Abstract
Research into brain-gut interactions, and the use of brain imaging, as potential investigative tools for functional gastrointestinal disorders, such as irritable bowel syndrome, is a promising new area. Studies are beginning to identify the structure and function of regions of the brain and their relationships to pain perception, stress, and other psychosocial variables. These imaging modalities may also have diagnostic potential, and perhaps even therapeutic applications, particularly with regard to understanding the benefit of centrally targeted modalities such as antidepressants and psychological treatments.
Keywords: brain imaging, positron emission tomography, amitriptyline, rectal pain, anterior cingulate cortex, irritable bowel syndrome, functional magnetic resonance imaging
The field of gastroenterology is moving beyond its traditional anatomical boundaries. New cross disciplinary studies, such as basic and translational research, epidemiological, cost, and decision analyses, and genetics are expanding our knowledge of the gastrointestinal disorders, and are creating new opportunities for clinical and health service applications. A particularly promising new development is research into brain-gut interactions, and the use of brain imaging, including positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), as potential investigative tools for functional gastrointestinal disorders (FGIDs).1 Such studies are beginning to identify the structure and function of regions of the brain and their relationships to pain perception, stress, and other psychosocial variables. These imaging modalities may also have diagnostic potential, and perhaps even therapeutic applications, particularly with regard to understanding the benefit of centrally targeted modalities such as antidepressants and psychological treatments.
In this issue of Gut, Morgan and colleagues2 make an original effort to integrate our evolving knowledge of central pain modulatory centres, stress influences on central nervous system (CNS) function, and the effects of antidepressant treatment in patients with irritable bowel syndrome (IBS) (see page 601). The results have important research and clinical implications. However, like all breakthrough studies, their findings may raise more questions than answers, and open the door to future investigations.
To understand the study results and their implications, it is important to first review our developing knowledge on brain-gut interactions as related to pain processing in IBS and the potential role for brain imaging. There are several major developments, listed from the most established to the more speculative areas.
PATIENTS WITH IBS HAVE GREATER GUT REACTIVITY TO VARIOUS STRESSORS
Greater stress reactivity is a hallmark of the disorder, and it is manifested as increased motility and visceral sensitivity to a variety of stimuli, including meals, visceral distension, physical activity, hormonal changes, and psychological stress.3,4 For example, IBS patients compared with control subjects rate rectal distension stimuli significantly higher in intensity and unpleasantness when given physical (for example, cold water immersion) or psychological (dichotomous listening) stress,5 and this is associated with higher emotional reactivity in terms of feeling more stress, anger, and anxiety than control subjects.6
INCREASED VISCERAL SENSITIVITY IS NOT SUFFICIENT TO EXPLAIN PAIN REPORTS OF IBS
The emerging evidence is that central processes, mediated by psychosocial distress, contribute to pain perception, at least as much or more than visceral signals.7 Patients with more severe IBS are distinguished from those with milder IBS by having greater psychological distress and disturbances yet with no differences in visceral sensation thresholds.8 Thus while chronic stress affects reports of pain perception, it does not appear to affect sensory thresholds. In fact, FGID patients with a history of sexual or physical abuse report greater pain9 but have higher visceral sensation thresholds.10 These data highlight the importance of central pain processing in amplifying the perception of visceral signals.
