Abstract
Background and aims: Splanchnic vein thrombosis is a significant source of complications in candidates for liver transplantation. The aims of this study were: (a) to determine the prevalence of and risk factors for splanchnic vein thrombosis in cirrhotic patients awaiting transplantation and (b) to assess the usefulness of anticoagulation.
Methods: A total of 251 cirrhotic patients listed for transplantation were analysed. All underwent systematic screening for thrombosis with Doppler ultrasonography. During the second period of the study, all patients with thrombosis received anticoagulation up to transplantation while during the first period none had received anticoagulation.
Results: The incidence of splanchnic vein thrombosis at evaluation was 8.4%. Seventeen additional patients (7.4%) developed de novo thrombosis after evaluation. Independent risk factors for thrombosis were low platelet count (77.4 (36.3) v 111.6 (69.2) 109/l; p = 0.001), a past history of variceal bleeding (47.4% v 29.1%; p = 0.003), and a prolonged interval from listing to transplantation (8.5 (6.8) v 4.8 (4.4) months; p = 0.002). The proportion of partial or complete recanalisation was significantly higher in those who received (8/19) than in those who did not receive (0/10, p = 0.002) anticoagulation. Survival was significantly lower in those who had complete portal vein thrombosis at the time of surgery (p = 0.04).
Conclusion: These results support a systematic screening for splanchnic vein thrombosis in patients awaiting transplantation. They suggest that in these patients, anticoagulation is safe and has a significant impact on recanalisation as well as prevention of extension of thrombosis.
Keywords: portal vein thrombosis, cirrhosis, portal hypertension, vitamin K antagonists, liver transplantation
Splanchnic vein thrombosis is not an uncommon complication in patients with end stage cirrhosis. Estimation of its incidence has been variable from series to series, ranging from 2% to 26 %,1,2 possibly due to different diagnostic criteria and different study populations. Several risk factors have been identified, including severity of liver insufficiency3 as well as inherited or acquired coagulation disorders (that is, factor V Leiden, factor II, and MTHFR gene mutations, antiphospholipid syndrome).4 Interestingly, two or more prothrombotic disorders appear to be frequently associated in patients with portal vein thrombosis.5
In candidates for liver transplantation, the patency of the portal vein is crucial.1,6–9 When partial, portal vein thrombosis is likely to make anastomoses technically difficult and to be a significant source of morbidity and mortality. In particular, it may favour early post transplantation portal vein thrombosis.10 When complete and extensive, portal vein thrombosis represents a definitive contraindication for transplantation unless complex alternative techniques such as caval transposition are used.11,12 However, even when applicable, these alternative techniques were shown to carry high rates of morbidity and mortality.12,13 As waiting times exceed six months in most liver transplant centres, early detection of and treatment for de novo thrombosis in those who did not have evidence of thrombosis at the time of listing is an important issue. Similarly, the ways in which extension of thrombosis could be prevented in those who have partial thrombosis at the time of listing have not been precisely investigated.
The efficacy and safety of anticoagulant therapy has been documented in cases of recent splanchnic vein thrombosis in those without underlying liver disease. It has been shown that anticoagulant therapy prevents the extension of thrombosis and, in some cases, allows complete recanalisation.14 Whether this approach is applicable, efficient, and safe in cirrhotic patients awaiting liver transplantation, especially those with a marked decrease in coagulation factors due to advanced liver insufficiency, has not been documented.
The aims of this study were to determine the prevalence of splanchnic vein thrombosis in candidates for transplantation at the time of evaluation, the incidence of de novo thrombosis while awaiting transplantation, and the usefulness and safety of anticoagulant therapy in patients with either pre-existing or de novo thrombosis.
PATIENTS AND METHODS
A total of 251 consecutive cirrhotic patients listed for a first liver transplantation from January 1996 to December 2001 were analysed. There were 196 males and 55 females. Mean age was 49 (8.5) years (range 15–63). Indications for transplantation were alcoholic cirrhosis in 59, hepatitis B virus (HBV) related cirrhosis in 22, hepatitis C virus (HCV) related cirrhosis in 42, hepatocellular carcinoma (HCC) in 84, primary biliary cirrhosis in nine, primary sclerosing cholangitis in 13, genetic haemochromatosis in three, α1-antitrypsin deficiency in one, and cirrhosis of unknown origin in 18. All patients with HCC had an underling cirrhosis and all had one nodule less than 5 cm or two or three nodules each less than 3 cm according to the current recommendations for transplantation.15 In this subgroup, the origin of the underlying cirrhosis was alcohol in 16, HBV infection in 18, HCV infection in 41, haemochromatosis in four, and miscellaneous causes in five. Whatever the number and size of the nodules, patients with evidence of portal invasion or extrahepatic metastases were excluded. Patients with Budd-Chiari syndrome evaluated during the study period were excluded.
The characteristics of the study population at the time of evaluation are shown in table 1 ▶.
Table 1.
