponding CD40 ligand (CD40L) gene, and with mice lacking either the CD40 or CD40L gene have demonstrated that interaction between CD40 and CD40L during T and B cell contact is essential for all events in thymus-dependent antigen responses such as Ig production, isotype switching, somatic hypermutation, and induction of B cell memory (2–5). Recently published studies indicate that CD40/CD40L interactions also play important roles in the activation of T cells, macrophages, natural killer cells, and endothelial cells (6). The molecular mechanisms for these biological effects of CD40/CD40L interactions, however, remain to be elucidated.
An important aspect of CD40 signaling in B cells is its ability to control apoptosis (7). Isolated germinal center B cells, which spontaneously undergo apoptosis in culture, are capable of surviving for extended periods when treated with anti-CD40 antibodies or CD40L. In addition, CD40 is known to rescue immature B cells, such as WEHI 231 cells, and mature B cells from surface IgM (sIgM) and Fas-mediated apoptosis, respectively (8–10).
Many studies indicate that the antiapoptotic function of CD40 is mediated by up-regulated expression of the Bcl-xL gene, an antiapoptotic member of the Bcl-2 family of proteins (11–13). This family includes other antiapoptotic members such as Bfl-1/A1, Mcl-1, and Bcl-w, as well as proapoptotic members such as Bax, Bad, Bid, and the alternatively spliced Bcl-xS (14). Studies show that overexpression of exogenous Bcl-xL (hereafter referred to as Bcl-x) is capable of preventing B cell apoptosis by various stimuli such as Fas activation, sIgM crosslinking, and exposure to various chemical inducers of cell death (15).
Studies by our laboratory, as well as those of others, have determined that TNF receptor-associated factor (TRAF) adapter proteins, most notably TRAF 2, are crucial for the activation of CD40-signaling pathways such as the NF-κB and stress-activated protein kinase (SAPK) pathways (16). The NF-κB pathway activates a number of related transcription factors collectively known as the Rel family, including five mammalian members: NF-κB1 (p50), NF-κB2 (p52), c-Rel, RelA (p65), and RelB (17). These molecules typically exist as inactive hetero-/homodimers in the cytoplasm complexed with an inhibitory IκB (inhibitor of NF-κB) molecule. Studies with fibroblast cell lines from RelA-deficient mice or cell lines overexpressing a dominant-active IκB-α mutant, with the critical serines at positions 32 and 36 converted to alanine residues, demonstrate that the NF-κB-signaling pathway plays an essential antiapoptotic role and that this function requires protein synthesis (18–20).
In an effort to identify genes up-regulated by CD40 signaling, our laboratory discovered, through representational difference analysis screening, that Bfl-1/A1, an antiapoptotic Bcl-2 family member, was up-regulated by CD40 activation. Along with Bcl-x, this represents the second Bcl-2 relative known to be up-regulated by CD40 signaling. Using cell lines overexpressing a super-repressor, dominant-active IκB, it was established that the NF-κB-signaling pathway is essential for CD40-induced up-regulation of both of these antiapoptotic Bcl-2 family members as well as for CD40-mediated rescue of Fas-induced B cell apoptosis. Our results also identify a potential NF-κB-binding region within the promoter of the Bcl-x gene, which binds NF-κB subunits and supports NF-κB-mediated transcription, as assessed through reporter gene analysis. Finally, by using cell lines expressing various levels of exogenous Bcl-x, it was also shown that the levels of Bcl-x induced by CD40 activation are sufficient to protect against Fas-mediated cell death in cells lacking functional NF-κB activity. Thus, our studies provide a crucial link in CD40-mediated antiapoptosis by linking the activation of the NF-κB-signaling pathway to the up-regulation of the antiapoptotic Bcl-2 family members.
MATERIALS AND METHODS
Chemicals and Antibodies.
Antibodies for detection of Bcl-x (sc 634) and for use in supershift assays were obtained from Santa Cruz Biotechnology, with the exception of the anti-c-Rel antibody, which was obtained from Nancy Rice (National Cancer Institute/Frederick Cancer Research and Development Center). The CH11, G28.5, and anti-FLAG antibodies were obtained from Upstate Biotechnology, American Type Culture Collection (Manassas, VA), and Babco (Richmond, CA), respectively. 4-Hydroxytamoxifen (4-OHT) was purchased from Calbiochem.
Plasmid Construction.
The 5′-FLAG-tagged IκB mutant was fused in-frame to the 5′ end of the mutant estrogen receptor ligand-binding domain (ER), and the chimera was cloned into the HindIII/EcoRI sites of pCDNA3 to generate the pCDNA3-IκBm-ER construct. The pEBB-puro-Bcl-x-HA construct was generated by PCR cloning of human Bcl-x, which then was inserted into the BamHI and NotI sites of pEBB-puro-HA in-frame with the 3′ influenza hemagglutinin (HA) tag. For luciferase assays, a 650-bp region of the Bcl-x promoter, spanning −640 to +9 relative to the transcriptional start site, was inserted between the XhoI and HindIII sites of the pGL2-Basic luciferase vector (Promega). The NF-κB mutant promoter had an internal deletion spanning the two potential NF-κB cis elements from −84 to −46 relative to the transcriptional start site.