STRESS IS A PERMISSIVE FACTOR IN THE DEVELOPMENT OF CERTAIN FUNCTIONAL GASTROINTESTINAL DISORDERS
This is best demonstrated with post infectious IBS (PI-IBS) where the persistence of functional gastrointestinal symptoms for several months after a bacterial infection is associated not only with increased inflammation in the gut mucosa but also with increased psychological distress present at the time of the initial infection.11,12 In fact, these associations were present even when the abnormal motility and visceral hypersensitivity were similar between PI-IBS patients and those who became asymptomatic after the infection.11 Thus it was the CNS amplification of these peripheral signals in the psychologically distressed group that raised them to conscious awareness, thereby perpetuating the symptoms.13
THE CNS IS “WIRED” TO MODULATE VISCERAL AFFERENT PAINFUL SIGNALS AND RESPONSES TO STRESS
The “gate control” pain system allows for bidirectional signals between the gut and brain. It begins with visceral signals ascending to the CNS via the dorsal horn of the spinal cord, through the thalamus, and then laterally to the somatosensory cortex and medially to the insula, medial thalamus, amygdala, and cingulate cortex. Amplification of these signals can occur at the level of the mucosa via sensitisation from inflammation or injury, at the dorsal horn (central sensitisation), or higher at midbrain structures. In addition, corticofugal pathways from the emotional motor system via the periaqueductal gray and nucleus raphe magnus descend to the dorsal horn where they can amplify or suppress afferent signals from the gut.14,15 Furthermore, these descending pain systems in addition to neuroendocrine (for example, hypothalamic-pituitary-adrenal axis), cognitive-attentional, and autonomic control loci are closely integrated, and mediate and affect stress responses.16 This occurs to a greater degree in patients with IBS who show increased motor, sensory, and autonomic reactivity via these central modulatory systems.16
SPECIFIC AREAS OF THE CORTICOLIMBIC MODULATORY SYSTEM REGULATE VISCERAL PAIN AND EMOTIONAL RESPONSES
Although our understanding of the areas of regional brain activation is only in its infancy, there is some consensus that while there are similar areas of cortical activation, visceral signals tend to activate regions associated with unpleasant affect and autonomic responses while somatic signals activate regions associated with skeletomotor responses and spatial orientation.17,18 Specifically, visceral stimulation activates corticolimbic modulatory systems, including the insular cortex, thalamus and hypothalalamus, right ventrolateral prefrontal cortex, amygdala, and anterior cingulate cortex (ACC).
“The anterior cingulate cortex (ACC) is the region of particular interest with regard to pain regulation, stress response, and the FGIDs”
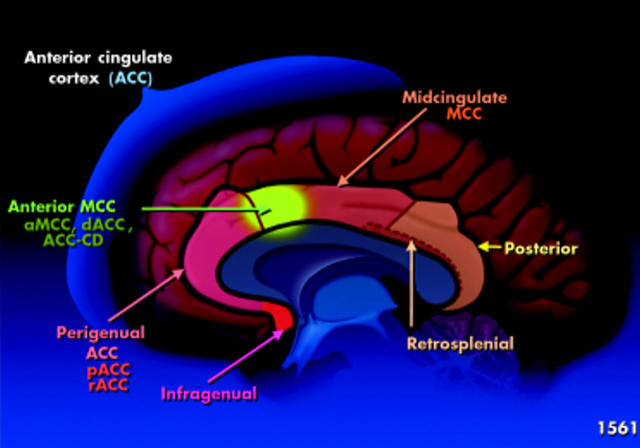
Relevant to this discussion is the cingulate cortex which consists of several structurally and functionally heterogeneous regions, as shown in fig 1 ▶.19,20 For the purpose of this discussion, the ACC is the region of particular interest with regard to pain regulation, stress response, and the FGIDs. This area processes information on stimulus intensity, emotion, mood, and attention, and is also the area involved with unpleasant affect and autonomic responses. It consists of two subregions: (1) an anterior perigenual ACC (pACC), also called the rostral ACC (rACC) and (2) a more dorsal and caudal portion located in the middle of the cingulate gyrus, the mid cingulate cortex (MCC).
Figure 1.
Structure of the cingulate cortex. The anterior region of the mid cingulate cortex (MCC, shown in green) is a subregion called the anterior mid cingulate cortex of the ACC (aMCC); it has a variety of other names including the caudal ACC, the dorsal ACC (dACC), or cognitive division of the ACC (ACC-CD). pACC, anterior perigenual ACC; rACC, rostral ACC.