Baseline characteristics of the study population
| Patients (n = 251) | |
| Age (y) | 49 (8) |
| Sex (M/F) | 196/55 |
| Past history of variceal bleeding (%) | 26 |
| Refractory ascites (%) | 28 |
| Bilirubin (μmol/l) | 66 (73) |
| Albumin (g/l) | 35 (8) |
| Prothrombin (% of normal) | 58 (20) |
| International normalised ratio | 1.7 (0.6) |
| Creatinine (μmol/l) | 86 (78) |
| Child’s grade (A/B/C, %) | 26/41/33 |
| MELD score | 13 (5) |
At the time of evaluation for transplantation, all candidates underwent detailed abdominal imaging investigations including Doppler ultrasonography (Doppler-US) and helical computed tomography (CT), techniques which have been proved to be highly accurate for diagnosing splanchnic vein thrombosis.16,17 Special attention focused on the patency of the portal vein and its branches, the mesenteric vein, the splenic vein, and the hepatic veins. When needed, these investigations were completed by angiography (n = 22) and/or magnetic resonance imaging (MRI) (n = 10). In addition, three patients with HCC and portal vein thrombosis for whom it was impossible to rule out malignant vascular invasion on the basis of imaging techniques, had US guided biopsy of the thrombus. In all three patients malignant vascular invasion was ruled out by histological examination.
From the time of listing up to transplantation, all patients underwent systematic screening at three month intervals while awaiting a donor. This screening included clinical assessment, biochemical tests, and Doppler-US. Patients with HCC and/or those who had evidence of splanchnic vein thrombosis at evaluation also had a CT scan every three months. Similarly, patients who were found to have de novo thrombosis with Doppler-US had a CT scan and/or MRI to document precisely the extent of thrombosis.
From 1996 to 1998, patients with either partial or complete thrombosis of the splanchnic veins did not receive anticoagulation until transplantation. However, these patients had a careful screening, as described above. Either at the time of evaluation or during follow up, evidence of extensive thrombosis involving the portal and mesenteric veins was considered a contraindication for transplantation, unless there was subsequent recanalisation. In contrast, patients in whom the proximal portion of the portal vein and mesenteric vein were patent were still considered as eligible for transplantation. Patients with partial thrombosis were also considered eligible.
From 1999 to 2001, we adopted a policy of administration of anticoagulant therapy to patients who had either splanchnic vein thrombosis at the time of listing or de novo thrombosis while awaiting a graft. This policy was adopted on the basis of the positive results which had been reported with anticoagulation in non-cirrhotic patients with portal or mesenteric vein thrombosis.14,18 Anticoagulation consisted of low molecular weight heparin (nadroparin, 5700 UI/day subcutaneously) followed by vitamin K antagonists (acenocoumarol). When vitamin K antagonists were started, low molecular weight heparin was discontinued after a minimum of five days and when the international normalised ratio (INR) was at least 2.0. Doses of vitamin K antagonists were adjusted in order to achieve an INR of 2.0–3.0. INR values were determined every 2–3 weeks for dose adjustments. Each dose adjustment consisted of an increase or decrease in the daily acenocoumarol dose of 1–2 mg. This modified protocol was approved by our local ethics committee. Contraindications for transplantation with respect to extent of thrombosis were similar during the two study periods. Indications for transplantation were not significantly different during the two time periods.
At the time of the transplantation procedure, splanchnic vessels were assessed by direct examination and intraoperative Doppler-US. If needed, coagulation disorders were reversed by administration of fresh frozen plasma during the initial phase of transplantation procedure.
Results for continuous variables are expressed as means (SD). Student’s t test, χ2 test, Fisher’s exact test, Mann-Whitney test, analysis of variance, and logistic regression analysis were used where appropriate. Survival analysis was performed by the Kaplan-Meier method and comparisons were made by log rank test. For all tests, p<0.05 was considered statistically significant. Analysis was performed using SPSS (Chicago, Illinois, USA) and SAS (Cary, North Carolina, USA) software.
RESULTS
Splanchnic vein thrombosis at the time of listing and incidence on the waiting list
Twenty one of 251 patients (8.4%) had splanchnic vein thrombosis at the time of listing for transplantation. Fourteen had partial portal vein thrombosis with an extension to the superior mesenteric vein in four and to the splenic vein in one. Thrombosis was limited to the right portal branch in five cases and involved both right and left branches in four.
Eight additional patients (3.2 %) developed de novo thrombosis while awaiting a donor, 12.1 (7.7) (range 4–27) months, on average, after listing. Six of these eight patients had partial portal vein thrombosis with an extension to the superior mesenteric vein in one. The two remaining patients developed complete portal vein thrombosis with an extension to the right and left intrahepatic branches. These two patients were not delisted because the proximal part of the portal vein as well as the mesenteric vein remained patent.
Overall, 28 died on the waiting list (11%), 4.8 (5.7) months, on average, after listing. Twelve (5%) were delisted because of a contraindication other than thrombosis and five (2%) were delisted because of a significant improvement. The remaining 206 patients were transplanted a mean of 5 (4.7) months after listing.
At the time of transplantation surgery, nine patients who did not have evidence of splanchnic vein thrombosis either at evaluation or during screening after listing were found to have extrahepatic portal vein thrombosis. Thrombosis was partial in eight patients and complete in one. All transplanted patients had detailed examination of the resected liver and none of those with HCC or thrombosis had evidence of tumoral vascular invasion.