Cell Culture and Transfection.
Cell lines were maintained in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin. For electroporations, cells were pulsed at 250 V/975 μF and then selected and maintained in 1 mg/ml active G418 (Mediatech, Washington, DC) or in 2.5 μg/ml puromycin (Sigma). 293T cells were maintained in DMEM (GIBCO/BRL) supplemented with 10% FBS and were transfected by using standard calcium phosphate methods.
Representational Difference Analysis.
Two identical cultures of 20 × 106 Daudi cells at a density of 5 × 105 cells per ml were incubated for 14 hr in the presence or absence of G28.5 at 1 μg/ml. Poly(A)+ RNA was extracted from whole cells by using a commercial mRNA isolation kit (Pharmacia), and cDNA was generated by using Superscript reverse transcriptase (GIBCO). Representational difference analysis was performed essentially as described previously (21, 22). Two rounds of hybridization–PCR amplification were performed, and the products of the second round of subtraction were cloned into pSKII Bluescript (Stratagene) for sequencing.
Electrophoretic Mobility-Shift Assays.
For gel-shift assays, cells were treated as indicated in the text. Nuclear extracts were prepared and used in binding reactions with either a γ-32P-labeled MHC II NF-κB-specific hairpin oligonucleotide (23) or with a γ-32P-labeled annealed sense and antisense oligonucleotide encoding the Bcl-x promoter from −84 to −46 by using previously published protocols (24). The sequence of the sense strand of the Bcl-x probe is GGCGGGGGGGACTGCCCAGGGAGTGACTTTCCGAGGAAG. For supershifts, 1 μl of the appropriate antibody was added to the nuclear extracts for 20 min on ice before addition of the radiolabeled oligonucleotide probe.
Laddering Assay.
For DNA laddering, cells were stimulated in 5 ml of medium for 24 hr with the appropriate stimuli before processing the samples by using previously published protocols (25).
Fluorescence-Activated Cell Sorter (FACS) Analysis.
Cells were stained with a hypotonic propidium iodide solution (3.4 mM sodium citrate/0.3% Triton X-100/0.1 mg/ml propidium iodide/20 μg/ml of ribonuclease A) and used immediately for cell cycle analysis on a FACScan flow cytometer (Becton Dickinson).
RESULTS
Identification of Bfl-1 as a CD40-Induced Gene in Human B Cells.
CD40 signaling is believed to activate a number of downstream pathways, which lead to the activation of specific transcription factors, including the SAPK/JNK pathway, the p38 kinase pathway, the extracellular signal-regulated kinase (ERK) pathway, and the NF-κB pathway. To identify target effector genes that mediate the complex biological responses of CD40 activation, representational difference analysis was undertaken.
Daudi cells, a human B cell lymphoma line, were either stimulated for 14 hr with the anti-human CD40 antibody, G28.5, or cultured under identical conditions without stimulation. Using cDNA isolated from both pools of cells, screening was performed through two rounds of hybridization–PCR amplification, after which the subtracted products representing CD40-induced genes were subcloned for sequencing. Among the genes identified were a number of known CD40-inducible molecules such as intercellular adhesion molecule-1 and various class II MHC chains.
Through this process, we identified a protein known as Bfl-1/A1/GRS. Bfl-1 is a member of the Bcl-2 family of apoptosis-regulatory proteins, which are expressed in various tissues during development and adult life. It was reported to be overexpressed in a number of cancer cell lines (26) and was shown to protect cells from apoptosis and cooperate with the adenoviral E1A protein to transform cells in culture (27). In addition to Bfl-1, our screen isolated another Bcl-2 family member, Bcl-x, which has been characterized previously as an antiapoptotic molecule induced by CD40.
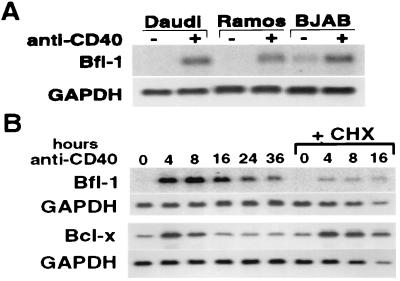
To confirm the induction of Bfl-1 in B cells, we probed Northern blots containing RNA from three human B cell lines: Daudi, Ramos, and BJAB (Fig. 1A). In all three cell lines, there is a dramatic increase in Bfl-1 RNA after 16 hr of CD40 stimulation. Thus, Bfl-1 expression, like that of Bcl-x, appears to respond strongly to CD40 stimulation in B cells.
Figure 1.
Bcl-x and Bfl-1 are rapidly induced primary response genes. (A) CD40 inducibility of Bfl-1 was confirmed in three human B cell lines cultured for 16 hr in the presence or absence of G28.5. All cells were treated with 1 μg/ml G28.5 at a density of 1 × 106 cells per ml. Eighteen micrograms of total RNA was loaded per lane, and the Northern blot was hybridized with a human Bfl-1 probe. The Northern blot was stripped, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was probed as a standard. (B) The kinetics of Bfl-1 and Bcl-x induction in Daudi cells were determined by examining gene expression after various periods of G28.5 stimulation. To determine the requirement for new protein synthesis in the CD40-mediated induction of these genes, cells were pretreated for 30 min with the protein synthesis inhibitor cycloheximide (CHX) at 25 μg/ml and then stimulated with G28.5 antibody for various periods of time in the presence of CHX. All cells were treated with 1 μg/ml G28.5 at a density of 1 × 106 cells per ml. Eighteen micrograms of total RNA was loaded per lane, and the Northern blots were hybridized with a human Bfl-1 or Bcl-x probe. Northern blots were stripped, and GAPDH expression was probed as a standard.