The anterior region of the MCC (shown in green in fig 1 ▶) is a subregion called the anterior midcingulate cortex of the ACC (aMCC); it has a variety of other names including the caudal ACC, dorsal ACC, or cognitive division of the ACC (ACC-CD). The pACC is linked and sensitively activates with emotions such as happiness and sadness, or in response to anticipation and anxiety. It is involved in many overlapping networks and may be activated or deactivated with pain. Possibly activation of the pACC could result from receiving increasing noxious information or may reflect efforts to try to modulate or decrease such information. It also has connections to the amygdala and may suppress its activity during fear/painful states.21 It contains endogenous opioids such as diprenorphine22 and has high concentrations of opioid receptors23 which in some way links it to pain regulation.
The more caudal or dorsal aMCC is associated with attentional processes, decision making (response selection), and pre-motor activities in response to visceral events that require a recoding of behaviour.20 Recent data also suggest that activation of this aMCC with visceral pain is associated with high levels of fear.20 Thus with painful visceral stimulation, there may be an increase in activity of the pACC associated with emotional distress and an increase in activity of the aMCC (ACC-CD) where pain is coupled with fear and with increased attention to the stimulus and inhibition of motor activity (response selection).15
FUNCTIONAL BRAIN IMAGING CAN DEMONSTRATE DIFFERENCES IN CENTRAL PAIN MODULATORY SYSTEMS BETWEEN PATIENTS WITH IBS AND CONTROLS
PET and fMRI, the most commonly used techniques in FGIDs, provide a window to increases (activation) or decreases (deactivation) in brain function based, respectively, on regional blood flow or degree of oxygenation. With IBS, this is usually done with rectal distension to provide a painful afferent signal that registers a response in the brain which is measured numerically or visually using three dimensional coordinates and t scores to show the degree of activation. The measurements are done with whole brain analyses or are targeted to specific anatomical regions of interest. Usually, the baseline value is subtracted from the stimulated value to give a level of activation (or deactivation), and with comparative analyses the activation level of the control group may be subtracted or contrasted with the target group.
“PET and fMRI, the most commonly used techniques in FGIDs, provide a window to increases (activation) or decreases (deactivation) in brain function based, respectively, on regional blood flow or degree of oxygenation”
With regard to IBS, several published studies indicate differences in levels of activation between patients with IBS and normal controls of these corticolimbic pain modulatory systems. Despite inconsistencies across the studies, in general there is an association of ACC activation to rectal distension in IBS relative to controls.1 Studies using both fMRI and PET show increases in activity of unspecified areas of the ACC compared with controls24 while others show increased activity of the aMCC18,25–28 or pACC29 compared with controls, thus linking emotion or fear with visceral pain in these patients, and others show increased pACC activity in controls relative to IBS.30,31 Some of these studies also show an expected correlation between ACC activation and greater pain reports to rectal distension.24,26,31,32
Unfortunately, the field of brain imaging in FGIDs is not yet developed enough to provide more specific information or to clarify the reported differences in regional brain activation in the FGIDs and their relationship to stress, pain, and emotion. The studies are confounded and methodologically limited because:
activations often involve neural circuitry of several interacting regions16,33,34 (which for simplicity are not discussed) which make it difficult to target single sites,
there may be imaging differences between PET and fMRI,
sex differences exist,35
there are confounding effects on the registration of images to rectal distension with anticipation of that event,36
there may be confounding central influences, such as placebo effects,33
there is clinical heterogeneity among patients with regard to diagnosis and severity of the disorders, and
there are methodological issues in technique, lack of instrument and protocol standardisation, low “signal to noise” ratios, and limitations in measuring functionally heterogeneous regions of the cingulate and other brain regions.
“Differences between IBS and controls do exist and topographical mapping of the regions of activation is an area for future study in IBS”
Thus it is difficult to obtain a consensus across studies on which brain subregions show increased or decreased activity.1 Nevertheless, differences between IBS and controls do exist and topographical mapping of the regions of activation is an area for future study in IBS.