In this population, a total of 38 patients (15.1%) were found to have splanchnic vein thrombosis at any time during the study period. Seventeen of 230 patients (7.4%) who did not have thrombosis at evaluation developed de novo thrombosis documented either during follow up on the waiting list or peroperatively. Neither at the time of initial evaluation nor during follow up did we observe discordance between Doppler-US and helical CT.
Risk factors for splanchnic vein thrombosis
Univariate analysis showed that the risk for splanchnic vein thrombosis was significantly associated with platelet count, a history of ascites, Child-Pugh score, a past history of variceal bleeding, and the technique used for endoscopic treatment (table 2 ▶). Platelet count was significantly lower in the 17 patients with de novo thrombosis or thrombosis at the time of surgery than in the remaining 229 patients (73 (43) v 108 (67) 109/l; p = 0.03). Multivariate analysis showed that platelet count and a past history of variceal bleeding were the only independent predictive factors for thrombosis (p = 0.001 and 0.003, respectively). It is worth noting that platelet count was inversely related to the probability of thrombosis.
Table 2.
Risk factors for splanchnic vein thrombosis: results of univariate analysis
| Splanchnic vein thrombosis | p Value | ||
| Yes (n = 38) | No (n = 213) | ||
| Age (y) | 49.2 (6.9) | 49.5 (8.8) | 0.86 |
| Sex (M/F) | 28/10 | 168/45 | 0.52 |
| Indication for transplantation | 9 (23.7%) | 50 (23.5 %) | 0.72 |
| Alcohol related cirrhosis | 6 (15.8 %) | 16 (7.5 %) | |
| HBV related cirrhosis | 6 (15.8 %) | 36 (16.9 %) | |
| HCV related cirrhosis | 11 (28.9 %) | 73 (34.3 %) | |
| HCC | 2 (5.3 %) | 20 (9.4%) | |
| Biliary (PBC and PSC) | 4 (10.5 %) | 18 (8.5 %) | |
| Other | |||
| Prothrombin time (% of normal) | 52.6 (17.8) | 58.7 (20.6) | 0.08 |
| Factor V (% of normal) | 54.5 (21.2) | 62.1 (25.7) | 0.09 |
| Bilirubin (μmol/l) | 54.6 (79.4) | 68.1 (71.6) | 0.29 |
| Albumin (g/l) | 33 (5.5) | 34.8 (7.9) | 0.20 |
| Creatinine (μmol/l) | 77.7 (19.5) | 88 (83.8) | 0.45 |
| Platelets count (109/l) | 77.4 (36.3) | 111.6 (69.2) | 0.001 |
| Episodes of encephalopathy | 12/38 (31.6%) | 49/213 (23%) | 0.18 |
| Chronic encephalopathy | 2/38 (5.3%) | 14/213 (6.6%) | 0.30 |
| Episodes of ascites | 30/38 (78.9%) | 126/213 (59.2%) | 0.009 |
| Refractory ascites | 15/38 (39.5%) | 55/213 (25.8%) | 0.11 |
| Spontaneous bacterial peritonitis | 6/38 (15.8%) | 33/213 (15.5%) | 0.96 |
| Child-Pugh score (A/B/C) | 4/21/13 | 61/83/69 | 0.04 |
| MELD score | 12.7 (5) | 11.5 (6.6) | 0.80 |
| Oesophageal varices | 0.22 | ||
| None/grade1/grade 2/grade 3 | 5/9/18/6 | 51/42/101/19 | |
| Variceal bleeding | 18/38 (47.4%) | 48/165 (29.1) | 0.001 |
| Endoscopic treatment | 0.07 | ||
| Sclerosis/ligation | 8/4 | 20/13 | |
| Abdominal surgery in the past | 13/38 (34.2%) | 56/213 (26.3%) | 0.32 |
HBV, hepatitis B virus; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; PBC, primary biliary cirrhosis, PSC, primary sclerosing cholangitis.
In patients who did not have splanchnic vein thrombosis at the time of listing, the risk of de novo thrombosis was significantly and independently associated with the time interval between listing and transplantation (8.5 (6.8) months v 4.8 (4.4) months; p = 0.002), along with the two factors cited above (that is, platelet count and a past history of variceal bleeding).
The direction of portal blood flow was precisely assessed by Doppler-US in 60 of 251 patients. Fifty five of these 60 patients (92%) (15 of whom had splanchnic vein thrombosis) had hepatopetal portal flow. None of the 15 patients with splanchnic vein thrombosis were found to have inverted portal flow. In parallel, none of the five patients with inverted portal flow on Doppler-US had portal vein thrombosis during follow up.
