Chronic idiopathic intestinal pseudo obstruction (CIIP) is a severe condition presenting with abdominal pain and dysmotility. Inflammatory or degenerative changes of the autonomic nervous system or of the muscles of the bowel have been observed in CIIP.1,2 As patients with inflammatory bowel disease (IBD) may show clinical3,4 and histological5 signs of autonomic neuropathy and dysmotility,6,7 the aim of this study was to examine whether there is an association between CIIP and IBD.
Six patients at our hospital presenting with signs and symptoms of intestinal dysmotility were diagnosed with CIIP based on clinical features, antroduodenojejunal manometry, and full thickness biopsies (table 1 ▶).8,9 Patient No 1 had an acute erosive colitis some years previously with bloody diarrhoea and an enhanced sedimentation rate, which was treated with steroids, and patient No 2 had relapsing proctitis treated with 5-aminosalicylic acid (5-ASA). Patient No 3 was totally and patient No 4 partially colectomised because of slow transit constipation. Patient No 6 was proctocolectomised due to refractory colitis. The patients were further investigated with magnetic resonance (MR) enterography10 and video capsule enteroscopy to establish whether there were any signs of IBD. If these examinations showed any pathology, push enteroscopy and ileocolonoscopy were also performed. All biopsies collected over the years were re-evaluated.
Table 1.
Summary of the findings in our patients
| Patient No: age (y)/sex | Debut age/CIIP diagn age (y) | Main symptoms | Clinical diagnosis | Endoscopic pathology | Histopathology | Antroduodenal manometry |
| 1 23/F | 16/22 | Pain, bloody diarrhoea | Crohn’s disease, CIIP | Small and large bowel | Degenerative neuropathy. Suspected Crohn’s disease | |
| 2 26/F | 15/25 | Pain, vomiting | Proctitis, CIIP | Rectum | Degenerative neuropathy | Abnormal |
| 3 35/F | Teenage/29 | Constipation, dyspepsia | Crohn’s disease, CIIP | Small and large bowel | Ganglionitis | |
| 4 44/F | 35/39 | Constipation, pain | Suspected Crohn’s disease, CIIP | Small bowel | Normal | Abnormal |
| 5 55/M | 39/41 | GORD, later pain and diarrhoea | Suspected Crohn’s disease, CIIP | Normal | Suspected Crohn’s disease | Abnormal |
| 6 67/M | 61/64 | Pain, weight loss | Crohn’s disease, CIIP | Large bowel | Ganglionitis. Crohn’s disease |
CIIP, Chronic idiopathic intestinal pseudoobstruction; GORD, gastro-oesophageal reflux disease.
MR enterography did not reveal any pathological changes in any of the subjects. In three patients (Nos 1, 3, and 4), video capsule enteroscopy revealed Crohn-like ulcerations/erosions in the stomach and small intestine. Further examination of patient No 1 by push enteroscopy confirmed the erosions in the stomach and one third of the proximal small intestine. In patient No 3, capsule enteroscopy showed aphthous ulcers typical of Crohn’s disease throughout the distal jejunum and ileum (fig 1A ▶). Ileocolonoscopy showed the same picture in the ileum and ileorectal anastomosis.
Figure 1.
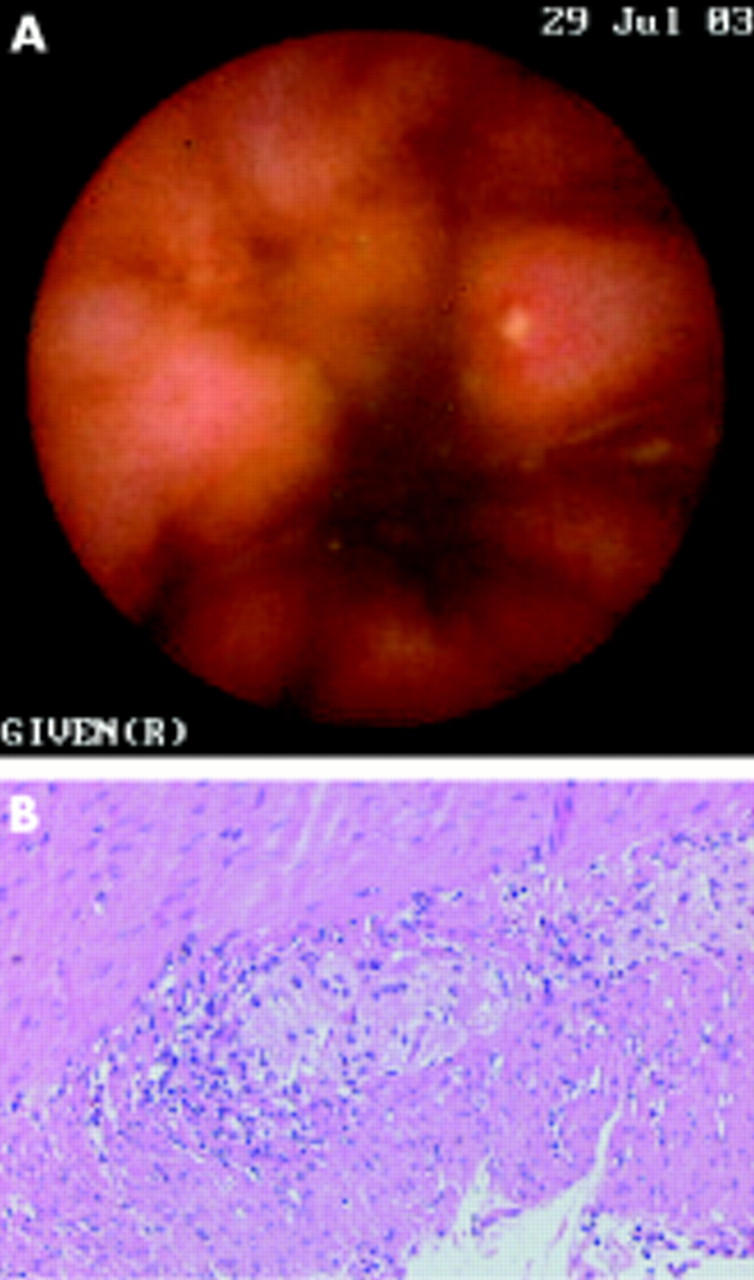
Patient No 3. (A) Capsule endoscopic view of the terminal ileum showing an aphthous ulceration in the ileum. (B) Moderate lymphocytic infiltrate around and within the myenteric ganglia (haematoxylin-eosin ×100).