THE BRAIN AREAS OF INTEREST IN IBS ARE ALSO AREAS LINKED TO AND ACTIVATED BY STRESS
Parallel research involving brain imaging within psychiatry relates activation of the ACC and related limbic structures to psychosocial disturbances, including post traumatic stress disorder,37 social rejection,34 and many studies relating to anxiety, depression, and cognitive functioning,38 including the recall of emotional experiences.39 With regard to FGIDs, preliminary data show that in IBS, ACC activation to rectal distension correlates with anxiety,40 stressful life events, maladaptive coping,32 and a history of abuse.30 Furthermore, abuse history and IBS diagnosis appear to have synergistic effects causing even greater activation of the pACC.29 These studies begin to support clinical observations of the connections between psychological distress, IBS, and greater pain reporting.9
BRAIN IMAGING MAY HELP EXPLAIN THE MECHANISMS FOR, AND PERMIT TARGETING OF, SUSCEPTIBLE GROUPS TO CENTRALLY TARGETED TREATMENTS IN IBS
Studies in IBS have begun to show the efficacy of antidepressants,41,42 cognitive behavioural therapy,41,43 hypnosis,44 and other psychological interventions.4 Given their putative actions with regard to central analgesia, stress reduction, and improvement in psychological symptoms, the use of brain imaging may help us understand their mechanisms of action and possibly monitor and predict their effects. With regard to IBS, one case report showed that clinical improvement associated with antidepressants and counselling occurred with a reduction in symptom reports, visceral pain threshold, and aMCC activity,26 and a study of cognitive behavioural treatment showed that when compared with pretreatment values, IBS patients had significant reductions in symptom severity, anxiety, and pACC activity.45 Within psychiatry, brain imaging is now being studied to predict which patients are more likely to respond to antidepressant and other psychotropic treatments, as psychiatric improvement is associated with metabolic changes in the brain that can be registered through these imaging techniques.46,47
“Brain imaging is now being studied to predict which patients are more likely to respond to antidepressant and other psychotropic treatments, as psychiatric improvement is associated with metabolic changes in the brain that can be registered through these imaging techniques”
Given this background, the results of the study of Morgan and colleagues2 in this issue of Gut can be put into clearer perspective. The authors set out to determine whether treatment with a low dose of amitriptyline (not sufficient to treat major depression) in female IBS patients compared with patients not receiving treatment would be associated with changes in ACC activation in response to rectal distension and psychological stress. They found that rectal pain induced significant activation of the pACC, right insula, and right prefrontal cortex, and treatment led to reduced pain related activations of the pACC and left posterior parietal cortex, but only during stress. The conclusion is notable: there is stress related inhibition of brain activation of pACC (along with other areas) in the treated group. This is logical as the pACC is part of the corticolimbic network that modulates the affective and cognitive components of pain perception and is activated in stress. Thus amitriptyline may uncouple the negative effects of stress on pain via the ACC.