Impact of anticoagulant therapy in patients with splanchnic vein thrombosis
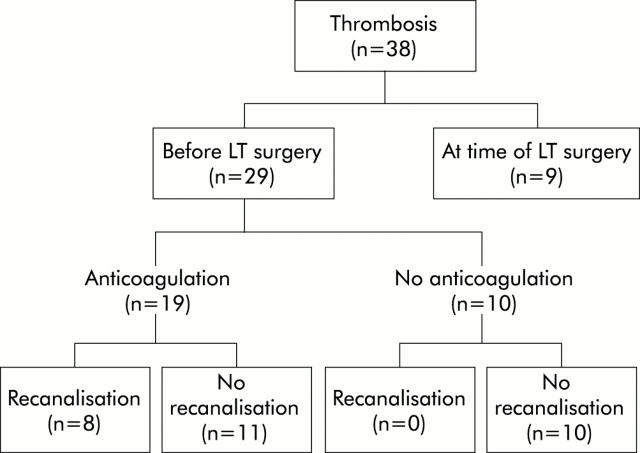
Among 29 patients who had either splanchnic vein thrombosis at evaluation or de novo thrombosis while on the waiting list, 19 (65.5%) received anticoagulation according to the protocol detailed above and 10 (34.5%) did not receive anticoagulation. Baseline criteria were comparable in the two groups except for past history of variceal bleeding which was significantly more frequent in those receiving anticoagulation (table 3 ▶). The location and extent of thrombosis was not significantly different whether or not patients received anticoagulation (table 3 ▶). In this group of patients with thrombosis, the waiting time was 4.4 (4.8) months in those who did not receive anticoagulation compared with 8.1 (6.5) months in those who received anticoagulation. This difference was not statistically significant.
Table 3.
Baseline characteristics of patients with splanchnic vein thrombosis diagnosed before transplantation surgery according to whether or not they received anticoagulation
| Anticoagulation | p Value | ||
| No (n = 10) | Yes (n = 19) | ||
| Age (y) | 52 (5.7) | 48.7 (7.5) | 0.22 |
| Sex (M/F) | 7/3 | 13/6 | 0.93 |
| Child-Pugh’s grade (A/B/C) | 2/6/2 | 2/13/4 | 0.78 |
| Meld score | 11.8 (6.2) | 12.8 (4.3) | 0.43 |
| Prothrombin time (% of normal) | 60.3 (17.9) | 51.9 (16.4) | 0.22 |
| International normalised ratio | 1.51 (6.25) | 1.76 (4.34) | 0.20 |
| Bilirubin (μmol/l) | 79.1 (146.5) | 43.5 (33.8) | 0.32 |
| Albumin (g/l) | 34.6 (2.5) | 34.3 (5.8) | 0.90 |
| Creatinine (μmol/l) | 81.6 (22.8) | 75.8 (19.9) | 0.49 |
| Platelet count (109/l) | 79.3 (33) | 74.4 (40.5) | 0.74 |
| Oesophageal varices | |||
| None/grade1/grade 2/grade 3 | 2/1/5/2 | 2/5/8/4 | 0.61 |
| Variceal bleeding (present/absent) | 2/8 | 14/5 | 0.01 |
| Initial extension of thrombosis (partial/complete) | 10/0 | 18/1 | 1 |
| De novo thrombosis | 2/10 | 6/19 | 0.57 |
| Interval from listing to transplantation or death (months) | 5.8 (4.6) | 7.9 (6.2) | 0.34 |
| Location of thrombosis | |||
| Portal vein trunk | 6/10 | 8/19 | 0.44 |
| Right branch | 4/10 | 9/19 | 0.70 |
| Left branch | 3/10 | 1/19 | 0.1 |
Among 19 patients who received anticoagulation, eight (42.1%) were found to have complete recanalisation, defined by the absence of intravascular material on imaging techniques in addition to restored blood flow on Doppler-US, between initiation of treatment and transplantation. Seven of these eight patients had partial thrombosis and one had complete thrombosis at the time of initiation of anticoagulation. Thrombosis was unchanged in 10 patients and extended despite anticoagulation in the remaining one. In this group of patients who received anticoagulation, the proportion of de novo thrombosis was not significantly different in those who had recanalisation (3/8) compared with those who had stabilisation or extension (3/11). Figure 1 ▶ (A, B) shows sequential MRI in a patient who had a partially obstructive thrombus at the junction of the splenic and mesenteric veins and had complete recanalisation with anticoagulation.
Figure 1.
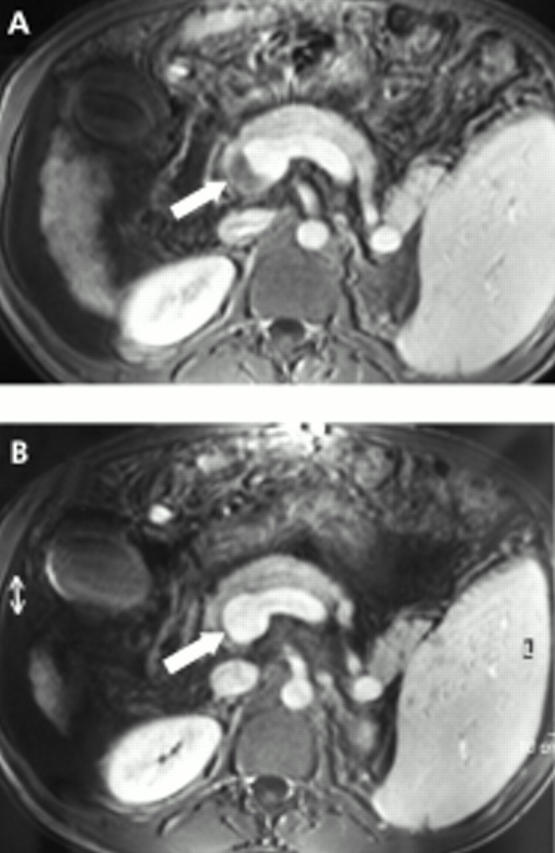
(A, B) Post contrast magnetic resonance imaging showing a partially obstructive thrombus at the junction of the splenic and mesenteric veins (A, white arrow) and complete resolution of the thrombus three months after initiation of anticoagulation (B, white arrow) in a patient with hepatitis C virus related cirrhosis.