Rapid and Transient CD40-Mediated Induction of Bfl-1 and Bcl-x Occurs Independently of New Protein Synthesis.
Because the activation of apoptosis occurs rapidly through the processing of preformed components, the induction of antiapoptotic molecules by CD40 also must be rapid to be effective. We investigated the temporal regulation of Bcl-x and Bfl-1 in CD40-stimulated Daudi cells by analyzing RNA samples collected at various time points after stimulation with G28.5. The same results were observed by using soluble CD40 ligand as the stimulus (data not shown). As seen in Fig. 1B, both molecules are induced rapidly by CD40 stimulation, with peak expression levels at 4–8 hr after activation, followed by a decline in the levels of both transcripts at later time points. At the protein level, however, Bcl-x still was induced strongly even at 24 hr (Fig. 2C).
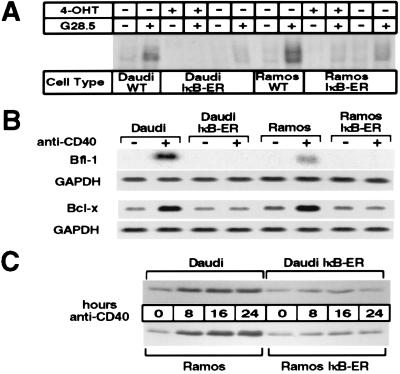
Figure 2.
Cells expressing IκB-ER have a functional block in NF-κB signaling and do not up-regulate Bcl-x and Bfl-1 in response to CD40 activation. (A) Cells were stimulated for 25 min in the presence or absence of 1 μg/ml G28.5 antibody, and the nuclear extracts were then processed as described previously. Six micrograms of nuclear extracts then were used in binding assays with the radiolabeled MHC II oligonucleotide. Cells induced with 4-OHT were pretreated with 200 nM of the synthetic estrogen for 3 hr before G28.5-mediated activation. (B) Daudi, Daudi-IκB-ER, Ramos, and Ramos-IκB-ER cells were pretreated with 4-OHT for 45 min and then cultured for 8 hr in 4-OHT alone or 4-OHT plus G28.5. Eighteen micrograms of total RNA was loaded per lane, and the Northern blots were hybridized with a human Bfl-1 or Bcl-x probe. Northern blots were stripped and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression was probed as a standard. (C) Daudi, Daudi-IκB-ER, Ramos, and Ramos-IκB-ER cells were pretreated with 4-OHT for 2 hr and then stimulated with G28.5 in the presence of 4-OHT for different periods of time. Total cell extracts from 1 × 106 cells were loaded per lane, and Western blotting was performed to detect Bcl-x expression.
To determine whether or not the induction of these genes requires the synthesis of intermediate factors, we stimulated Daudi cells for different time periods in the presence of the protein synthesis inhibitor cycloheximide (CHX). Northern blot analysis of the products of these cells revealed that both Bcl-x and Bfl-1 transcripts continue to be synthesized in the absence of new protein synthesis (Fig. 1B); however, the use of CHX affects the expression of these two genes in different ways. Although the magnitude of induction of Bcl-x remains the same, the response of Bfl-1 to CD40 stimulation is blunted, suggesting that full induction of Bfl-1 requires the participation of transcription factors whose activity is sensitive to protein synthesis inhibition. These results suggest that the regulatory mechanisms of these two genes are not identical, but they have in common a rapid and transient induction phase that is not abolished by protein synthesis inhibition.
Establishing NF-κB-Blocked Daudi and Ramos Stable Cell Lines.
In addition to our data showing the up-regulation of Bfl-1 and Bcl-x by CD40 signaling, Bfl-1 also has been shown to be induced by various stimuli such as TNF-α, lipopolysaccharide, granulocyte/macrophage colony-stimulating factor, and IL-1, whereas Bcl-x has been shown to be up-regulated by CD40 and sIgM signaling (11, 28–30). All of these stimuli share the common feature of signaling through NF-κB. Because of the established link between NF-κB signaling and the regulation of apoptosis, we sought to determine the role of NF-κB signaling in CD40-mediated up-regulation of both Bcl-2 family members.
To determine this role, we established human B cell lines with a functional block in the NF-κB-signaling pathway. The strategy involved the overexpression of a chimeric fusion protein consisting of a dominant-active IκB-α mutant (with serine-to-alanine mutations at positions 32 and 36) fused to a mutated estrogen receptor ligand-binding domain (ER) (31). The IκB mutant is incapable of being phosphorylated at the critical serine residues and, therefore, is not targeted for proteosomal degradation upon activation of the IκB kinase complex. The fused ER confers inducible activation of the gene of interest upon exposure to the synthetic estrogen 4-OHT. The FLAG-IκB-mutant-ER construct was cloned into the pCDNA3 expression vector and stably transfected into Daudi and Ramos cells. Single clones were isolated, and those that expressed the construct by Western analysis (data not shown) were chosen for further characterization by electrophoretic mobility-shift assay.