The findings are potentially important but far from conclusive due to several methodological limitations, in part related to the infancy of this area of research. Firstly, there was no healthy control group to determine whether these are generalised effects or are more specific to IBS. Secondly, the study may have been underpowered and this may explain only trends in terms of reductions in pain responses to rectal distension in the treated group or in the reduction of aMCC (called “ACC-cognitive” in this study) activation, and the lack of correlation of brain activity with anxiety or depression, all of which might be anticipated to have occurred. Thus this study is difficult to interpret with regard to the clinical relevance of the noted brain activation changes, or whether the effects relate to pain reduction pathways, stress effects, or both. Thirdly, it is unclear the degree to which the treatment effects on depression or other psychological symptoms might confound the brain activation results; although a low dose of amitriptyline was chosen to obviate this possibility, psychological scores were not evaluated for possible interaction effects in this study. Fourthly, we do not know the generalisability of the effects on other clinical populations, including males, or patients with more or less severe symptoms or differing stool patterns. For examples, sex differences in brain activation has been reported,35 and there may be differences in brain responses between milder patients and more severe patients with high degrees of psychosocial morbidity, Fifthly, given the possibility of anticipation and placebo effects, and their independent influences on brain activation, it would be necessary to replicate this study by a parallel design with baseline assessment and to also consider a non-drug control group. Finally, it is beyond our current methodology to evaluate the complex relationships between the relevant regions of interest in the pain neuromatrix33 and also to determine whether the treatment effects are primarily central, peripheral, or both.48
“In the end, it is likely that we may develop more effective treatments for our patients with FGIDs and also truly understand how these treatments are working”
Nevertheless, the study is creative, novel, and at the forefront of investigation in this field. The authors have taken a large step to initiate what will hopefully be a series of investigations to link augmentation of pain and symptom reporting to stress, and responses to centrally active treatments via their central mechanistic associations in IBS. For the future it is likely that the best yield for such studies will occur by identifying specific subgroups amenable to more targeted investigations and then conducting focused assessments. This will involve more precise characterisation of patients and their symptom features, standardisation of physiological and psychological methods, and the use of profiles characterised by specific psychophysiological and biological features.1 In the end, it is likely that we may develop more effective treatments for our patients with FGIDs and also truly understand how these treatments are working.
Abbreviations
FGIDs, functional gastrointestinal disorders
IBS, irritable bowel syndrome
PI-IBS, post infectious IBS
PET, positron emission tomography
fMRI, functional magnetic resonance imaging
CNS, central nervous system
ACC, anterior cingulate cortex
pACC, anterior perigenual ACC
MCC, mid cingulate cortex
aMCC, anterior midcingulate cortex of the ACC
ACC-CD, cognitive division of the ACC
Conflict of interest: None declared.
REFERENCES
- 1.Hobson AR, Aziz Q. Brain imaging and functional gastrointestinal disorders: has it helped our understanding? Gut 2004;53:1198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan V, Pickens D, Gautam S, et al. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut 2005;54:601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drossman DA, Camilleri M, Mayer EA, et al. AGA technical review on irritable bowel syndrome. Gastroenterology 2002;123:2108–31. [DOI] [PubMed] [Google Scholar]
- 4.Drossman DA, Creed FH, Olden KW, et al. Psychosocial aspects of the functional gastrointestinal disorders. In: Drossman DA, Corazziari E, Talley NJ, et al, eds. Rome II. The functional gastrointestinal disorders: diagnosis, pathophysiology and treatment; A multinational consensus, 2nd edn. McLean, VA: Degnon and Associates 2000:157–245.
- 5.Murray CD, Flynn J, Ratcliffe L, et al. Effect of acute physical and psychological stress on gut autonomic innervation in irritable bowel syndrome 1. Gastroenterology 2004;127:1695–703. [DOI] [PubMed] [Google Scholar]
- 6.Dickhaus B, Mayer EA, Firooz N, et al. Irritable bowel syndrome patients show enhanced modulation of visceral perception by auditory stress. Am J Gastroenterol 2003;98:135–43. [DOI] [PubMed] [Google Scholar]
- 7.Whitehead WE, Palsson OS. Is rectal pain sensitivity a biological marker for irritable bowel syndrome: psychological influences on pain perception. Gastroenterology 1998;115:1263–71. [DOI] [PubMed] [Google Scholar]
- 8.Drossman DA, Whitehead WE, Toner BB, et al. What determines severity among patients with painful functional bowel disorders? Am J Gastroenterol 2000;95:974–80. [DOI] [PubMed] [Google Scholar]
- 9.Drossman DA, Li Z, Leserman J, et al. Health status by gastrointestinal diagnosis and abuse history. Gastroenterology 1996;110:999–1007. [DOI] [PubMed] [Google Scholar]
- 10.Ringel Y, Whitehead WE, Toner BB, et al. Sexual and physical abuse are not associated with rectal hypersensitivity in patients with irritable bowel syndrome. Gut 2004;53:838–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gwee KA, Leong YL, Graham C, et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut 1999;44:400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunlop SP, Jenkins D, Neal KR, et al. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfections IBS. Gastroenterology 2003;125:1651–9. [DOI] [PubMed] [Google Scholar]
- 13.Drossman DA. Mind over matter in the postinfective irritable bowel. Gut 1999;44:306–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melzack R, Wall P. Gate-control and other mechanisms. In: Melzack R, Wall P, eds. The challenge of pain, 2nd edn. London: Pelican Books, 1988:165–93.