Among the 10 patients who did not receive anticoagulation, none had complete recanalisation. Thrombosis was unchanged in four and extended in six. Overall, the proportion of patients who had recanalisation was significantly higher in those who received (8/19) than in those who did not receive (0/10, p = 0.002) anticoagulation.
Among the 19 patients who received anticoagulation, only one had an upper digestive tract bleeding episode after prophylactic variceal band ligation. This bleeding episode was due to a post ligation ulceration in the oesophagus and the course was favourable with antisecretory agents and transfusion of two units of packed red cells. No other side effect occurred during waiting time and anticoagulation did not have to be discontinued in any patient.
Impact of thrombosis and anticoagulation therapy on surgery and post transplantation survival
Among 29 patients who had splanchnic vein thrombosis at evaluation or de novo thrombosis while on the waiting list and were transplanted, mean duration of the procedure was not significantly different whether or not they received (11.2 (2) hours) or did not receive (10.6 (1.3) hours) anticoagulation. Similarly, red blood cell transfusions were not significantly different in the two groups (5.4 (3.7) v 5.2 (3) units in patients with and without anticoagulation, respectively). Therefore, there was no evidence that anticoagulation by itself increased peroperative blood loss or duration of surgery. However, considering the whole population, blood transfusions were significantly more important in those who had splanchnic vein thrombosis at the time of surgery (7.6 (4.8) units) compared with those who did not have splanchnic vein thrombosis (2.8 (3.6) units, p = 0.0004).
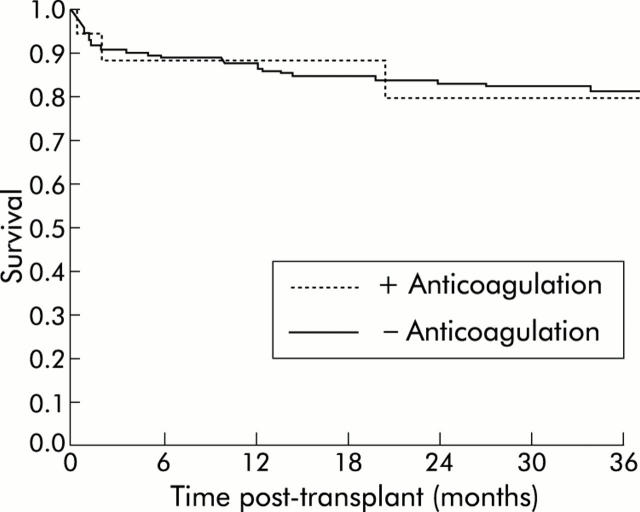
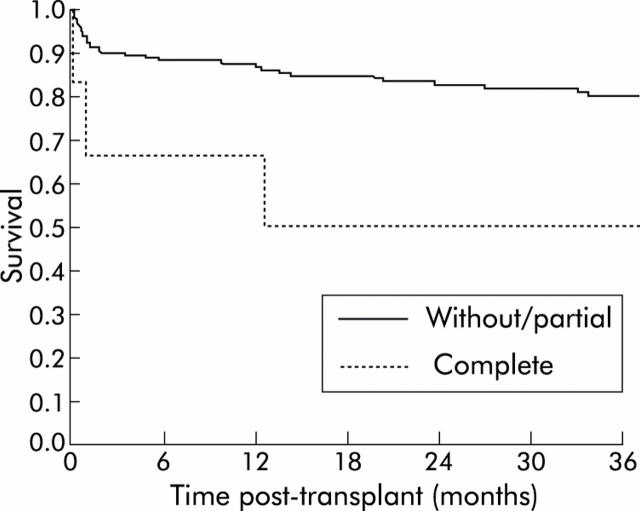
Two year post transplantation survival was 83% in the group with a patent portal vein at the time of transplantation, 82% in the group with partial portal vein thrombosis, and 50% in the group with complete portal vein thrombosis (fig 2 ▶). Post transplantation survival was not significantly different in those without portal vein thrombosis compared with those with partial thrombosis. In contrast, survival was significantly higher in patients with either patent portal vein or partial thrombosis compared with those with complete thrombosis (83% v 50% two year survival, respectively; p = 0.04) (fig 3 ▶). In parallel, in the subgroup of patients who had portal vein thrombosis at the time of transplantation, complete thrombosis was significantly more frequent in those who did not receive anticoagulation (4/5) compared with those who did (0/12, p<0.0001). In patients without portal vein thrombosis or partial thrombosis, survival was not significantly different whether or not they received anticoagulation (79% v 83% two year survival rate, respectively). In patients who did not have splanchnic vein thrombosis either at evaluation or during follow up, two year survival rate was similar during the two time periods of the study (1996–1998 v 1999–2001, 84% v 84%).