To determine whether the IκB-ER construct was functioning to block NF-κB signaling, nuclear extracts from cells either untreated or treated with the human CD40-activating G28.5 antibody were assessed for binding activity to a radiolabeled MHC II NF-κB oligonucleotide probe (H2 probe) through electrophoretic mobility-shift assay. As shown in Fig. 2A, nuclear extracts from parental Ramos and Daudi cells treated with the G28.5 antibody were capable of generating gel-shift complexes representing inducible NF-κB activity. These gel-shift complexes formed just as strongly in the presence of 4-OHT (data not shown), indicating that the drug did not interfere with NF-κB signaling. In stable Daudi and Ramos clones expressing the IκB-ER construct, however, CD40-mediated NF-κB induction was completely abolished in the presence of 4-OHT. These cell lines also had significantly decreased NF-κB induction in the absence 4-OHT, indicating some leakage in the system. Because 4-OHT treatment produced maximal suppression of NF-κB activity, all subsequent experiments were performed in the presence of 4-OHT.
The NF-κB Pathway Is Essential for CD40-Mediated Bcl-x and Bfl-1 Induction.
Using the NF-κB-blocked cell lines described above, we examined CD40 regulation of both Bcl-x and Bfl-1 through Northern blot analysis. In the presence of 4-OHT, parental Daudi and Ramos cells, as well as Daudi-IκB-ER and Ramos-IκB-ER cells, were left unstimulated or treated with the G28.5 antibody for 8 hr. As seen in Fig. 2B, induction of both genes in response to CD40 treatment was abolished in 4-OHT-treated Daudi-IκB-ER and Ramos-IκB-ER cells but not in parental Daudi and Ramos cells, suggesting that these cells require NF-κB signaling to induce both of these genes.
Expression of Bcl-x also was examined at the protein level in the same cell lines stimulated through CD40 in the presence of 4-OHT for different time periods. In agreement with the above results, Western blot analysis showed that Bcl-x expression could not be induced by CD40 in the NF-κB-blocked cell lines (Fig. 2C). We also observed that although the induction of Bcl-x, both at the RNA and protein levels, was completely abolished in IκB-ER cells, its basal expression was not affected. This suggests that other transcription factors may be able to substitute for NF-κB in the baseline expression of this gene or that there is enough leak-through NF-κB activity, even in the presence of 4-OHT, to enable low-level Bcl-x expression.
An NF-κB Consensus Sequence in the Bcl-x Promoter Binds NF-κB Complexes and Supports Transcriptional Activity.
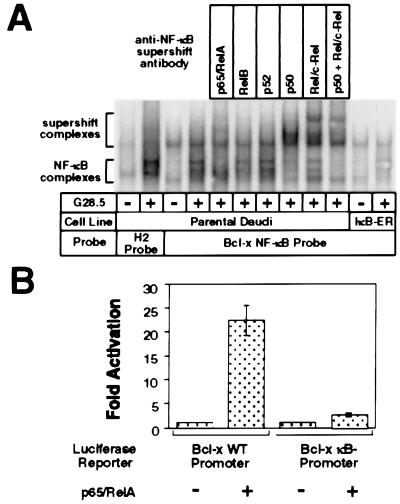
The Northern and Western blot analyses with the stable cell lines strongly support the role of NF-κB signaling in CD40-mediated up-regulation of Bfl-1 and Bcl-x. In agreement with these results, visual inspection of the human Bcl-x promoter identified two tandem, potential NF-κB-binding sites, one starting at position −77 relative to the transcriptional start site, with the sequence GGGACTGCCC, and the other starting at position −62, with the sequence GTGACTTTCC. A radiolabeled oligonucleotide spanning the two potential NF-κB-binding sites was used with nuclear extracts from unstimulated and G28.5-stimulated Daudi cells to assess binding by electrophoretic mobility-shift assay. As shown in Fig. 3A, nuclear extracts from parental Daudi cells stimulated with the G28.5 antibody were capable of generating several band shifts, probably corresponding to the different NF-κB dimer complexes bound to the probe; however, extracts from stable cell lines expressing the IκB-ER construct did not produce an inducible shift, indicating that NF-κB activity is necessary to bind to the Bcl-x NF-κB probe upon CD40 activation. Supershifted bands were detected with the anti-p50, anti-p65, and anti-c-Rel antibodies, but only weakly, if at all, with the other anti-Rel antibodies, indicating that the gel-shift complexes consisted predominantly of p50, p65, and c-Rel. Further gel-shift analysis indicated stronger NF-κB binding to the upstream site spanning positions −77 to −68 than to the downstream site (data not shown).
Figure 3.