- 15.Vogt BA, Sikes RW. The medial pain system, cingulate cortex, and parallel processing of nociceptive information. In: Mayer EA, Saper CB, eds. The biological basis for mind body interactions. Los Angeles: Elsevier Science BV, 2000:223–35. [DOI] [PubMed]
- 16.Mayer EA, Naliboff BD, Chang L, et al. Stress and the gastrointestinal tract v. stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2001;280:G519–24. [DOI] [PubMed] [Google Scholar]
- 17.Derbyshire SW. Visceral afferent pathways and functional brain imaging. Sci World J 2003;3:1065–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hobday DI, Aziz Q, Thacker N, et al. A study of the cortical processing of ano-rectal sensation using functional MRI. Brain 2001;124:361–8. [DOI] [PubMed] [Google Scholar]
- 19.Vogt BA, Hof PR, Vogt LJ. Cingulate gyrus. In: Paxinos G, Mai JK, eds. The human nervous system, 2nd edn. San Diego: American Press, 2002:915–46.
- 20.Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci 2003;18:3134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrovic P, Carlsson K, Petersson KM, et al. Context-dependent deactivation of the amygdala during pain. J Cogn Neurosci 2004;16:1289–301. [DOI] [PubMed] [Google Scholar]
- 22.Vogt BA, Watanabe H, Grootoonk S, et al. Topography of diprenorphine binding in human cingulate gyrus and adjacent cortex derived from coregistered PET and MR images. Hum Brain Mapp 1995;3:1–12. [Google Scholar]
- 23.Petrovic P, Ingvar M. Imaging cognitive modulation of pain processing. Pain 2002;95:1–5. [DOI] [PubMed] [Google Scholar]
- 24.Mertz H, Morgan V, Tanner G, et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology 2000;118:842–8. [DOI] [PubMed] [Google Scholar]
- 25.Naliboff BD, Derbyshire SWG, Munakata J, et al. Cerebral activation in irritable bowel syndrome patients and control subjects during rectosigmoid stimulation. Psychosom Med 2001;63:365–75. [DOI] [PubMed] [Google Scholar]
- 26.Drossman DA, Ringel Y, Vogt B, et al. Alterations of brain activity associated with resolution of emotional distress and pain in a case of severe IBS. Gastroenterology 2003;124:754–61. [DOI] [PubMed] [Google Scholar]
- 27.Chang L, Berman S, Mayer EA, et al. Brain responses to visceral and somatic stimuli in patients with irritable bowel syndrome with and without fibromyalgia. Am J Gastroenterol 2003;98:1354–61. [DOI] [PubMed] [Google Scholar]
- 28.Verne GN, Himes NC, Robinson ME, et al. Central representation of cutaneous and visceral pain in irritable bowel syndrome. Gastroenterology 2001;120:A713. [Google Scholar]
- 29.Ringel Y, Drossman DA, Leserman J, et al. IBS diagnosis and a history of abuse have synergistic effect on the perigenual cingulate activation in response to rectal distention. Gastroenterology 2003;124:A531. [Google Scholar]
- 30.Ringel Y, Drossman DA, Turkington TG, et al. Regional brain activation in response to rectal distention in patients with irritable bowel syndrome and the effect of a history of abuse. Dig Dis Sci 2003;48:1774–81. [DOI] [PubMed] [Google Scholar]
- 31.Silverman DHS, Munakata JA, Ennes H, et al. Regional cerebral activity in normal and pathologic perception of visceral pain. Gastroenterology 1997;112:64–72. [DOI] [PubMed] [Google Scholar]