Figure 2.
Post transplantation survival in patients with patent portal vein or partial portal vein thrombosis who received or did not receive anticoagulation.
Figure 3.
Post transplantation survival in patients without portal vein thrombosis or with partial portal vein thrombosis and in those with complete portal vein thrombosis (p = 0.04).
The disposition of patients according to the presence of thrombosis and response to anticoagulation is shown in figs 4 ▶ and 5 ▶.
Figure 4.
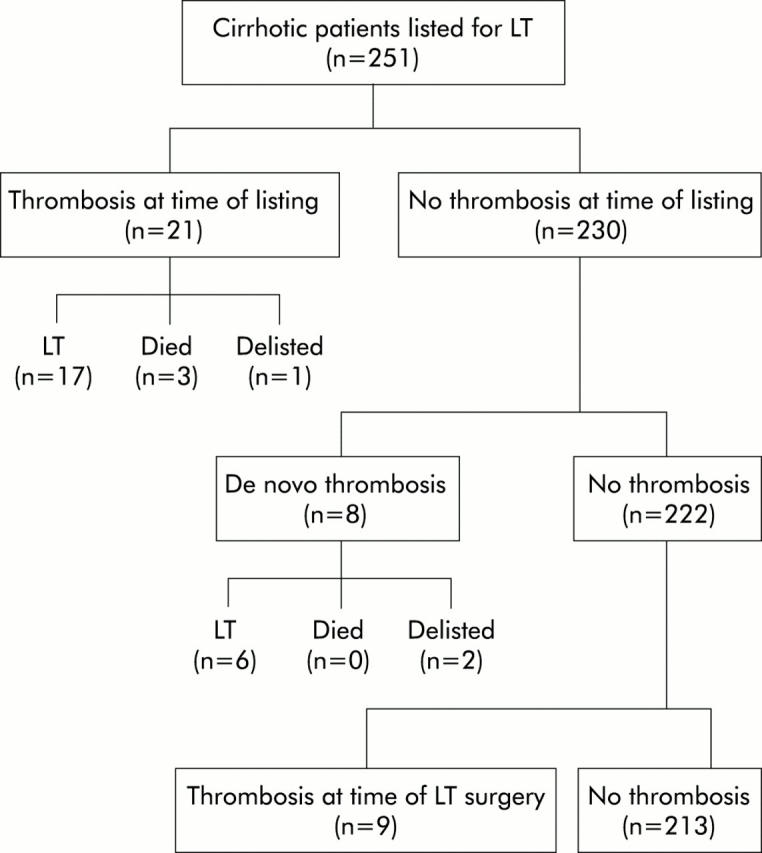
Disposition of the patients in the study population according to the presence or absence of thrombosis and the timing of diagnosis of thrombosis. LT, liver transplantation.
Figure 5.
Disposition of the patients with thrombosis according to anticoagulation and response to therapy. LT, liver transplantation.
DISCUSSION
In the present series, 8.4% of patients were found to have splanchnic vein thrombosis at evaluation. This proportion probably underestimates the true incidence of splanchnic vein thrombosis during end stage cirrhosis. Indeed, a significant proportion of patients with extensive thrombosis may not have been referred to our centre because they were not considered as suitable candidates for transplantation and therefore were not taken into account for analysis. None the less, eight of 230 (3.2%) patients who did not have thrombosis at evaluation developed thrombosis while on the waiting list. Importantly, the majority of these patients (6/8) only had partial portal vein thrombosis. Although pretransplantation Doppler-US screening was performed at three month intervals, nine additional patients were found to have thrombosis at the time of surgery. Again, eight of these nine patients had only partial thrombosis and transplantation was technically feasible in all cases.
Multivariate analysis showed that the only independent predictive factors for thrombosis were platelet count and a past history of variceal bleeding. Surprisingly, the risk of thrombosis was inversely correlated to platelet count. It was significantly higher in those patients who had the lowest platelet count. This paradoxical finding illustrates the fact that, with respect to splanchnic vein thrombosis, the impact of portal hypertension, as reflected by hypersplenism, outweighs the proper effects of low platelet count in preventing thrombosis. It also illustrates the fact that cirrhotic patients with a marked decrease in platelet count as well as decreased coagulation factors are not protected against portal vein thrombosis. In this series, we failed to show a significant relation between reversed portal blood flow on Doppler-US and de novo thrombosis. Although the direction of portal flow was only assessed in a subgroup of 60 patients, in this subgroup, hepatofugal portal flow was present in less than 10%. This proportion is in line with that observed in other series.19–21 A more precise evaluation of the direction of portal flow and blood velocity with Doppler-US could help predict more accurately the risk of thrombosis. We found an independent correlation between a past history of variceal bleeding and thrombosis. It could be argued that the increased incidence in variceal bleeding might be a consequence of thrombosis, leading to an increase in portal hypertension. However, we also found that among those who did not have thrombosis at evaluation, a history of variceal bleeding prior to evaluation was also an independent risk factor for de novo thrombosis. These findings suggest that episodes of variceal bleeding, reflecting the severity of portal hypertension, are more likely to be predisposing factors for, rather than a consequence of, thrombosis itself. Therefore, variceal bleeding seems unlikely to be secondary to thrombosis. The proportion of patients who had endoscopic treatment of varices, either sclerosis or elastic band ligation, was not significantly different between those who had thrombosis and those who did not. However, it remains impossible to exclude definitively that in some cases endoscopic treatment favoured the occurrence of thrombosis.