Rel members are capable of mediating transcriptional activation through the Bcl-x promoter by binding to NF-κB cis elements. (A) The NF-κB complex binding to the putative NF-κB-binding sites within the Bcl-x promoter consists predominantly of p50, p65, and c-Rel. Nuclear extract harvests and binding reactions were performed as described previously. Supershifts were performed by the addition of the appropriate antibody to the nuclear extracts on ice 20 min before the binding reaction. (B) p65 is capable of mediating transcriptional activation through the Bcl-x promoter. A Bcl-x promoter fragment spanning −640 to +9 relative to the transcriptional start site (Bcl-x WT promoter) and another fragment missing the NF-κB-binding sequence (Bcl-x κB- promoter) were cloned into the pGL2-Basic luciferase reporter vector. 293T cells were transfected with 100 ng of the indicated reporter plasmids and either 2 μg of p65/RelA expression vector or 2 μg of pBABE-puro control vector as carrier. Samples were harvested 36 hr after transfection and assessed for luciferase activity.
Luciferase reporter assays also were performed to assess the ability of Rel family members to drive transcription from the Bcl-x promoter. A 650-bp DNA fragment spanning the Bcl-x 5-prime promoter region was inserted into the pGL2-Basic luciferase vector (Bcl-x WT). Another reporter with an internal deletion spanning the potential NF-κB-binding region (Bcl-x κB-) also was created. 293T cells were transfected with the indicated reporters (Fig. 3B) and p65-expressing DNA vector or control vector DNA. Cotransfection with p65 up-regulated reporter activity from the Bcl-x WT promoter by 22.3-fold (SD = 3.1) relative to the control-transfected samples, whereas p65 cotransfection up-regulated reporter activity by only 2.6-fold (SD = 0.36) from the Bcl-x κB- reporter. Similar results were observed in transiently transfected Jurkat cells (data not shown). These experiments suggest that Rel members are capable of directly activating transcription from the Bcl-x promoter.
NF-κB Activity Is Required for Protection of B Cells from Fas-Mediated Apoptosis.
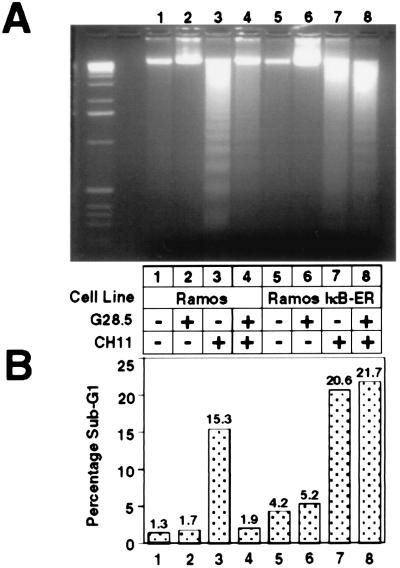
To study the role of NF-κB signaling in CD40-mediated rescue from cell death, we investigated whether the NF-κB pathway was crucial for CD40-mediated inhibition of Fas-activated cell death. Parental Ramos cells, as well as the IκB-ER stable cell line, were either left untreated, treated with the G28.5 antibody, treated with the human Fas-activating antibody, CH11, or treated with both G28.5 and CH11. After incubation for 24 hr, the samples were harvested and processed for apoptotic markers, including oligonucleosomal DNA laddering (Fig. 4A) and analysis of sub-G1 fraction by propidium iodide staining and FACScan cell cycle analysis (Fig. 4B).
Figure 4.
CD40-mediated rescue of Fas-induced apoptosis is ablated in the absence of NF-κB signaling. Cells (6 × 106) in 5 ml of medium were either untreated or treated for 24 hr with 1 μg/ml G28.5, 500 ng/ml CH11 Fas-activating antibody, or both. Cells were harvested, and DNA fragmentation (A) and the sub-G1 fraction (B) were assayed as described in Materials and Methods.
Wild-type Ramos cells treated with the CH11 antibody display the characteristic laddering profile of apoptotic cells and also had a high sub-G1 fraction (15.3%). This profile is inhibited upon CD40 activation, which reduces the sub-G1 fraction to 1.9% and decreases the amount of DNA laddering. In the Ramos IκB-ER cells, the laddering and the increased sub-G1 fraction is still observed upon treatment with the CH11 antibody, but cotreatment with the G28.5 antibody does not inhibit the laddering process or decrease the sub-G1 fraction, indicating that cell death is not rescued by CD40 activation in the IκB-ER cells. Daudi cells were not sensitive to Fas-mediated killing (data not shown) and, thus, were not used to assess CD40-mediated inhibition of this process. These results show that in Ramos cells, the CD40-mediated inhibition of Fas-activated cell death requires signaling through the NF-κB-signaling pathway.
Expression of Bcl-x at Physiologically Induced Levels Is Capable of Protecting Against Fas-Mediated Cell Death in the Absence of NF-κB Activity.