- 32.Ringel Y, Drossman DA, Leserman J, et al. Association of anterior cingulate cortex (ACC) activation with psychosocial distress and pain reports. Gastroenterology 2003;124:A97. [Google Scholar]
- 33.Lieberman MD, Jarcho JM, Berman S, et al. The neural correlates of placebo effects: a disruption account. Neuroimage 2004;22:447–55. [DOI] [PubMed] [Google Scholar]
- 34.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science 2003;302:290–2. [DOI] [PubMed] [Google Scholar]
- 35.Naliboff BD, Berman S, Chang L, et al. Sex-related differences in IBS patients: Central processing of visceral stimuli. Gastroenterology 2003;124:1738–47. [DOI] [PubMed] [Google Scholar]
- 36.Porro CA, Baraldi P, Pagnoni G, et al. Does anticipation of pain affect cortical nociceptive systems? J Neurosci 2002;22:3206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taber KH, Rauch SL, Lanius RA, et al. Functional magnetic resonance imaging: application to posttraumatic stress disorder. J Neuropsychiatry Clin Neurosci 2003;15:125–9. [DOI] [PubMed] [Google Scholar]
- 38.Bub DN. Methodological issues confronting PET and fMRI studies of cognitive function. Cognit Neuropsychol 2000;17:467–84. [DOI] [PubMed] [Google Scholar]
- 39.Phan KL, Wager TD, Taylor SF, et al. Functional neuroimaging studies of human emotions. CNS Spectr 2004;9:258–66. [DOI] [PubMed] [Google Scholar]
- 40.Morgan V, Pickens D, Shyr Y. Anxiety is associated with increased anterior cingulate but not thalamic activation during rectal pain in IBS and controls. Gastroenterology 2001;120:A714. [Google Scholar]
- 41.Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy vs. education and desipramine vs. placebo for moderate to severe functional bowel disorders. Gastroenterology 2003;125:19–31. [DOI] [PubMed] [Google Scholar]
- 42.Jackson JL, O’Malley PG, Tomkins G, et al. Treatment of functional gastrointestinal disorders with anti-depressants: A meta-analysis. Am J Med 2000;108:65–72. [DOI] [PubMed] [Google Scholar]
- 43.Greene B, Blanchard EB. Cognitive therapy for irritable bowel syndrome. J Consult Clin Psychol 1994;62:576–82. [DOI] [PubMed] [Google Scholar]
- 44.Gonsalkorale WM, Miller V, Afzal A, et al. Long term benefits of hypnotherapy for irritable bowel syndrome. Gut 2003;52:1623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lackner JM, Lockwood A, Coad M, et al. Alterations in GI symptoms, psychological status, and brain function folllowing participation in cognitive therapy for IBS. Gastroenterology 2004;126:A477. [Google Scholar]
- 46.Mayberg HS, Brannan SK, Mahurin RK, et al. Cingulate function in depression: A potential predictor of treatment response. Neuroreport 1997;8:1057–61. [DOI] [PubMed] [Google Scholar]
- 47.Ketter TA, Wang PW. Predictors of treatment response in bipolar disorders: evidence from clinical and brain imaging studies. J Clin Psychiatry 2002;63 (suppl 3) :21–5. [PubMed] [Google Scholar]
- 48.Hobson AR, Furlong PL, Worthen SF, et al. Real-time imaging of human cortical activity evoked by painful esophageal stimulation. Gastroenterol 2005;128:610–9. [DOI] [PubMed] [Google Scholar]