In this series, the global incidence of de novo thrombosis (7.4%, including thrombosis diagnosed at the time of surgery) could be considered relatively low. However, this low incidence must be interpreted in the light of the relatively short duration on the waiting list (mean 3.8 (3) months for those who were transplanted) in the present series. As the interval between listing and transplantation was an independent predictive factor for thrombosis, the incidence of de novo thrombosis could be much higher in populations for which waiting times exceed six months, which gives further support for a systematic screening policy.
The most important finding in this study was that the proportion of partial or complete recanalisations was significantly higher in those who had received anticoagulation than in those who did not. The two groups were not randomised but they corresponded to two different time periods with two different policies concerning anticoagulation. The characteristics of the patients were comparable in the two groups except for past variceal bleeding. However, past variceal bleeding was more frequent in those who received anticoagulation. Finally, none of the patients who did not receive anticoagulation had partial or complete recanalisation. Therefore, together with the positive results which have been reported in non-cirrhotic patients with portal vein thrombosis,14,18 these results strongly suggest that anticoagulation had a significant advantage over absence of specific therapy. A randomised study would avoid the potential limitations related to the use of an historical control group. It can be estimated that, given the data from the present series, a study population of 120 patients (two groups of 60 patients each) would be necessary to demonstrate a significant difference in the rate of recanalisation with or without anticoagulation.
Systematic screening based on repeated Doppler-US in those without thrombosis at evaluation made it possible to detect de novo thrombosis, to start anticoagulation, and to prevent an extension of thrombosis or, in some cases, allow complete recanalisation. Therefore, early detection of de novo thrombosis followed by anticoagulation reduced the risk of complete portal vein thrombosis, a recognised technical difficulty at the time of transplantation.
Until now, most groups have been reluctant to use anticoagulation in candidates for transplantation with portal hypertension because, among other inconveniences, it could potentially precipitate variceal bleeding episodes. In addition, it has been suspected that anticoagulation could favour bleeding at the time of surgery. Finally, the usefulness of anticoagulation itself in patients with decreased coagulation factors and low platelet count was regarded as questionable. However, our results as well as those from other studies suggest that anticoagulation may gain wider acceptance. Firstly, there is no clear evidence that, within the therapeutic range, anticoagulation has a significant impact on the risk of variceal bleeding.18 Indeed, variceal bleeding is essentially influenced by portal hypertension and in our study population only one of 19 patients receiving anticoagulation had an episode of bleeding. Secondly, we did not find evidence that anticoagulation itself increased the duration of transplantation procedure or blood loss. In contrast, those who had portal vein thrombosis, whether or not they received anticoagulation, had significantly more transfusions of red blood cell units. Again, these findings indicate that, as well as variceal bleeding, the effects of anticoagulation on splanchnic vein patency may overcome its potential impact on haemostasis and surgical bleeding. The optimal anticoagulation regimen in the pre-transplantation setting has to be determined. However, one of the advantages of vitamin K antagonists over heparin derivates was that their effects could be rapidly corrected by transfusion of fresh frozen plasma at the beginning of the transplant procedure.
This study confirms the results of others showing that complete portal vein thrombosis is associated with a significant decrease in post transplantation survival.6,22 In contrast, we found that partial portal vein thrombosis did not have a significant impact on survival. The reasons for this difference in terms of mortality are probably multifactorial, including among other factors, technical difficulties during hepatectomy (due to the presence of a cavernoma and marked portal hypertension) and vascular reconstruction, abundant transfusions, early recurrence of portal vein thrombosis, and graft dysfunction. None the less, it is worth noting that this survival difference was essentially marked by an excess of early post transplantation mortality, as shown by an early drop in the survival curve (fig 3 ▶). Provided the proximal portion of the portal vein and/or superior mesenteric vein remain patent, complete portal vein thrombosis might not be considered as a definitive contraindication for liver transplantation. In patients who are known to have complete portal vein thrombosis, harvesting of the maximum length of splanchnic veins in the donor and, if necessary, the use of veno-venous bypass during the procedure may improve technical feasibility.
In conclusion, the results of this study support systematic screening for de novo thrombosis in patients awaiting transplantation. Complete portal vein thrombosis, although it may not represent a definitive contraindication for transplantation in all cases, is associated with significant morbidity and mortality. Even if the incidence of de novo thrombosis is relatively low, Doppler-US allows the detection of thrombosis at an early stage and anticoagulation to start, a treatment which, on the basis of our findings, might help prevent extension or achieve recanalisation. As portal vein patency had a significant impact on survival, our results also suggest that the benefits of anticoagulation outweigh its potential inconveniences in terms of bleeding and peroperative transfusions. A three month interval between Doppler-US examinations does not exclude the fact that de novo thrombosis may be discovered at the time of surgery. Monthly screening, although incurring additional costs, might reduce this risk. Overall, a more precise assessment of portal blood flow as well as prothrombotic states may help better define high risk populations for splanchnic vein thrombosis among candidates for transplantation.