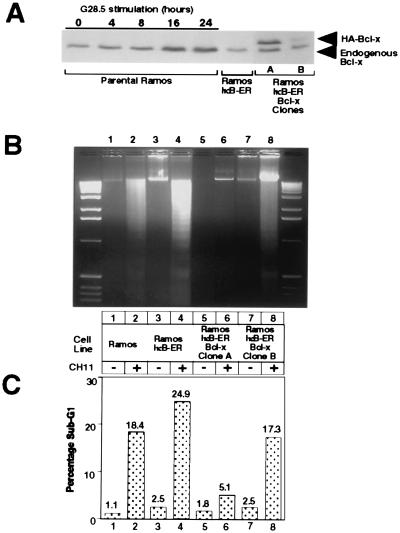
As shown in the previous sections, CD40-mediated rescue of Fas-induced apoptosis proceeds through an NF-κB-dependent pathway that may involve the up-regulation of Bcl-x and Bfl-1. To determine the contribution of Bcl-x to CD40-mediated antiapoptosis, we introduced exogenous HA-tagged Bcl-x into the Ramos IκB-ER cells and then determined the sensitivity of individual clones to Fas-mediated cell death. The HA-tagged Bcl-x protein migrates slightly slower than the endogenous Bcl-x, allowing for comparative analysis of expression levels in the isolated clones (Fig. 5A). Ramos IκB-ER-Bcl-x clone A expresses exogenous Bcl-x at levels similar to those seen in parental Ramos cells after 8–16 hr of CD40 stimulation. Ramos IκB-ER-Bcl-x clone B, however, expresses exogenous Bcl-x at relatively lower levels.
Figure 5.
Expression of exogenous Bcl-x protects against Fas-mediated cell death. (A) Ramos IκB-ER cells were transfected by electroporation with a pEBB-puro-Bcl-x-HA construct, and stable clones were isolated by limiting dilution plating and selection with puromycin. Expression of exogenous Bcl-x then was assessed by Western analysis and compared with the levels of endogenous Bcl-x that are up-regulated in response to CD40 activation by the G28.5 antibody. (B) DNA fragmentation was assessed in parental Ramos, Ramos IκB-ER, and Ramos IκB-ER-Bcl-x clones A and B after being untreated or treated for 24 hr with CH11. (C) The sub-G1 fraction was assessed by FACScan analysis after propidium iodide staining in Ramos cells, Ramos IκB-ER cells, and Ramos IκB-ER-Bcl-x clones A and B after being untreated or treated for 24 hr with the CH11 antibody.
To determine the protection afforded by Bcl-x against Fas-mediated death, parental Ramos cells, Ramos IκB-ER cells, and the two Bcl-x-expressing IκB-ER cell lines were stimulated with the human Fas-activating CH11 antibody for 24 hr, and cell death was assessed by DNA laddering and propidium iodide staining. As shown in Fig. 5 B and C, apoptosis was induced in both the parental Ramos and Ramos IκB-ER cell lines after treatment with the CH11 antibody. Ramos IκB-ER-Bcl-x clone A, which expresses exogenous Bcl-x near physiologically induced levels, is resistant to Fas-mediated cell death, whereas the lower-expressing Ramos IκB-ER-Bcl-x clone B was still sensitive to Fas-mediated apoptosis. Other clones, which expressed exogenous Bcl-x at levels between those seen in clones A and B, likewise were protected at intermediary levels (data not shown). These results suggest that the levels of Bcl-x that are induced by CD40 activation are sufficient for mediating its antiapoptotic effects against Fas activation.
DISCUSSION
In addition to their roles in inflammation and immunity (17), members of the Rel family of transcription factors also are believed to play important roles in preventing programmed cell death. Enhanced cytotoxicity in response to TNF treatment has been observed in RelA−/− fibroblasts and macrophages, as well as in a variety of cells, including fibroblasts, fibrosarcoma cells, and lymphoma cells, in which NF-κB signaling or protein synthesis has been blocked specifically through the overexpression of dominant-negative pathway components or through the use of cycloheximide (18–20). Taken together, these results would indicate that NF-κB may prevent cell death through the up-regulation of specific gene products, whereas apoptosis can proceed in the absence of new protein synthesis through the activation of the preformed caspase cascade. Indeed, the rapid induction kinetics characteristic of the NF-κB family are most likely crucial to its ability to compete with the transcription-independent apoptosis pathway.
A large number of genes, including many inflammatory cytokines and adhesion molecules, are regulated by NF-κB (32), but the mediators of this pathway’s antiapoptotic effect have remained a mystery until the recent identification of a few candidate molecules, including the antiapoptotic inhibitor of apoptosis molecules 1 and 2 (c-IAP 1 and 2), TRAFs 1 and 2, IEX-1L, and A20 (33–36). Although members of the IAP family recently have been suggested to act as direct inhibitors of caspases, their mechanisms of action—and certainly those of the TRAFs, A20 and IEX-1L—are not yet clearly understood.
Here we report the identification of two antiapoptotic members of the Bcl-2 family as targets of NF-κB signaling. We find Bfl-1 to be induced by CD40 signaling in multiple B cell lines (Fig. 1A). In addition, Bcl-x has been well characterized as a CD40-responsive inhibitor of apoptosis in B cells (11–13). Both Bcl-x and Bfl-1 are induced rapidly by CD40 activation (Fig. 1B) via an NF-κB-dependent signaling pathway (Fig. 2B).