Histopathological examination of the full thickness biopsies from patient Nos 1 and 2 showed visceral degenerative neuropathy, combined with vacuolisation of the interstitial cells of Cajal (ICCs). In patient Nos 3 and 6, lymphocytic ganglioneuronitis was found in both neural plexa of the resected colon and ileum (fig 1B ▶), with signs of neurone degeneration, and 50% and 80% reduction of ICCs in the perimyenteric ICC-plexus and deep muscular plexus of the circular muscle layer, respectively. Patient No 4 had a normal biopsy, and patient No 5 was not biopsied. Examination of mucosal biopsies from patient No 1 revealed focal active inflammation in the duodenum and caecum, and chronic inflammation in the rectum; patient No 5 had multifocal mild antral cryptitis, and both patients were diagnosed with suspected Crohn’s disease. Colon biopsies from patient No 6 revealed epithelioid cell granulomas and multinucleated giant cells, as well as multifocal transmural lymphoid hyperplasia consistent with Crohn’s disease.
In three patients (Nos 1, 3, and 4), dysmotility preceded the mucosal changes. In patient Nos 2 and 5, these two entities occurred simultaneously, while in patient No 6, dysmotility developed after proctocolectomy. Ganglionitis in patient No 3 could have been caused by Crohn’s disease before other symptoms of the disease developed. Treatment with 5-ASA has reduced her abdominal pain. The normal histology of the sigmoideum in patient No 4 does not exclude the possibility of ganglionitis in other parts of the bowel due to the known patchy involvement of the gut in Crohn’s disease.
The present observations indicate that apart from inflammation, even purely degenerative neuronal and ICCs changes seen in CIIP can occur in patients who also have IBD/an IBD-like condition. At present, it is not known whether the observed abnormalities are part of IBD or independent of each other. This small patient sample prevents us from drawing any definite conclusion regarding this question. Further observations are needed to establish whether or not this connection is causal.
Conflict of interest: None declared.
References
- 1.Patel R, Christensen J. Chronic intestinal pseudo-obstruction: Diagnosis and treatment. The gastroenterologist 1995;3:345–56. [PubMed]
- 2.De Giorgio R, Sarnelli G, Corinaldesi R, et al. Advances in our understanding of the pathology of chronic intestinal pseudo-obstruction. Gut 2004;53:1549–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindgren S, Lijla B, Rosén I, et al. Disturbed autonomic nerve function in patients with Crohn’s disease. Scand J Gastroenterol 1991;26:523–8. [DOI] [PubMed] [Google Scholar]
- 4.Isgar B, Harman M, Kaye MD, et al. Symptoms of irritable bowel syndrome in ulcerative colitis in remission. Gut 1983;24:190–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dvorak AM, Osage JE, Monahan RA, et al. Crohn’s disease: transmission electron microscopic studies. III. Target tissues. Proliferation of and injury to smooth muscle and the autonomic nervous system. Hum Pathol 1980;11:620–34. [DOI] [PubMed] [Google Scholar]
- 6.Manousos ON, Salem SN. Abnormal motility of the small intestine in ulcerative colitis. Gastroenterology 1965;104:249–57. [DOI] [PubMed] [Google Scholar]
- 7.Rao SSC, Read NW, Brown C, et al. Studies on the mechanism of bowel disturbance in ulcerative colitis. Gastroenterology 1987;93:934–40. [DOI] [PubMed] [Google Scholar]
- 8.Stanghellini V, Camilleri M, Malagedala J-R. Chronic idiopathic intestinal pseudo-obstruction: clinical and intestinal manometric findings. Gut 1987;28:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mann SD, Debinski HS, Kamm MA. Clinical characteristics of chronic idiopathic intestinal pseudo-obstruction in adults. Gut 1997;41:675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gourtsoyiannis N, Papanikolaou N, Grammatikakis J, et al. MR eneteroclysis: technical considerations and clinical applications. Eur Radiol 2002;12:2651–8. [DOI] [PubMed] [Google Scholar]