Abbreviations
HBV, hepatitis B virus
HCV, hepatitis C virus
HCC, hepatocellular carcinoma
Doppler-US, Doppler ultrasonography
MRI, magnetic resonance imaging
CT, computed tomography
INR, international normalised ratio
Conflict of interest: None declared.
REFERENCES
- 1.Nonami T, Yokoyama I, Iwatsuki S, et al. The incidence of portal vein thrombosis at liver transplantation. Hepatology 1992;16:1195–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Okuda K, Ohnishi K, Kimura K, et al. Incidence of portal vein thrombosis in liver cirrhosis. An angiographic study in 708 patients. Gastroenterology 1985;89:279–86. [DOI] [PubMed] [Google Scholar]
- 3.Yerdel MA, Gunson B, Mirza D, et al. Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation 2000;69:1873–81. [DOI] [PubMed] [Google Scholar]
- 4.Amitrano L, Brancaccio V, Guardascione MA, et al. Inherited coagulation disorders in cirrhotic patients with portal vein thrombosis. Hepatology 2000;31:345–8. [DOI] [PubMed] [Google Scholar]
- 5.Denninger MH, Chait Y, Casadevall N, et al. Cause of portal or hepatic venous thrombosis in adults: the role of multiple concurrent factors. Hepatology 2000;31:587–91. [DOI] [PubMed] [Google Scholar]
- 6.Lerut J, Tzakis AG, Bron K, et al. Complications of venous reconstruction in human orthotopic liver transplantation. Ann Surg 1987;205:404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMaster P . Liver transplantation in the presence of portal vein thrombosis. HPB Surg 1992;5:217–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karatzas T, Lykaki-Karatzas E, Demirbas A, et al. Management of portal vein thrombosis in liver transplantation. Transplant Proc 1997;29:2866–7. [DOI] [PubMed] [Google Scholar]
- 9.Robles R, Parrilla P, Hernandez Q, et al. Liver transplantation in cirrhotic patients with thrombosis of the portal vein. Transplant Proc 1999;31:2415. [DOI] [PubMed] [Google Scholar]
- 10.Davidson BR, Gibson M, Dick R, et al. Incidence, risk factors, management, and outcome of portal vein abnormalities at orthotopic liver transplantation. Transplantation 1994;57:1174–7. [DOI] [PubMed] [Google Scholar]
- 11.Langnas AN, Marujo WC, Stratta RJ, et al. A selective approach to preexisting portal vein thrombosis in patients undergoing liver transplantation. Am J Surg 1992;163:132–6. [DOI] [PubMed] [Google Scholar]
- 12.Olausson M, Norrby J, Mjornstedt L, et al. Liver transplantation using cavoportal hemitransposition—a life-saving procedure in the presence of extensive portal vein thrombosis. Transplant Proc 2001;33:1327–8. [DOI] [PubMed] [Google Scholar]
- 13.Stieber AC, Zetti G, Todo S, et al. The spectrum of portal vein thrombosis in liver transplantation. Ann Surg 1991;213:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Condat B, Pessione F, Denninger MH, et al. Recent portal or mesenteric venous thrombosis: increased recognition and frequent recanalization on anticoagulant therapy. Hepatology 2000;32:466–70. [DOI] [PubMed] [Google Scholar]
- 15.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693–9. [DOI] [PubMed] [Google Scholar]
- 16.Miller VE, Berland LL. Pulsed Doppler duplex sonography and CT of portal vein thrombosis. AJR Am J Roentgenol 1985;145:73–6. [DOI] [PubMed] [Google Scholar]
- 17.Marn CS, Francis IR. CT of portal venous occlusion. AJR Am J Roentgenol 1992;159:717–26. [DOI] [PubMed] [Google Scholar]
- 18.Condat B, Pessione F, Hillaire S, et al. Current outcome of portal vein thrombosis in adults: risk and benefit of anticoagulant therapy. Gastroenterology 2001;120:490–7. [DOI] [PubMed] [Google Scholar]
- 19.Gaiani S, Bolondi L, Li Bassi S, et al. Prevalence of spontaneous hepatofugal portal flow in liver cirrhosis. Clinical and endoscopic correlation in 228 patients. Gastroenterology 1991;100:160–7. [DOI] [PubMed] [Google Scholar]
- 20.Kawasaki T, Moriyasu F, Nishida O, et al. Analysis of hepatofugal flow in portal venous system using ultrasonic Doppler duplex system. Am J Gastroenterol 1989;84:937–41. [PubMed] [Google Scholar]
- 21.Taourel P, Blanc P, Dauzat M, et al. Doppler study of mesenteric, hepatic, and portal circulation in alcoholic cirrhosis: relationship between quantitative Doppler measurements and the severity of portal hypertension and hepatic failure. Hepatology 1998;28:932–6. [DOI] [PubMed] [Google Scholar]
- 22.Manzanet G, Sanjuan F, Orbis P, et al. Liver transplantation in patients with portal vein thrombosis. Liver Transpl 2001;7:125–31. [DOI] [PubMed] [Google Scholar]