We also have identified an NF-κB consensus sequence in the promoter of Bcl-x, and an inducible nuclear complex that is recognized by antibodies against p50/NF-κB1, c-Rel, and p65/RelA was shown to bind this sequence in stimulated control cells but not in 4-OHT-treated, IκB-ER-expressing cells (Fig. 3A). In addition, a luciferase reporter construct driven by the Bcl-x upstream promoter region responded strongly to p65 expression; however, this response was inhibited by the removal of the putative NF-κB-binding sequence from the promoter (Fig. 3B). Thus, we have demonstrated that the Bcl-x promoter contains an element that binds NF-κB transcription factors and supports transcriptional activation by a member of this family.
CD40 signaling increases NF-κB activity and is known to play instrumental roles in the activation, proliferation, and differentiation of B cells (3, 37). Activated proliferating germinal center B cells undergo a process of somatic hypermutation of Ig variable regions followed by antigen-driven selection of high-affinity clones as they migrate to the periphery of the germinal center. Low-affinity clones, as well as those that acquire self-reactivity through the mutation process, are believed to be removed through apoptosis. CD40 stimulation is able to rescue germinal center B cells from programmed cell death (7). Cross-linking of Fas leads to B cell death, and CD40 signaling can protect against this (9). Interestingly, CD40 also has been reported to up-regulate Fas expression in mature B cells and, thus, actually sensitize them to Fas-mediated killing under some conditions (38). Thus, CD40 activation intensifies the death signal of Fas by increasing Fas expression while, at the same time, protecting against this effect through the induction of Bcl-x above basal levels and possibly through the expression of additional antiapoptotic molecules such as Bfl-1. This protective effect of CD40 alone, however, is transient in nature, and prolonged activation of Fas can overcome it, unless other stimuli, such as sIgM stimulation through high-affinity antigen binding, are also present (39). This is consistent with our data demonstrating the transient induction of Bcl-x and Bfl-1 at the RNA level (Fig. 1B). We demonstrate that a blockade of the NF-κB pathway, which leads to a loss of Bcl-x and Bfl-1 induction (Fig. 2B), abolishes the protective effect of CD40 against Fas-mediated apoptosis in Ramos B cells (Fig. 4).
When Bcl-x is reintroduced into NF-κB-blocked cells, they are protected against the apoptotic effects of Fas ligation (Fig. 5). The level of ectopic Bcl-x protein required for this protective effect is roughly equivalent to the levels of endogenous Bcl-x induced by CD40 treatment in cells with intact NF-κB signaling. Together with the data discussed above, these results suggest that NF-κB-mediated induction of Bcl-x expression is sufficient for the ability of CD40 to protect B cells from Fas-mediated apoptosis.
The ability of NF-κB to induce antiapoptotic proteins is consistent with its cytoprotective nature. Interestingly, the level of Bcl-x expression capable of protecting against Fas-mediated killing represents an induction of only a few fold above baseline, emphasizing the quantitatively fine nature of this balance. Because the NF-κB pathway must act rapidly to counteract the amplification of the apoptosis signal, it may be important that quantitatively minor changes in protein expression level be sufficient to achieve this end. Finally, although physiological levels of Bcl-x alone are sufficient to protect against apoptosis, the fact that Bfl-1 also is induced strongly by CD40 suggests that this protein also may play a role in protecting B cells. Because the response of NF-κB against apoptosis must be rapid, leaving little room for error, the induction of two Bcl-2 family members where one is sufficient may represent a precautionary reserve of antiapoptotic activity.
Acknowledgments
We thank Drs. David Baltimore, Charles Sawyers, and Stephen Smale for their critical reading and valuable suggestions on this manuscript. We also thank Drs. Chris Denny and David Chang for their technical advice on the representational difference analysis protocol and Shomyseh Sanjabi, as well as Drs. Thomas Parks, Katrina Catron, Joshy Jacob, and Nancy Rice, for providing valuable reagents. This work was supported by the Margaret E. Early and Stop Cancer Foundations. H.H.L. and H.D. are supported by a University of California at Los Angeles Medical Scientist Training Program Training Grant (GM 08042).
ABBREVIATIONS
- IκB
inhibitor of NF-κB
- TNF
tumor necrosis factor
- TRAF
TNF receptor-associated factor
- HA
hemagglutinin
- 4-OHT
4-hydroxytamoxifen
- ER
estrogen receptor ligand-binding domain
- sIgM
surface IgM
Note
. During the preparation of this manuscript, two separate studies showed up-regulation of Bfl-1/A1 by NF-κB signaling through other stimuli (40, 41).
References
- 1.Smith C A, Farrah T, Goodwin R G. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 2.Callard R, Armitage R, Fanslow W, Spriggs M. Immunol Today. 1993;14:559–564. doi: 10.1016/0167-5699(93)90188-Q. [DOI] [PubMed] [Google Scholar]
- 3.Durie F H, Foy T M, Masters S R, Laman J D, Noelle R J. Immunol Today. 1994;15:406–411. doi: 10.1016/0167-5699(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 4.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Sumatsu S, Yoshida N, Kishimoto T, Kikutani H. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 5.Xu J, Foy T M, Lsaman J D, Elliott E A, Dunn J J, Waldschmidt T J, Elsemore J, Noelle R J, Flavell R A. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 6.Grewal I S, Flavell R A. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 7.Kehry M R. J Immunol. 1996;156:2345–2348. [PubMed] [Google Scholar]
- 8.Tsubata T, Wu J, Honjo T. Nature (London) 1993;364:645–648. doi: 10.1038/364645a0. [DOI] [PubMed] [Google Scholar]
- 9.Cleary A, Fortune S, Yellin M, Chess L, Lederman S. J Immunol. 1995;155:3329–3337. [PubMed] [Google Scholar]
- 10.Lagresle C, Mondiere P, Bella C, Krammer P, Defrance T. J Exp Med. 1996;183:1377–1388. doi: 10.1084/jem.183.4.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi M, Boise L, Gottschalk A, Quintans J, Thompson C, Klaus G. Eur J Immunol. 1995;25:1352–1357. doi: 10.1002/eji.1830250533. [DOI] [PubMed] [Google Scholar]
- 12.Merino R, Grillot D, Simonian P, Muthukkumar S, Fanslow W, Bondada S, Nunez G. J Immunol. 1995;155:3830–3838. [PubMed] [Google Scholar]
- 13.Wang Z, Karras J, Howard R, Rothstein T. J Immunol. 1995;155:3722–3725. [PubMed] [Google Scholar]
- 14.Adams J, Cory S. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 15.Schneider T, Grillot D, Foote L, Nunez G, Rothstein T. J Immunol. 1997;159:4834–4839. [PubMed] [Google Scholar]
- 16.Lee H, Dempsey P, Parks T, Zhu X, Baltimore D, Cheng G. Proc Natl Acad Sci USA. 1999;96:1421–1426. doi: 10.1073/pnas.96.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baeuerle P, Baltimore D. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 18.Beg A A, Baltimore D. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 19.Wang C-Y, Mayo M W, Baldwin A S. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 20.Van Antwerp D, Martin S, Kafri T, Green D, Verma I. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 21.Hubank M, Schatz D. Nucleic Acids Res. 1994;22:5640–5648. doi: 10.1093/nar/22.25.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang D, Park N, Denny C, Nelson S, Pe M. Oncogene. 1998;16:1921–1930. doi: 10.1038/sj.onc.1201715. [DOI] [PubMed] [Google Scholar]
- 23.Fujita T, Nolan G p, Liou H-C, Scott M L, Baltimore D. Genes Dev. 1993;7:1354–1363. doi: 10.1101/gad.7.7b.1354. [DOI] [PubMed] [Google Scholar]
- 24.Liou H, Sha W, Scott M, Baltimore D. Mol Cell Biol. 1994;14:5349–5359. doi: 10.1128/mcb.14.8.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrmann M, Lorenz H, Voll R, Grunke M, Woith W, Kalden J. Nucleic Acids Res. 1994;22:5506–5507. doi: 10.1093/nar/22.24.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park I, Lee S, Whang D, Hong W, Choi S, Shin H, Choe T, Hong S. Anticancer Res. 1997;17:4619–4622. [PubMed] [Google Scholar]
- 27.D’Sa-Eipper C, Chinnadurai G. Oncogene. 1998;16:3105–3114. doi: 10.1038/sj.onc.1201851. [DOI] [PubMed] [Google Scholar]
- 28.Lin E, Orlofsky A, Berger M, Prystowsky M. J Immunol. 1993;151:1979–1988. [PubMed] [Google Scholar]
- 29.Karsan A, Yee E, Kaushansky K, Harlan J. Blood. 1996;87:3089–3096. [PubMed] [Google Scholar]
- 30.Hu X, Yee E, Harlan J, Wong F, Karsan A. Blood. 1998;92:2759–2765. [PubMed] [Google Scholar]
- 31.Littlewood T, Hancock D, Danielian P, Parker M, Evan G. Nucleic Acids Res. 1995;23:1686–1690. doi: 10.1093/nar/23.10.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldwin A J. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 33.Chu Z, McKinsey T, Liu L, Gentry J, Malim M, Ballard D. Proc Natl Acad Sci USA. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C, Mayo M, Korneluk R, Goeddel D, Baldwin A J. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 35.Wu M, Ao Z, Prasad K, Wu R, Schlossman S. Science. 1998;281:998–1001. doi: 10.1126/science.281.5379.998. [DOI] [PubMed] [Google Scholar]
- 36.Krikos A, Laherty C, Dixit V. J Biol Chem. 1992;267:17971–17976. [PubMed] [Google Scholar]
- 37.Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi J P, Kooten C v, Liu Y J, Rousset F, Saeland S. Annu Rev Immunol. 1994;12:881–992. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 38.Rothstein T. Curr Opin Immunol. 1996;8:362–371. doi: 10.1016/s0952-7915(96)80126-9. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Li L, Choe J, Krajewski S, Reed J, Thompson C, Choi Y. Cell Immunol. 1996;173:149–154. doi: 10.1006/cimm.1996.0260. [DOI] [PubMed] [Google Scholar]
- 40.Grumont R, Rourke I, Gerondakis S. Genes Dev. 1999;13:400–411. doi: 10.1101/gad.13.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zong W, Edelstein L, Chen C, Bash J, Gelinas C. Genes Dev. 1999;13:382–387. doi: 10.1101/gad.13.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]